To the Editor,
It is well known that acute haemolysis is a cause of acute renal failure due to tubular damage caused by pigments being deposited in the proximal tubule. Maintained haemolysis can produce chronic renal damage, caused by different mechanisms.
We present two patients with intravascular haemolysis produced by different causes, both with acute renal failure but different progression.
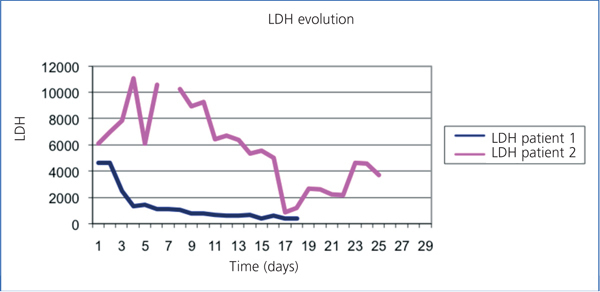
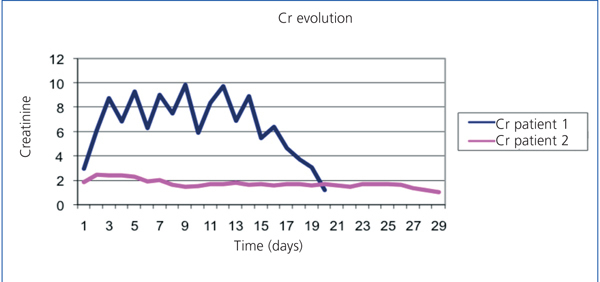
A 57-year-old male, crop-sprayer, was admitted to the haematology department due to non-immune haemolytic anaemia (negative direct Coombs test). He had organophosphate poisoning, which had lasted 4 days, with oligoanuria and acute renal failure. Haemolysis stopped after two plasmapheresis sessions. Upon admission he presented with: haemoglobin (Hb): 6.3g/dl, haemocrit (Ht): 19%, leukocytes 30 630/µl (Ne 77.7%), platelets: 217 000/µl. Blood smear: intense anisocytosis, polychromatophilia, microspherocytes (7-8/field). occasional basophilic stippling, presence of erythroblasts. There were no schistocytes. Haptoglobin: 10.4mg/dl. Total bilirubin: 8.60mg/dl. Conjugated bilirubin: 2.70mg/dl. Indirect bilirubin: 5.90mg/dl. Myoglobin 170.5µg/l, lactate dehydrogenase (LDH) 4637IIU/l (Figure 1). Iron: 242µg/dl; ferritin: 2754ng/ml; Urea: 188mg/dl; creatinine: 2.95mg/dl (Figure 2). Urine: proteins +++. Haemoglobin: ++++, 10 red blood cells/field. Renal function worsened gradually, presenting with anuria and a glomerular filtration rate of 6ml/min 24 hours after, which required 17 sessions of haemodialysis. Renal function was completely recovered after 2 months.
A 71-year-old male, with a metal aortic and mitral prosthesis because of a rheumatic valve disease. He was admitted to the cardiology department to stop a periprosthetic mitral valve leak. He presented with acute haemolytic anaemia secondary to attempting a percutaneous closure and chronic haemolysis. He had baseline Hb: 10.6g/dl and LDH: 1500-2000IIU/l. Baseline renal function: 71.86ml/min, with Cr: 1.85mg/dl. During this episode he presented with Hb: 7.6g/dl; haematocrit: 25.2% ; leukocytes : 7070/µl (Ne: 74.5%) ; platelets: 261 000. Smear: abundant schistocytes. Haptoglobin: <7.56mg/dl. Total bilirubin: 4.80mg/dl; direct bilirubin: 0.6mg/dl; LDH: 10 500IIU/l (Figure 1); normal iron profile; urea: 83mg/dl; Cr: 2.46mg/dl (Figure 2). Urine: proteins: 150mg/dl; haemoglobin: ++++, 47 red blood cells/field. Renal function was maintained stable with conservative treatment. Following the surgical closure, the leak stopped the haemolysis and the renal function recovered up to a glomerular filtration rate of 57ml/min, with Cr 1.7mg/dl.
Intravascular haemolysis of any cause can produce acute tubular necrosis, due to haemoglobinuria. It presents with red/brown urine and plasma, low haptoglobin, elevated LDH, deteriorated renal function and fractional excretion of sodium less than 1%. The incidence is unknown, reaching 50% in massive haemolysis.1,2
Haemoglobin is released to the plasma, binds to haptoglobin and is degraded by the reticuloendothelial system. When the haptoglobin is saturated, the free haemoglobin goes from its usual tetrameric form to a dimeric form. It filters through the glomerulus and goes inside the proximal tubule when it binds to the apical surface of megalin-cubulin receptor.3 It is there that globin and haem group are dissociated. The intracellular increase of proteins in the haem group produces nephrotoxicity caused by renal hypoperfusion, direct cytotoxicity and formation of intratubular casts interacting with Tamm-horsfall protein, which obstruct the tubules.4
During massive haemolysis, deleterious effects of the nitric oxide depletion are observed: smooth muscle tone imbalance, vascular constriction, thrombosis and intrarenal vasoconstriction.5
Chronic damage is produced because the kidney is continually exposed to the haem group, mediated by monocyte chemoattractant protein-1 (MCP-1) and TGFb1, which recruit monocyte and macrophages and provoke fibrosis.4
These cases show the two forms kidney damage expression in haemolysis: acute and chronic. The first had acute haemolysis and required haemodialysis, with complete recovery of the renal function. The second had chronic haemolysis and chronic renal damage due to maintained exposure to the haem group, and needed conservative treatment, maintaining certain renal failure.
Figure 1. Comparing LDH evolution in two patients.
Figure 2. Comparing evolution of creatinine figures in two patients.