Calciphylaxis, also known as calcific uremic arteriolopathy, is a rare syndrome that typically causes skin necrosis and usually affects dialysis patients. Its pathogenesis is multifactorial and is the consequence of many factors causing ectopic calcifications in patients with chronic kidney disease, such as calcium-phosphate metabolism disorders, hyper- or hypo-parathyroidism, diabetes, obesity, systemic inflammation and the use of vitamin K antagonists, among others. From a clinical point of view, calciphylaxis may progress from painful purpura to extensive areas of skin necrosis that can potentially lead to superinfection and the death of the patient due to sepsis. Treatment is primarily based on managing the wounds, eliminating all the possible precipitating factors of ectopic calcification and administering agents which are capable of inhibiting the process of calcification.
La calcifilaxis, también denominada arteriolopatía urémica calcificante, es un síndrome raro que causa típicamente necrosis cutánea y que afecta principalmente a los pacientes en diálisis. La patogénesis es multifactorial y depende de la suma de todos los factores que producen calcificaciones ectópicas en el paciente con enfermedad renal crónica, como las alteraciones del metabolismo calcio-fósforo, el hiper o el hipoparatiroidismo, la diabetes, la obesidad, la inflamación sistémica y el uso de inhibidores de vitamina K, entre otros. Desde un punto de vista clínico, la calcifilaxis puede evolucionar desde una púrpura dolorosa hasta extensas áreas de necrosis cutánea que pueden sobreinfectarse y llegar a causar el fallecimiento del paciente por sepsis. El tratamiento se basa fundamentalmente en el manejo de las heridas, la eliminación de todos los elementos que puedan precipitar la calcificación ectópica y el uso de agentes inhibidores del proceso de calcificación.
Calciphylaxis, also called calcific uremic arteriolopathy (CUA), is a clinical syndrome characterized by necrotic ulceration of the skin due to arteriolar calcification of the media plus fibrosis of the intima and subsequent cutaneous ischemia due to thrombosis of the arteriole. It is manifested in patients on renal replacement therapy or with low glomerular filtration, whose alteration of phosphorus and calcium metabolism seems to represent the main cause of this pathology. However, other risk factors concur in the pathogenesis of calciphylaxis so it can be manifested in patients with normal glomerular filtration rate or kidney transplanted patients, especially given the progressive increase in the average age and the high prevalence of cardiovascular pathology in these patients.1–3
It is a rare pathology, whose real incidence is unknown, although it seems to be increasing, but this could simply reflect a better knowledge of the disease. There are many undiagnosed cases, and not all are properly collected.4 The data from the American United States Renal DataSystem (USRDS) estimate that its annual incidence is between 0.01% and 0.05% of hemodialysis patients.5 More recently, a study of the dialysis units from Fresenius Medical Care North America reported an annual incidence of 0.34%,6 while the German registry reflects an annual incidence of 0.04%.7 The incidence in peritoneal dialysis patients is not well known; a study focused on this group shows an annual incidence of 0.9%.8 Less information is available on the incidence of calciphylaxis in patients with CKD not on dialysis.
It is associated with a high morbi-mortality. Morbidity is mainly due to the intense pain caused by the skin injuries and the need for hospitalization. Mortality is between 30% and 80% depending on the comorbidities and the configuration of cutaneous involvement. In recent years, mortality seems to have improved, especially since the pathology is better known, action is taken more quickly and some treatment alternatives are available. A more recent study shows a 50% mortality at 6 months and 58% after a year being septicemia the main cause of mortality.9
Pathogenesis and risk factorsIschemia and the subsequent necrosis are produced by thrombotic occlusion of the cutaneous arterioles with a thickening of their wall due to the progressive calcification of the tunica media. While the process of thrombosis is an acute event and coincides with the clinical development of the pathology, the process of calcification takes much longer, in which many factors involved in ectopic calcification act together to produce the thickening of the media throughout a longer period of time. These factors usually coexist in the patient in renal replacement therapy and are practically the same as those that are responsible for the calcification of large arteries.
