Objetivos: Determinar la frecuencia y tipo de alteraciones de la función tiroidea en niños con insuficiencia renal crónica (IRC) en programa de diálisis peritoneal (DP) o hemodiálisis (HD), así como establecer la utilidad de bocio como marcador clínico para identificar pacientes con IRC que cursan con alteraciones de la función tiroidea. Pacientes y métodos: Estudio transversal y descriptivo, realizado en un hospital pediátrico de tercer nivel de atención. Se incluyeron pacientes menores de 17 años, con IRC y con más de tres meses en DP o HD. En cada paciente se evaluó su crecimiento y desarrollo, así como la presencia de bocio. Las alteraciones tiroideas se detectaron mediante la cuantificación de los niveles séricos de tirotropina (TSH), tiroxina (T4L) y triyodotironina (T3T). Resultados: Se incluyeron 50 pacientes, 25 del sexo masculino, con edad promedio de 3 años. Hubo 14 (28%) pacientes con alteración en la función tiroidea, nueve con hipotiroidismo subclínico, tres con síndrome de enfermo eutiroideo y dos con hipotiroidismo primario. En 13 pacientes se detectó bocio, siete con disfunción tiroidea y seis con función normal. La sensibilidad del bocio para la detección de alteraciones tiroideas fue del 50%, y la especificidad del 83.3%. Dos de los pacientes con hipotiroidismo presentaron la mayor afectación en su crecimiento. Conclusiones: Debido a la alta frecuencia de alteraciones tiroideas en niños con IRC, es necesaria su valoración de manera sistemática, a fin de mejorar la calidad de su atención.
INTRODUCTION
In children, CRF is caused by slow, progressive kidney diseases such as obstructive uropathy, renal dysplasia, glomerulosclerosis, reflux nephropathy or systemic autoimmune diseases.1-4 In the guidelines for handling paediatric patients with CRF it is recommended that when renal function is < 15ml/min/1.73m2, renal replacement therapy must be initiated, which includes peritoneal dialysis (PD), haemodialysis (HD) and kidney transplant.5
Two thirds of catabolism of hormones takes place in the kidneys. In patients with renal failure, renal clearance decreases at the same time as renal blood flow; as this progresses, renal tubular and peritubular transport of hormones decreases, causing disparity in serum hormone concentrations.6 Different studies have shown thyroid dysfunction in patients with CRF which includes: low circulating concentrations of thyroid hormones, altered peripheral thyroid hormone metabolism and altered binding to transporting proteins, as well as a reduction in thyroid hormone content and an increase in the iodide reserves in the thyroid glands.7-9
In clinical terms, euthyroid sick syndrome (ESS) is the most common condition, followed by subclinical hypothyroidism.7-9 ESS affects subjects without thyroid disease and with a biochemistry that is commonly characterised by a decrease in triiodothyronine (T3T) and occasionally, free thyroxine 4 (FT4), as well as an increase in reverse T3 levels; whereas thyroid stimulating hormone levels (TSH) remain normal. Subclinical hypothyroidism is characterised by an increase in levels of TSH, however T3T and FT4 values remain within the normal ranges.10
Given the similarity of signs and symptoms, sometimes it is difficult to identify subjects with CRF who also present hypothyroidism,11,12 therefore, different studies have been carried out to establish the incidence of these conditions. Most of these studies involve adult populations and indicate a prevalence that ranges between 5 and 30%.7-9,13,14 Few studies involving children with CRF have been published, however an incidence of thyroid dysfunction that ranges between 10 and 55%15,16 has been found. However, these studies have typically involved a population of less than ten children, whereas those involving adults describe up to 200 patients. With regard to the type of condition, in the case of both children and adults, ESS was most common, followed by primary hypothyroidism. Secondary hypothyroidism9 and goiter10,13 was only described in adults.
Since the information available regarding the paediatric population is limited, the aim of this study was to establish the incidence and type of thyroid dysfunctions that affect children with CRF in PD or HD, and to determine whether goiter could be used as a clinical marker to help identify patients with CRF and thyroid dysfunction.
