El presente estudio fue diseñado para evaluar la percepción actual de los nefrólogos españoles en el manejo clínico de las alteraciones del metabolismo óseo y mineral en la enfermedad renal crónica (CKD-MBD). Para ello se empleó un procedimiento semiestructurado de consenso profesional a distancia, por correo electrónico (método Delphi modificado), a un panel representativo del colectivo nefrológico, bajo la dirección de un comité coordinador. Para analizar la opinión grupal y el tipo de consenso alcanzado sobre cada cuestión planteada, se empleó la posición de la mediana de puntuaciones del grupo y el «nivel de concordancia» alcanzado por los encuestados. Sobre un total de 86 cuestiones se logró un consenso en acuerdo y desacuerdo en 70 (81,4 %), de los cuales un 60,5 % (52 ítems) lo fueron en términos de acuerdo con la aseveración y un 20,9 % (18 ítems) en desacuerdo. En 16 ítems (18,6 %) no se consiguió suficiente unanimidad de criterio en el panel, bien por disparidad de opinión profesional, bien por falta de criterio establecido en una mayoría del comité de expertos. Aceptando las limitaciones del estudio, consideramos que los ítems en los que hubo consenso refuerzan algunos conceptos de CKD-MBD con su repercusión en la práctica clínica diaria y permiten valorar el grado de homogeneidad que podríamos esperar en esta área. Los ítems en los que no hubo consenso nos ayudan a conocer las áreas de incertidumbre y resultan de gran utilidad para precisar en qué aspectos existe una mayor necesidad de profundización y de emprender estudios prospectivos que permitan mejorar el manejo de estas alteraciones.
Abstract
This study was designed to assess the current perception of Spanish nephrologists in the clinical management of mineral and bone metabolism disorders in chronic kidney disease (CKD-MBD). As such, we used a semi-structured distance professional consensus procedure via e-mail (modified Delphi method) on a representative nephrologist panel, under the direction of a coordinating committee. To analyse the group’s opinion and the type of consensus reached on each issue raised, we used the median of the group’s scores and the “level of agreement” reached by those surveyed. On a total of 86 issues, a consensus agreement and disagreement was achieved in 70 (81.4%), of which 60.5% (52 items) agreed with the statement and 20.9% (18 items) disagreed. In 16 items (18.6%), there was insufficient unanimity in the panel’s opinion, either due to professional opinion disparity or due to the lack of opinion established in the majority of the expert committee. Accepting the study’s limitations, we considered that the items for which there was a consensus reinforce some CKD-MBD concepts with their impact on daily clinical practice and allow the degree of homogeneity that we could expect in this area to be assessed. The items in which there was no consensus help us to know the areas of uncertainty and are very useful for clarifying which aspects have a greater need for further knowledge and which areas require prospective studies to be conducted to improve the management of these disorders.
INTRODUCTION
Mineral and bone metabolism disorders in chronic kidney disease (CKD-MBD) is a very dynamic field of study, which has experienced a lot of changes, especially over the last five years.
Other factors have been added to the group considered to be the “classic regulators of mineral and bone metabolism” (calcium, phosphorus, parathyroid hormone [PTH] and calcitriol), some already known, such as calcidiol, and others new, such as fibroblast growth factor 23 (FGF-23) and klotho.1 Furthermore, other disorders that until recently were considered outside the area of CKD-MBD, such as vascular calcification, cardiovascular disease and bone fractures, have progressively become part of the CKD-MBD2 group.
Along with these changes and advances in the knowledge of CKD-MBD, new drugs have been marketed for controlling its disorders, which, although they potentially offer more flexibility and a fuller therapeutic range, have increased the number of question marks about their efficacy and limitations of use in daily practice.
Most of these issues have been addressed in the recent clinical practice guidelines, amongst them those of 2009 Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD, which will be revised again at the end of 2013,2 and the CKD-MBD Guidelines of the Spanish Society of Nephrology (S.E.N.).3 However, there is still significant uncertainty that results in major variations in the clinical management of these disorders, and we will be exploring this area in our study.
This study was designed to assess Spanish nephrologists’ current perception of the clinical management of CKD-MBD disorders.
MATERIAL AND METHOD
To assess Spanish nephrologists’ perception of mineral and bone metabolism, we used a semi-structured remote professional consensus procedure via e-mail (modified Delphi method),4,5 allowing for contemplative and equal participation by a representative nephrologist panel. It required successive rounds of a structured survey (with closed-ended question scales), with processing and return of intermediate results to participants in order that they could confidentially compare their personal opinions with those of the other panellists.
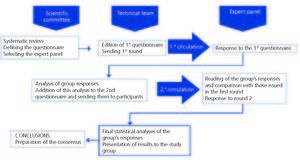
The project was developed over four phases (Figure 1): 1) Formation of a scientific committee comprising 10 nephrologists with a special interest in the area of mineral and bone metabolism, responsible for leading the project and the task of developing a questionnaire, proposing expert panellists, analysing and interpreting the results and drawing final conclusions. 2) Selection of an expert panel: the scientific committee chose 59 nephrologists representing all regions of Spain, interested in the area of mineral and bone metabolism, who met the requirements detailed above on the basis of a pre-selection of 192 nephrologists and who worked mainly in the areas of dialysis, pre-dialysis and renal transplantation. 3) The expert panel received the survey with the statements, which they responded to via e-mail in two rounds. 4) Analysis and discussion of these results in a joint face-to-face meeting between the scientific and expert committees.