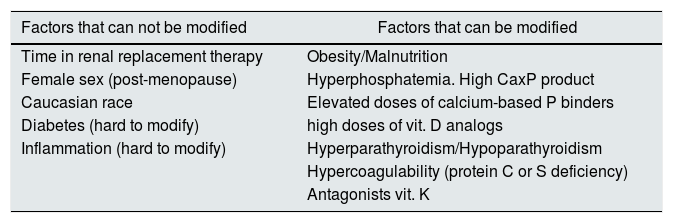
We could group these factors as non-modifiable factors and modifiable factors (Table 1). Non-modifiable modifiable factors include: time in renal replacement therapy, female gender (postmenopausal), Caucasian race, diabetes and systemic inflammation.7,9,10 The most relevant modifiable factors include obesity, malnutrition, hyperphosphatemia, high calcium-phosphorus product, use of high doses of calcium-based phosphate binders, treatment with vitamin D analogs, hyperparathyroidism or hypoparathyroidism, anticoagulation with antagonists of vitamin K and hypercoagulability states.11
Risk factors associated with the development of CUA in patients with CKD.
| Factors that can not be modified | Factors that can be modified |
|---|---|
| Time in renal replacement therapy | Obesity/Malnutrition |
| Female sex (post-menopause) | Hyperphosphatemia. High CaxP product |
| Caucasian race | Elevated doses of calcium-based P binders |
| Diabetes (hard to modify) | high doses of vit. D analogs |
| Inflammation (hard to modify) | Hyperparathyroidism/Hypoparathyroidism |
| Hypercoagulability (protein C or S deficiency) | |
| Antagonists vit. K |
Being female is considered a risk factor that suggests that women, especially post-menopause, for genetic or hormonal reasons (estrogen deficiency), have a certain predisposition to a pattern of ectopic calcification that involves the smaller vessels.
High doses of Vitamin D analogs may accelerate vascular calcification by facilitating osteogenic differentiation of vascular smooth muscle cells but, fundamentally by increasing the serum levels of phosphate and calcium. Analogs such as paricalcitol or maxicalcitol seem to induce less calcification.12
Although hyperparathyroidism has traditionally been considered as an important risk factor for uremic calciphylaxis, hypoparathyroidism has been also associated more recently with the development of calciphylaxis, in fact, adynamic bone disease has been associated with more severe vascular calcifications.13 In the German registry of calciphylaxis, 47% of the patients had PTH levels below the range recommended by the KDIGO guidelines.7
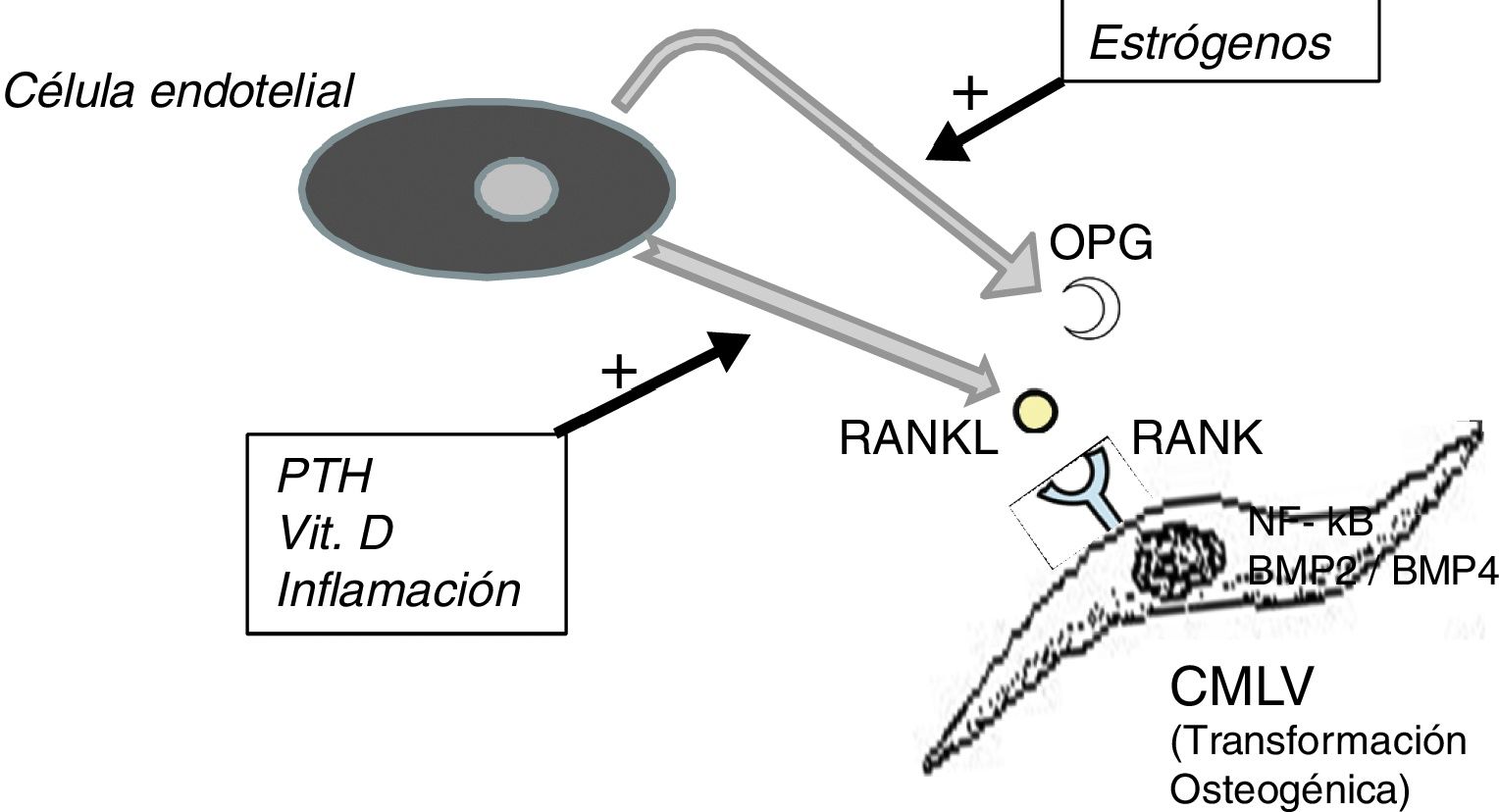
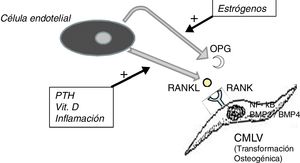
It should not be forgotten that the process of calcification of the tunica media does not consist only in the passive deposition of calcium crystals, the process of calcification is preceded by the phenotypic transformation of the mesenchymal cells of the arterial wall into osteoblastic cells, that are able to produce bone tissue inside the tunica media exactly as if it was bone. This is a finely regulated process in which all the above mentioned factors act against the inhibitory mechanisms that should have prevented ectopic calcification, such as osteoprotegerin (OPG) and Matrix G1 Protein (MGP).