MATERIAL AND METHOD
A cross-sectional, prospective study was designed and carried out in the Mexican Institute of Social Security and the Departments of Nephrology and Paediatric Endocrinology of the Paediatric Hospital in the Twenty-First Century National Medical Centre, located in Mexico City. This is a third level, reference hospital that sees patients from Mexico City and several other states in inland Mexico. Before starting this study, the protocol was approved by the Local Ethics and Research Committee of the aforementioned hospital; parents and patients agreed to participate in the study and signed a letter of informed consent.
All children aged 4 to 17 with CRF in PD or HD for more than three months were included in the study. Age, sex, weight, height and the stage of sexual maturity using the Tanner scale17 were recorded, as well as the cause of CRF and the duration of renal replacement treatment, specifically, PD and HD. The nutritional status was evaluated according to the weight for age, height for age and weight for height Z scores, using the anthropometric program of the statistics package Epi-Info version 6.0.
Goiter was detected by direct palpation of the thyroid glands and confirmed when they were larger in size than the distal phalange of the child¿s thumb.18,19 This assessment was carried out independently by two Endocrinology specialists. Their assessments concurred in over 80% of cases examined. When there was any discrepancy regarding the presence of goiter the opinion of a third specialist with over 20 years experience was sought.
In order to evaluate thyroid function, a blood sample was taken; in HD patients the sample was taken before dialysis was carried out. T3 and T4 concentrations were established using radioimmunoassay (Inmunotech Beckman Coulter, Czech Republic); TSH was measured using immunoradiometric assay (Inmunotech Beckman Coulter, Czech Republic). For the purpose of this study, normal values of TSH values were set at 0.17-4.06mU/ml; at 0.89- 1.8ng/ml for T4; and at 78-182ng/ml for T3. Having established these values,euthyroidism was defined as levels of T3, T4 and TSH within the normal ranges; primary hypothyroidism was characterised by levels of TSH > 10mU/ml and T3 and T4 below normal levels; subclinical hypothyroidism was characterised by TSH between 4.07 and 9.9mU/ml and normal T3 and T4 levels, and ESS was characterised by normal TSH levels and T4 or T3 below the normal ranges.10
Statistical analysis. Since the distribution of variables was not normal, quantitive variables are expressed as a median and minimum and maximum values. The comparison of different groups of qualitative variables was carried out using the Chi-squared or Fisher¿s exact test. The U-Mann- Whitney and Spearman¿s Rho test were used for quantitative variables. In order to establish the usefullness of goiter as a clinical marker of hypothyroidism, the diagnostic test used was a blood test, therefore the sensitivity, specificity, as well as the positive predictive value and the negative predictive value were calculated. A value of p < 0.05 was considered statistically significant.
RESULTS
During the period of study, 74 patients with CRF were in chronic dialysis, and of these 50 met the selection criteria.
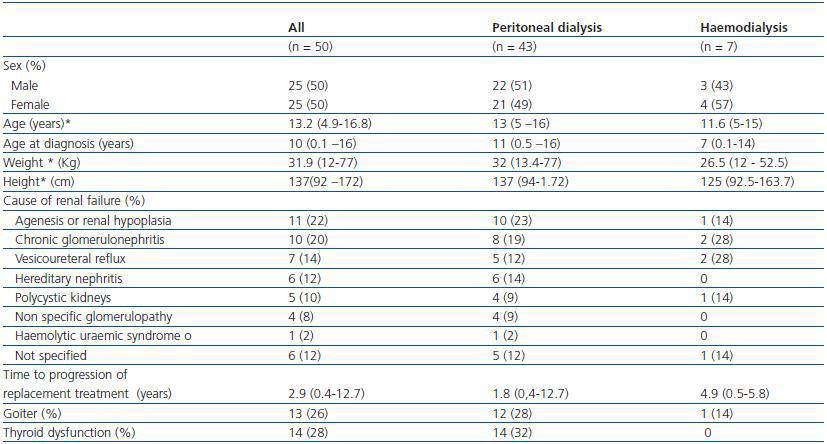
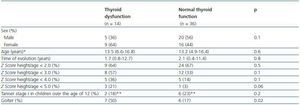
Twenty-five of the patients (50%) were male and the mean age was 13, with ages ranging from 4 years and 9 months to 16 years and 8 months. As shown in Table 1, the cause of CRF was identified in 44 patients (88%); the three most common causes were agenesis or renal hypoplasia, chronic glomerulonephritis and vesicoureteral reflux. On average, patients were diagnosed with CRF at the age of 10. Forty-three out of fifty patients (86%) were in PD dialysis and seven (14%) were in haemodialysis. At the time of evaluation, the median time in replacement therapy was one year. Table 1 also shows that, in general, there were no differences between those patients in PD and those in HD, with the exception of age at the time of diagnosis, which was lower for children in HD.
