Carfilzomib is a proteasome inhibitor drug with antiproliferative and pro-apoptotic activity. It is one of the drugs used in the treatment of refractory multiple myeloma.1–4 The onset of atypical haemolytic uremic syndrome (aHUS) associated with the use of carfilzomib is a complication previously described in the literature.5–10 We describe a case of aHUS secondary to carfilzomib effectively treated with eculizumab.
This case involves a 71-year-old woman with refractory Bence-Jones Kappa multiple myeloma treated with carfilzomib, daratumumab and dexamethasone (KdD). The patient was admitted to the Haematology Department due to onset of malaise and fever 2 days after receiving the second cycle of KdD. Despite negative microbiology results, her blood pressure increased to over 180/100mmHg on the fourth day. Blood tests found a decrease in haemoglobin of up to 6.8g/dl and in platelets of up to 12,000, with an increase in LDH of up to 900IU/l, accompanied by a deterioration of renal function, with serum creatinine levels of 2.6mg/dl. Suspecting thrombotic microangiopathy (TMA), treatment with prednisone (1mg/kg/day) and fresh plasma was started, with no improvement, and the patient was referred to the Nephrology Department for assessment.
Studies performed by the Nephrology Department showed 5–6% schistocytes per field on peripheral blood smear, no signs of malignant arterial hypertension on fundoscopy, undetectable haptoglobin in blood, and negative direct Coombs test. ADAMTS13 test was normal (ADAMTS13>50%). Other causes of aHUS and typical haemolytic uraemic syndrome (STEC-HUS) were ruled out, and genetic studies were not performed due to the high suspicion of a pharmacological cause. Carfilzomib was discontinued and treatment with eculizumab was started after administration of meningococcal prophylaxis with oral penicillin. Plasmapheresis was not performed due to the poor status of the patient and to avoid the comorbidity associated with the technique. One week after the third dose of eculizumab, serum creatinine levels had normalised (1.1mg/dl) without the need for any haemodialysis sessions, and schistocytes had disappeared. From a haematological point of view, partial improvement was observed in haemoglobin and platelet levels, probably due to the underlying disease, although no schistocytes were found. The patient received 3 weekly doses of 900mg of eculizumab, which was discontinued after improvement of renal function and stabilisation of haemolysis parameters.
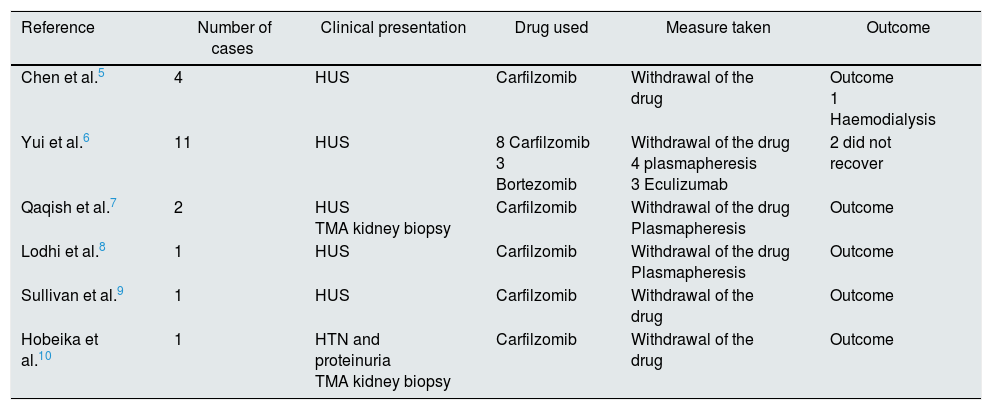
Based on the accumulated evidence relating to the pathogenesis of this condition, we wonder whether treatment with eculizumab is justified. In the years since it was released, carfilzomib has been associated with several cases of TMA5–10 (Table 1). The mechanism by which the damage occurs is thought to be related to the accumulated dose or to an alloimmune effect. Some authors suggest, however, that proteasome inhibitors decrease production of vascular endothelial growth factor (VEGF) by inhibiting the activity of proteins that regulate its genetic transcription. This results in endothelial and podocyte damage similar to that caused by anti-VEGF drugs.6,8 The latter is the most plausible of the 3 proposed mechanisms, since aHUS has occurred in patients who have only received 2 doses, as in our case, and not in other patients with higher cumulative doses. Likewise, the alloimmune mechanism is an unlikely culprit, given the context of administration of a plasma cell depleting drug in patients that are highly immunosuppressed due to previous chemotherapy and, as in our case, do not respond to immunosuppressive treatment (prednisone).
Review of cases of TMA associated with the use of carfilzomib.
| Reference | Number of cases | Clinical presentation | Drug used | Measure taken | Outcome |
|---|---|---|---|---|---|
| Chen et al.5 | 4 | HUS | Carfilzomib | Withdrawal of the drug | Outcome 1 Haemodialysis |
| Yui et al.6 | 11 | HUS | 8 Carfilzomib 3 Bortezomib | Withdrawal of the drug 4 plasmapheresis 3 Eculizumab | 2 did not recover |
| Qaqish et al.7 | 2 | HUS TMA kidney biopsy | Carfilzomib | Withdrawal of the drug Plasmapheresis | Outcome |
| Lodhi et al.8 | 1 | HUS | Carfilzomib | Withdrawal of the drug Plasmapheresis | Outcome |
| Sullivan et al.9 | 1 | HUS | Carfilzomib | Withdrawal of the drug | Outcome |
| Hobeika et al.10 | 1 | HTN and proteinuria TMA kidney biopsy | Carfilzomib | Withdrawal of the drug | Outcome |
Secondary aHUS should be treated by withdrawing the causative agent.1,2 In some series, plasmapheresis has been used, with no clear benefit for symptoms.6,7 In many cases, TMA is resolved by withdrawing the drug. However, as shown by the review published by Cavero et al., early use of eculizumab may be effective in cases of refractory TMA in which impaired renal function persists or worsens, or in life-threatening situations.2 Recent studies have shown that the alternative pathway of the complement system is temporarily activated in cases of secondary aHUS. Based on this evidence, the new classification of thrombotic microangiopathies proposed in the KDIGO guidelines1 now includes all secondary TMAs with ADAMTS13>10% activity in the context of aHUS. Therefore, in cases of secondary aHUS that does not improve despite withdrawal of the causative agent, we recommend early, short-term administration of eculizumab.
Please cite this article as: Moliz C, Gutiérrez E, Cavero T, Redondo B, Praga M. Síndrome hemolítico urémico atípico secundario al uso de carfilzomib tratado con eculizumab. Nefrologia. 2019;39:86–88.