The OPG is a soluble protein, synthesized mainly by osteoblasts, but also by stromal cells and endothelial cells. It acts as a decoy receptor preventing the binding of RANKL (ligand of the activating receptor of the nuclear factor κβ) to its natural receptor RANK, thus inhibiting the RANK-mediated activation of NF-kB (nuclear factor kappa B), a fundamental transcription factor for the production of growth factors, mediators of inflammation and cytokines and playing an essential role in the bone development and physiological turnover. Its inhibition is associated with the development of hypermineralized bone (osteopetrosis), while its hyperactivity is associated with loss of bone tissue and ectopic deposit of mineral. In fact, in animal models with OPG deficit, extensive vascular calcification is observed due to the hyperactivity of the RANKL-RANK-NF-kB axis14 that also promotes the activation of bone morphogenic proteins 2 and 4 (BMP2 and BMP4) favoring the osteogenic transition of smooth muscle cells (Fig. 1). In the patient with chronic kidney disease there are many factors acting on this pathway; for example, PTH, vitamin D and systemic inflammation activate RANKL and NF-kB.15 The decrease in serum estrogen levels is also associated with a decreased expression of OPG,16 which could explain the aforementioned higher incidence of calciphylaxis in post-menopausal women. Denosumab, a monoclonal antibody against RANKL, has been shown experimentally capable of preventing vascular calcification,17 although this beneficial effect has not yet been confirmed in clinical studies.18
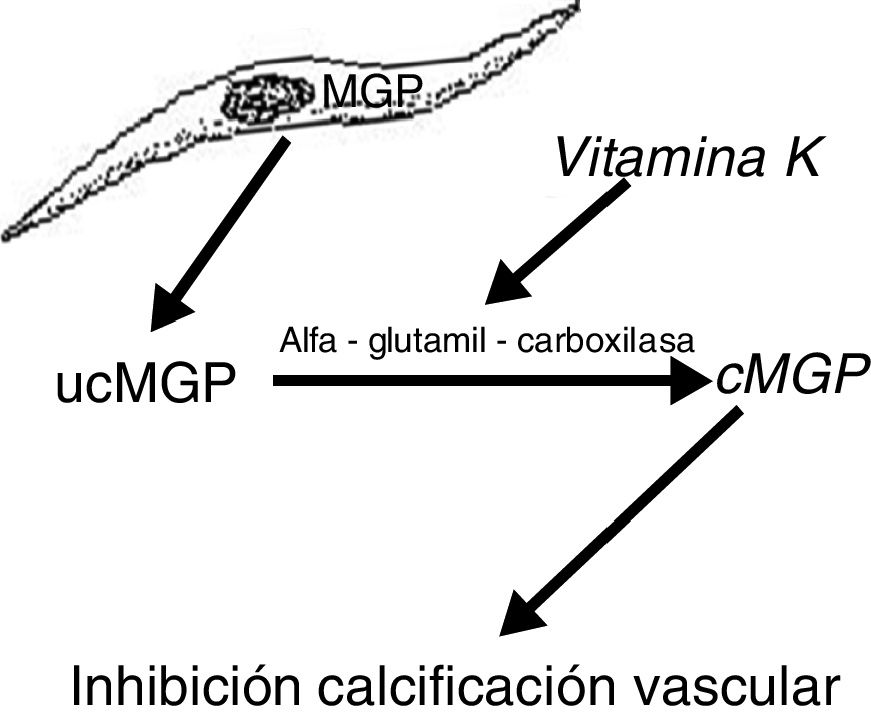
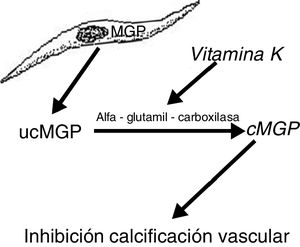
Another protein, MGP, produced by vascular smooth muscle cells, prevents transdifferentiation of the vascular smooth muscle cells into osteoblastic cells. MGP also interacts with hydroxyapatite crystals preventing calcification.19,20 This protein is activated by a vitamin K dependent carboxylation (gamma-glutamyl carboxylase). Thus vitamin K prevents vascular calcification by serving as a cofactor for the carboxylation of the MGP protein, a process that converts the decarboxylated MGP (ucMGP) into carboxylated MGP (cMGP) (Fig. 2). This explains why vitamin K antagonists, such as oral anticoagulants (acenocoumarol, warfarin), favor ectopic