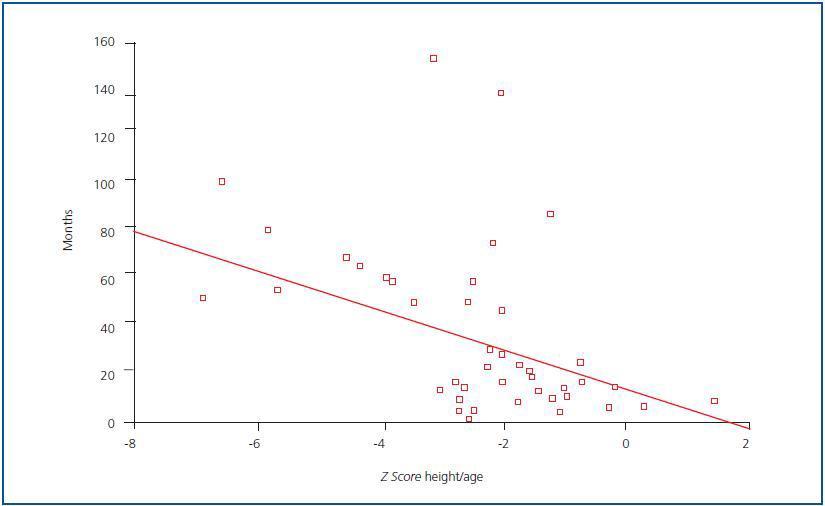
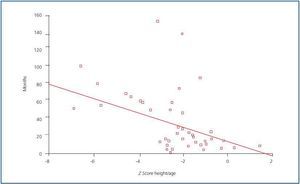
With regard to growth, a higher proportion of patients experienced growth problems, given that height for age was affected in 66% of cases. The weight for height of all patients was normal. When the Z score of height for age was correlated with diseaseprogression, a negative correlation was found (figure 1); in other words, the longer the progression time of CRF, the greater the effect on height: r = -0.42; p = 0.001.
With regard to maturity or sexual development, only takinginto consideration those patients over the age of 12 (n = 33 patients; 66%), it was established that there was a delay in sexual development in 8/33 cases (24.2%), since they were identified as Tanner stage I. Of these, five were male and three were female patients.
Evaluation of thyroid function
Out of the total number of patients, 36 (72%) had normal thyroid function, and dysfunctional thyroid incidence affecting children with CRF was 28% (14 patients) in this study.With regard to the type of dysfunction, nine (64.2%) were diagnosed with subclinical hypothyroidism, three (21.4%) with ESS and two (14.2%) with primary hypothyroidism. All patients with thyroid dysfunction were in PD.