calcification, something that seems to be confirmed by clinical findings. Thus, a case-control study showed a strong association between the use of warfarin and the development of calciphylaxis, with an odds ratio of 11.421 and in another case control study, vitamin K deficiency, was associated with a decrease in serum levels of cMGP that were significantly reduced in patients who presented calciphylaxis. In this last study, a reduction of 0.1 units in the relative concentration of cMGP was associated with an increase of more than twice in the risk of calciphylaxis.22
It has been suggested that thrombosis of the stenosed vessel, which entails the acute onset of the clinical syndrome, may also be a consequence of a state of hypercoagulability, which may be caused by deficiency of protein C or S or even lupus anticoagulant.23,24 Thrombosis may be also associated to hypotensive episodes which may occur during the hemodialysis session.
In some cases, patients have had a previous trauma in areas where subsequently develop necrosis or, in the case of diabetics, necrosis is observed in areas of repeated insulin injections such as abdomen or upper thighs.6 This means that specific factors can precipitate the acute cutaneous necrosis, causing the thrombosis of arterioles previously stenosed over time as a consequence of all the aforementioned mechanisms.
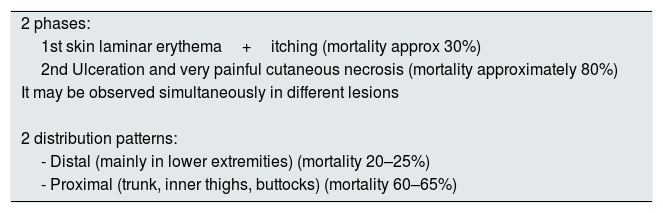
Clinical manifestations and diagnosisGenerally, the clinical manifestation of CUA presents 2 phases: the first one is manifested by cutaneous laminar erythema and pruritus, the second with ulceration and very painful cutaneous necrosis (a pain disproportionate to the cutaneous lesion), however both types of lesions may occur simultaneously. While the first lesions are associated with a 30% mortality, the second carries a much higher mortality rate, approximately 80%.25
The distribution of skin lesions is not homogeneous, there are two classic patterns of disease (Table 2). The first, a distal pattern, which occurs in approximately 90% of cases, the lesions are observed in the lower extremities, especially between the ankle and calf, although there are cases affecting the fingers, hands and even genitals. The second type of pattern, is proximal, less frequent and affects areas with more adipose tissue such as the trunk, inner thighs, buttocks and, occasionally, the breasts. In general, this pattern is considered to have a worse prognosis than the distal pattern. However, there are series that report similar mortality rates (distal 70%, proximal 76%).26
Clinical manifestations of the CUA.
| 2 phases: |
| 1st skin laminar erythema+itching (mortality approx 30%) |
| 2nd Ulceration and very painful cutaneous necrosis (mortality approximately 80%) |
| It may be observed simultaneously in different lesions |
| 2 distribution patterns: |
| - Distal (mainly in lower extremities) (mortality 20–25%) |
| - Proximal (trunk, inner thighs, buttocks) (mortality 60–65%) |
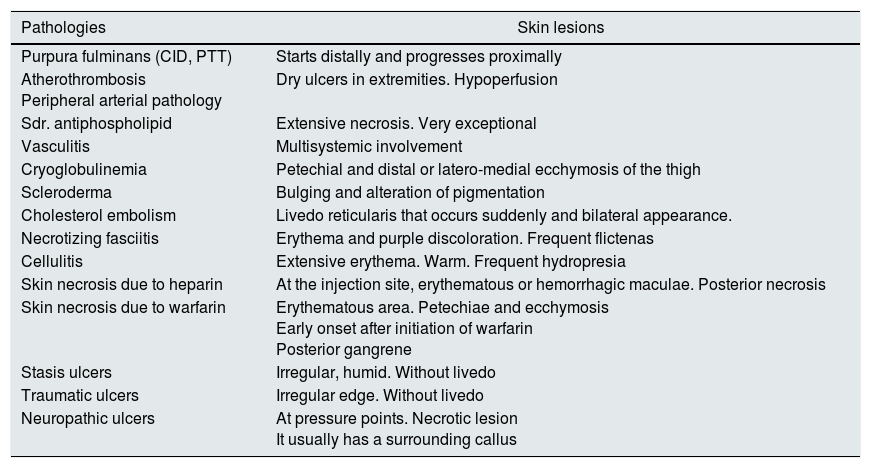
In the differential diagnosis, it is necessary to take into account other pathologies that may present with a similar cutaneous Picture.11Table 3 groups the dermatological manifestations of pathologies that could represent some kind of confusion with CUA; however, most of them can be ruled out by considering differences in the back ground, the clinical history, the blood chemistry or ultimately by histological findings.
Differential diagnosis of CUA lesions.
| Pathologies | Skin lesions |
|---|---|
| Purpura fulminans (CID, PTT) | Starts distally and progresses proximally |
| Atherothrombosis Peripheral arterial pathology | Dry ulcers in extremities. Hypoperfusion |
| Sdr. antiphospholipid | Extensive necrosis. Very exceptional |
| Vasculitis | Multisystemic involvement |
| Cryoglobulinemia | Petechial and distal or latero-medial ecchymosis of the thigh |
| Scleroderma | Bulging and alteration of pigmentation |
| Cholesterol embolism | Livedo reticularis that occurs suddenly and bilateral appearance. |
| Necrotizing fasciitis | Erythema and purple discoloration. Frequent flictenas |
| Cellulitis | Extensive erythema. Warm. Frequent hydropresia |
| Skin necrosis due to heparin | At the injection site, erythematous or hemorrhagic maculae. Posterior necrosis |
| Skin necrosis due to warfarin | Erythematous area. Petechiae and ecchymosis Early onset after initiation of warfarin Posterior gangrene |
| Stasis ulcers | Irregular, humid. Without livedo |
| Traumatic ulcers | Irregular edge. Without livedo |
| Neuropathic ulcers | At pressure points. Necrotic lesion It usually has a surrounding callus |
The skin biopsy would confirm the diagnosis of calciphylaxis. The tissue sample should be obtained from the edges of the lesion to avoid necrotic tissue, as this may spoil the diagnosis, but it cannot be taken too far away from the edge because in this case it may result in false negatives. The biopsy should be obtained with a depth of at least 4–5mm in order to include subcutaneous fat in the sample, so the affected arterioles are examined facilitating the diagnosis. It has been described that occasionally the skin biopsy may be complicated by superinfection or further necrosis. Therefore, the risks and benefits of performing a biopsy should be weighed in each patient, especially those who have a very revealing clinical picture that can make it de biopsy unnecessary. The classic anatomopathological findings are defined by the calcification of the tunica media of arterioles from the cutaneous or subcutaneous fat tissue, associated with an important fibro-intimal thickening that decreases the lumen of the vessel which is associated to thrombosis of residual lumen in some vessels. The calcification of the media can be evidenced with a positive Von Kossa staining27 or may be seen as a violet pattern with hematoxylin and eosin staining.