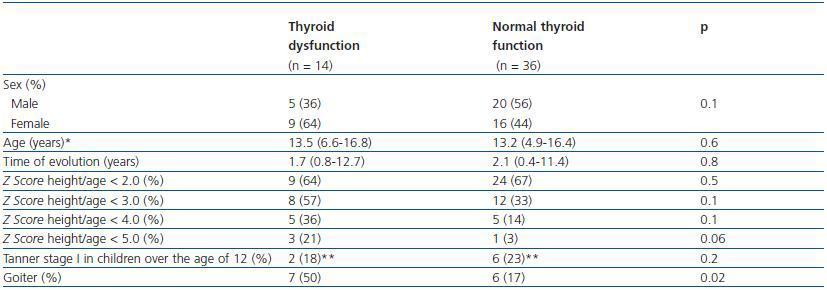
Table 2 shows that when certain characteristics of patients with and without hypothyroidism were compared, even when the time to progression of CRF (median 20 months vs. median 25 months) and the duration of renal replacement therapy (median 12 months vs. median 16 months) was lower in the group with thyroid dysfunction, the differences were not statistically significant (p = 0.82 and 0.28, respectively). It was also observed that the proportion of children with a height for age Z score < 2.0 was similar in both groups, 64 vs. 66% (p > 0.05), respectively.
There was also no difference in the proportion of children with delayed sexual development and thyroid dysfunction (2/14; 14.2%), when compared with children with delayed sexual development and normal thyroid function (6/16; 16.6%). It is worth highlighting that of the 50 patients studied, the greatest effect on height (Z score < 6.0) was found in two girls with thyroid dysfunction, one who had primary hypothyroidism and the other subclinical hypothyroidism. Neither of the two girls has started puberty. Their age at the time of assessment was 12 years and 3 months and 13 years and 4 months.
Goiter as an indicator of thyroid dysfunction
Goiter was detected in 13/50 patients (26%), who were all from the PD group. With regard to thyroid dysfunction, 6/36 (16.6%) patients with normal thyroid function had goiter, whereas of the 14 patients with thyroid dysfunction, seven (50%) presented goiter. From a statistical point of view, the difference in the proportions was significant (p = 0.02). However, when analysing goiter as a diagnostic test for identifying thyroid dysfunction in patients with CRF, its sensitivity was 50%, with a positive predictive value of 53.8%; and a specificity of 83.3%, and a negative predictive value of 81.1%. It is worth highlighting that of the seven patients that presented goiter and thyroid dysfunction at the same time, six had subclinical hypothyroidism and one primary hypothyroidism.
DISCUSSION
CRF is a disease that involves the progressive loss of renal function and is characterised by an increase in serum of nitrogen compounds and other toxins which can cause endocrine and metabolic alterations. In general, some of the signs and symptoms in patients with CRF are similar to those presented by patients with hypothyroidism, such as asthenia, intolerance to the cold, dry, brittle hair, somnolence, delay in growth, lethargy and hypercholesterolaemia.11,12 Therefore, during the eighties the first studies were published which demonstrated that these two conditions could be present at the same time. To date, studies are scarce, particularly those involving children, however all of them agree that the incidence of thyroid dysfunction in patients with CRF is greater than that found among the general population unaffected by renal conditions. It is estimated that the prevalence of hypothyroidism among children of school age and adolescents ranges from 1 in 500 (0.2%) to 1 in 1000 (0.1%);20 the results obtained in this study confirm that paediatric patients with CRF present a higher incidence of hypothyroidism (28%). In light of the information obtained from this and other studies, it seems that the incidence of thyroid dysfunction both in children and in adults is similar, given that in previous studies involving adults its incidence ranges between 5.413 and 37.5%,7 whereas in the two previous studies involving children it ranged from 1016 to 55%.15 In view of the fact that this study involved the largest series of paediatric patients (50 patients) to date, (in previous studies the number of children included was less than ten per study),15,16 we can assume that the results are more reliable and support the finding that thyroid dysfunction incidence in patients with CRF seems to be similar in children and adults. Interestingly, and in contrast with the findings of this and other studies, Castellano et al., in 1996, did not identify any case of hypothyroidism in their study.21 They analysed the thyroid hormones levels of a group of 59 pre-pubescent and post-pubescent subjects with renal failure, of which 26 were in HD, 18 had undergone a kidney transplant and 13 were receiving conservative treatment. In general, the authors observed a decrease in hormone concentration, which was slightly more significant among patients who were in HD.