Recently, there have been proposed diagnostic criteria, that have some limitations but these are the only one available. According to these criteria, the diagnosis of calciphylaxis can be made if the three following clinical features are present: (1) patient on hemodialysis or with glomerular filtration <15ml/min/1.73m2 (2) with two painful and non-curable ulcers with associated painful purpura (3) present in the trunk, extremities or penis. In case there are only two of the criteria above mentioned, the diagnosis must be confirmed with is a biopsy showing the histological signs of calciphylaxis.26
Imaging tests may contribute to the diagnosis of calciphylaxis. A simple X-ray of the affected area can demonstrate the presence of calcifications of the vessels of medium-small caliber. In a retrospective multicenter study of 29 patients, the presence of a net-like pattern of calcifications in the soft tissues showed a very high specificity, around 90%.28 Bone scintigraphy has also shown some utility, with the tracer accumulating diffusely in the areas corresponding to the clinical findings. It could also be used to monitor the effect of treatment.29 An X-ray performed with mammography technique, due to its better graphic definition, may show calcifications of the small cutaneous vessels and can be very useful in the differential diagnosis of these lesions.30 Ultrasound, being an inexpensive tool, easily available and safe, can contribute to the diagnosis in patients with high clinical suspicion. In these cases, ultrasound of the affected areas may show vascular calcifications in the subcutaneous tissues as hyperechoic deposits with shadow cones.30 There are also cases where the presence of calcified vessels of small and medium caliber has been demonstrated using a high resolution CT. However, considering the risk to the patient, the cost and the greater sensitivity of the simple radiography or mammography technique, routine use of CT is not recommended.31 In short, today there is no image test with enough specificity/sensitivity for the diagnosis of calciphylaxis, perhaps the simple X-ray with mammography technique seems to offer the best results.
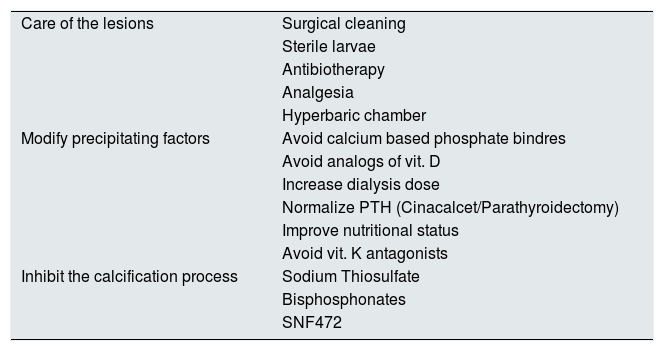
Therapeutic managementThe treatment of calciphylaxis could be articulated in three complementary levels (Table 4). First is the management of the lesions from the medico-surgical point of view, a second therapeutic approach should aim to modify all the possible precipitating factors of the ectopic calcification, and a third level of care should be to use all those compounds that actively inhibit the cutaneous calcification process.
Possible alternatives for the treatment of CUA in patients with CKD.
| Care of the lesions | Surgical cleaning |
| Sterile larvae | |
| Antibiotherapy | |
| Analgesia | |
| Hyperbaric chamber | |
| Modify precipitating factors | Avoid calcium based phosphate bindres |
| Avoid analogs of vit. D | |
| Increase dialysis dose | |
| Normalize PTH (Cinacalcet/Parathyroidectomy) | |
| Improve nutritional status | |
| Avoid vit. K antagonists | |
| Inhibit the calcification process | Sodium Thiosulfate |
| Bisphosphonates | |
| SNF472 |
Surgical wound cleaning is the first step in the management of injuries. Two retrospective studies have shown that the one-year survival of patients who underwent debridement was 60% at 6 months and 70% a year, while in patients who did not perform debridement it was 27% at 6 months and 50% after a year, respectively.9,32
The sterile larvae of Lucilia sericata have been shown to be useful for the debridement of wounds such as non-scarring ulcers of diabetic patients, pressure ulcers or post-surgical ulcers. These larvae produce and secrete digestive enzymes that selectively dissolve the necrotic tissue, disinfect the wound and stimulate healing.33 They have been used successfully as adjuvant therapy in calciphylaxis lesions, especially in lesions with extensive necrotic areas and panniculitis.34
It also seems reasonable to try to isolate microbiological agents to guide a specific antibiotic therapy or, if this is not possible, broad-spectrum therapy should be used with coverage for Gram-positive and anaerobics.
The mechanism of pain is poorly understood, it is believed to be caused by both ischemia and neuropathic pain secondary to nerve inflammation. The pain is very severe and quite resistant to opiates. There is no controlled study that shows an optimal analgesic approach. The need for powerful analgesia without toxicity requires drugs that do not depend on renal function or that are easily dialysable. However, case series suggest that an analgesic regimen that includes opioids, ketamine, and non-opioid adjuvants (e.g., gabapentin or tricyclic antidepressants) may be effective.35 However, perhaps fentanyl, buprenorphine and methadone may be more recommended than opioids since, in patients with CKD, they are associated with less toxicity. The use of topical ketamine or topical opioids, such as gels with morphine infusion, can offer reasonable local control of pain, with potentially less systemic side effects, but there is very limited information.
It has been reported that the use of the hyperbaric chamber may promote the recovery of necrotic lesions as it usually happens in large burns, also favoring the proliferation of fibroblasts, collagen and angiogenesis.36
A second step, and taking into account that many of the factors that produce calciphylaxis are reversible, it modify those factors involved in the calcification process. In relation to calcium-phosphorus metabolism, it will be crucial to avoid hypercalcemia and hyperphosphatemia. Then, depending on the case, it will be necessary to discontinue the treatment with vitamin D analogs and/or selective VDR agonists and to use phosphate binders, carefully avoiding those that are calcium based.
In case of patients undergoing hemodialysis, the dose of dialysis should be increased to augment clearance of phosphate and uremic toxins. Regarding hyper or hypoparathyroidism, it is advisable to reach the values suggested by the Recommendations of the Spanish Society of Nephrology37 which in the dialysis patient considers that iPTH values should be between 150 and 300pg/ml, being acceptable up to 500pg/ml. It should not be forgotten that both high and low bone remodeling are associated with ectopic calcification. In case of severe hyperparathyroidism, in addition to treating hypocalcemia and hyperphosphatemia, Cinacalcet should be prescribed and parathyroidectomy may be considered. It has been reported that the management of secondary hyperparathyroidism with Cinacalcet may decrease the number of calciphylaxis.38 In patients with severe secondary hyperparathyroidism, subtotal parathyroidectomy, compared with patients without parathyroidectomy, has been associated with significantly better survival at both 6 months (90% versus 42%) and at 5 years (53% versus 11%).9 In the case of hypoparathyroidism, it is mandatory to avoid hypercalcemia and hyperphosphataemia.
As nutritional status is associated with the development of calciphylaxis, it is very important to maintain patients nutrition even with added supplements or total parenteral nutrition if necessary.
Antagonists of vitamin K (acenocoumarol, warfarin) are probably one of the best-known factors that precipitate calciphylaxis. The treatment with vitamin K antagonists, should be discontinued at all costs and, if anticoagulation is necessary, assess the conversion to subcutaneous heparin or non-vitamin K antagonist oral anticoagulants. The latter present the problem of the difficulty in dose adjustment. Rivaroxaban, Apixaban and Edoxaban (factor Xa inhibitors) are approved in Europe, with a corresponding dose reduction, for patients with stage 4 chronic kidney disease. Apixaban and Edoxaban seem to be the most recommended. Apixaban is eliminated by 27% in urine and Edoxaban by 50%, but in general terms a dose reduction of 50% is recommended for both. For patients on dialysis, positive experiences have been reported with Apixaban 2.5mg every 12h and with Rivaroxaban 10mg every 24h. Other alternatives or higher doses carries a considerable risk of hemorrhage.39
The third level of therapy includes maneuvers to improve/inhibit the calcification process.