Xess et al.8 found that there was no difference in thyroid hormone levels in subjects in HD when compared with control subjects. In this study, there were similar findings, given that none of the seven patients in HD presented thyroid dysfunction. In the two studies involving children which identified cases of hypothyroidism, the type of renal replacement therapy that the patients received is not described.15,16 Therefore, given that the information available is limited, we are unable to determine whether PD could be responsible for thyroid dysfunction.
In this study, 14 patients with three different types of thyroid dysfunction were identified; the incidence of dysfunctions identified is similar to that recorded in previous studies involving children.15,16 Subclinical hypothyroidism (60%) was the most common condition, followed by ESS (26.6%) and primary hypothyroidism (13.3%). The dysfunctions that affect children described in this study contrast with those described in studies involving adults, which indicate that the most common condition is ESS, followed by subclinical hypothyroidism and primary hypothyroidism. This could suggest that CRF in children is associated with a greater incidence of real thyroid dysfunction, rather than a condition that is considered a reaction to a chronic disease, like ESS.
The high incidence of thyroid dysfunction in patients with CRF highlights the need to systematically assess thyroid hormone levels,22 especially since recently it is has been observed that they can have a negative impact on the prognosis of adults with CRF.23 However, it is worth clarifying that in all the studies published an assessment has only been carried out on one occasion, therefore the regularity or best moment for assessing thyroid hormone levels has not yet been clearly defined. In this study, the time to progression of the disease or renal replacement therapy was associated with thyroid dysfunction. Therefore, we recommend that an assessment is carried out at least once a year. Since the information available is limited, it would be useful to carry out longitudinal studies in order to establish the incidence of this comorbidity, as well as the possible factors associated with its development.
Taking into consideration the importance of thyroid hormones, both in terms of growth as well as in terms of sexual development, the possible impact of thyroid dysfunction on children with CRF should also be taken into account in future studies, since both growth and sexual development are often affected in children. This study analysed this possibility, however it did not find any relationship between the variables, which may be due to the fact that other factors were delaying growth and development in these subjects or because the size of the sample was limited. Table 2 shows that height for age was more likely to be affected in patients with thyroid problems, however the results were not statistically significant. In addition, a delay in pubescent development was also identified in the two cases which recorded the greatest effect on height.
As a secondary objective of this study, we assessed whether goiter could be used a clinical marker to identify thyroid dysfunction in children with CRF. The results showed that even though there was a higher proportion of subjects with goiter and thyroid dysfunction, it is not a sign that helps us to identify patients with this kind of dysfunction (50% sensitivity). However, the absence of goiter (83% specificity) could help to identify subjects with thyroid dysfunction with greater certainty. One finding which stood out was the high incidence of goiter, given that in this study 13 patients (26%) presented the condition, which was more that the number of cases recorded for a population of children of school age (from 2,8 to 6%).24-26 The high incidence of goiter had already been described by other authors and in studies involving children.6,21 The reasonbehind this alteration could be decreased iodide clearance, which increases plasma levels of inorganic iodide, increases the thyroid reserve and decreases iodide uptake by the thyroid glands. The increase in total organic iodide may partially inhibit the synthesis of thyroid hormones (Wolf-Chaicoff effect).27
The identification of cases with thyroid dysfunction does not imply that these patients should be treated, with the exception of primary hypothyroidism, which requires specific renal replacement therapy. In the case of ESS, there is no evidence to suggest that treatment has any effect on patient progress, whereas the recommendations for renal replacement therapy in subclinical hypothyroidism should be taken into consideration when this is persistent or when it becomes more serious.10,19 Therefore, it is necessary to assess the thyroid function of these patients and check for any symptoms that usually accompany the condition, like dyslipidaemia, predisposition to hypoglycaemia or alterations in neuroconduction at least once a year.
To conclude, in children with CRF in dialysis, the incidence of thyroid dysfunction is high, therefore it is necessary to introduce the assessment of thyroid function in order to improve the overall quality of care of these patients.
Table 1. General description of patients studied
Table 2. Comparison of certain characteristics of patients with and without thyroid dysfunction
Figure 1.