The administration of sodium thiosulfate is probably the most common off-label treatment of calciphylaxis. Its mechanism of action is based on its ability to form soluble complexes with many metals and minerals, as well as a possible effect as vasodilator, antioxidant and direct inhibitor of the calcification process.11 In the largest retrospective study of 172 patients on hemodialysis with calciphylaxis, a total recovery or improvement was observed in 70% of the treated patients.40 The most widely accepted dosage is 25g (100ml of 25% solution) intravenously 3 times per week (after each hemodialysis session). A multicenter randomized clinical trial of sodium thiosulfate versus placebo in patients with calciphylaxis is currently in the recruitment phase. Other drugs that have been shown to be effective in the treatment of calciphylaxis are bisphosphonates, which have an inhibitory action on calcification through their binding to hydroxyapatite crystals. In a retrospective study, 11 consecutive patients treated with bisphosphonates in the period 2002–2010 all obtained a restitution ad integrum.41 Of course, the effectiveness of this treatment must be weighed against the risk of inducing an adynamic bone disease in patients with end-stage renal disease. However, taking into account the mortality associated with calciphylaxis, the risk/benefit ratio plays undoubtedly in favor of the use of bisphosphonates. Our current treatment scheme is to administer intravenous Pamidronate 60mg (in 100ml of saline to pass during the last hour of dialysis), a dose that is repeated at 15 days and after a month. In case of absence of complete restitution of the lesions we propose a new dose of 60mg at three months.
The SNF472 is currently in the development phase, as an alternative treatment for Calciphylaxis in patients in hemodialysis. SNF472 is an intravenous formulation of myo-inositol hexaphosphate (IP6). SNF472 selectively inhibits the formation and growth of hydroxyapatite crystals and thus vascular calcification. In a phase 2 trial it has been shown to be effective in reversing calciphylaxis lesions.42
ConclusionsCalciphylaxis is a rare disease that mainly affects patients with CKD on dialysis and is due to the progressive and chronic calcification of small vessels followed by sudden thrombosis. The risk factors mostly associated with the development of calciphylaxis are the time in renal replacement therapy, alterations in calcium and phosphate metabolism, female gender, obesity and treatment with vitamin K antagonists. The most reliable diagnostic technique is skin biopsy, although often the clinical presentation may be sufficient to make the diagnosis. The initiation of a rapid treatment is essential to prevent the progression of the lesions and their superinfection. The surgical cleaning of the wounds, the antibiotic therapy, the elimination of the precipitating factors of the ectopic calcification and the administration of drugs such as sodium thiosulfate or bisphosphonates, are the current therapeutic options for their management.
To improve the knowledge of calciphylaxis at present there are several international Registries, and specifically in Spain, the registry of the Spanish society of nephrology. It is fundamental that, when a patient is diagnosed, the data are entered in the registry, because it is the only way to carry out studies with larger number of patients.
Key concepts- •
Calcifilaxis, also known as calcifying uremic arteriolopathy, is a rare pathology that mainly affects patients with chronic kidney disease, especially in dialysis. The incidence, although with limited information, seems to be increasing.
- •
It is a clinical syndrome typically characterized by necrotic ulceration of the skin due to calcification of the media plus fibrosis of the arteriolar intima and posterior cutaneous ischemia due to thrombosis.
- •
There are many factors that contribute to the pathogenesis and development of calcifications of the wall of small caliber vessels of the skin and subcutaneous tissue.
- •
Modifiable risk factors are obesity, malnutrition, elevated CaxP product, especially hyperphosphatemia, high doses of calcium based phosphate binders, vitamin D analogs, hypercoagulability and, most important, the use of vitamin K antagonists.
- •
It is usually manifested in two phases; first, cutaneous erythema and pruritus and the second with very painful ulceration and skin necrosis. The latter is asociated with a high mortality rate.
- •
The distribution of the lesions may be proximal (areas with more adipose tissue) or distal which is the most frequent and with a better prognosis.
- •
The diagnosis is mainly clinical, although the role of skin biopsy is very important, especially in doubtful cases.
- •
Therapeutic alternatives include the medical–surgical management of injuries, modify all the possible precipitating factors of the calcification and employ compounds that actively inhibit the process of cutaneous calcification such as sodium thiosulfate or bisphosphonates.
The authors approve the submission of the article for publication to the Nefrologia Journal and declare that it has not been sent to any other journal or sent for publication simultaneously. The authors also declare that there has been no funding for the completion of this work and that their intellectual property is assigned to Nefrologia. Both authors (DC and JVT) have participated to the same degree in the writing of the article. The authors approve the final form thereof and are responsible for the content of the text.
Conflicts of interestThe authors declare that they do not have any type of conflict of interest.
Please cite this article as: Cucchiari D, Torregrosa J-V. Calcifilaxis en pacientes con enfermedad renal crónica: una enfermedad todavía desconcertante y potencialmente mortal. Nefrología. 2018;38:579–586.