A technical team was also required, which was responsible for implementing the method, editing and distributing the first questionnaire, analysing the responses of the first round, the provisional report and distributing the second questionnaire, analysing the second questionnaires and statistical interpretation of the consensus reached.
Developing the questionnaire and method of response
We initially defined the systematic literature review and questionnaire development procedure. Each item on the questionnaire was a statement (positive or negative) on an opinion regarding CKD-MBD disorders in any aspect of interest or controversy. After reviewing and grouping the items proposed by subject, we developed a final version of the questionnaire, which included 86 items and was accepted by the scientific committee and divided into the following subject sections:
- Phosphorus, calcium and magnesium metabolism.
- Bone and cardiovascular system.
- Vitamin D.
- Calcimimetics, parathyroidectomy and associations with vitamin D.
- Renal transplantation.
Assessment scale of the clinical recommendations being judged
In order to assess the issues, one 9-point Likert-type ordinal scale was proposed, which was similar to the conventional format (UCLA-Rand Corporation) used for comparative assessment and prioritisation of different health options (technologies, etc.).6,7 The response categories were defined using linguistic qualifiers of agreement/disagreement grouped into three regions, with the proposals presented as the following possible conclusions:
- 1-3: I disagree with the statement (the lower the score, the higher the degree of disagreement).
- 4-6: I neither agree nor disagree with the statement; my opinion on the issue is not fully defined (4 or 6 is selected when the respondent is closer to disagreeing or agreeing, respectively).
- 7-9: I agree with the statement (the higher the score, the higher the degree of agreement).
The survey also offered the possibility of adding free comments to each item and a final section of new proposals for the committee to assess. For statistical purposes, unanswered statements were analysed as lost cases.
Analysis and interpretation of results
We performed a descriptive analysis of the responses using the median, mean and interquartile range with the specifications listed below. The comparisons were carried out using the mean with a 95% confidence interval. To assess the group’s opinion and the type of consensus reached on each issue raised, we used the median group score and the “level of agreement” reached by those surveyed, in accordance with the following criteria: an item was considered to be agreed upon by consensus whenever there was “concordance” of opinion within the panel. In this case, the median value determined the group consensus reached, which was based on the three aforementioned groups: majority “disagreement” with the item if the median was ≤3; majority “agreement” with the item if the median was ≥7. Cases in which the median was between 4 and 6 were considered to be “uncertain” items for the majority of the group and were not agreed upon by consensus. We established that there was “discordance” in the panel whenever the percentage of panellist responses against (those whose score was outside the region containing the median for this item) was higher than 33%. The remaining items in which we did not observe concordance or discordance were considered to have an “undetermined” level of consensus.
All the items for which the group did not achieve a clear consensus for or against the issue raised (uncertain items, those in which discordance was observed and those that showed an undetermined level of consensus) were proposed for the panel’s reconsideration in the second Delphi round (Figure 1). The items in which a significant spread of opinions was observed amongst those surveyed were also re-assessed, with an interquartile range ≥4 points (range of scores between the p25 and p75 values of the distribution).
Between both rounds, the panellists were informed of the detailed distribution of the group’s anonymous responses in the first survey (through bar graphs) and comments and clarifications contributed by each participant were provided anonymously.
After reviewing this information, we requested a new personal assessment of the items not agreed upon by consensus in the first round. After the second round of the survey, identical criteria were applied in order to distinguish the items definitively agreed upon by consensus from those in which it was not possible to homogenise the panel’s opinion. The total time in which the two rounds were carried out was two months.
For the purpose of comparing graphs between items, we calculated the panellists’ average scores for each statement with a 95% confidence interval. The more extreme the average score of an item (closer to 1 or 9), the more we considered that either an agreement or a disagreement consensus, respectively, was achieved on the proposal expressed by each item.
A smaller confidence interval was interpreted as an expression of greater unanimity of opinions in the group. The items in which no consensus was achieved after the process described was completed were analysed descriptively to distinguish whether this situation was due to discordance of opinion or due to a majority of the panel’s opinion being uncertain with respect to the item (the majority of the group said they did not have a definitive opinion; response = 4-6).
RESULTS
Out of a total of 86 statements, an agreement and disagreement consensus was achieved in 70, that is, a sufficient consensus was achieved in 81.4%, of which 60.5% (52 items) agreed with the statement and 20.9% (18 items) disagreed. In 16 items (18.6%), sufficient unanimity was not achieved in the panellist’s opinions, either due to a disparity of professional opinion, or due to the lack of opinion in a majority of the expert committee. In the first round, there was a consensus in 40 out of the 86 statements analysed (34 in agreement and 6 in disagreement). Of the 46 remaining items proposed for the experts’ reconsideration in the second round, a consensus was reached in 30 more (18 in agreement and 12 in disagreement) (Table 1). The results/conclusions detailed below group together the key aspects of this consensus.
Statements in which a consensus was reached
The 52 items agreed on by consensus can be grouped into the following 36 following points:
10. Lanthanum and sevelamer carbonate have an advantage over calcium binders in terms of survival in the subpopulation over 65 years of age.
11. The high price of lanthanum and sevelamer carbonate may mean that they are not considered the first choice in all cases.
12. Gastrointestinal tolerance and the degree of adherence to the prescription are very important factors when using a phosphate binder.
13. Serum calcium does not represent the net balance of calcium intake, as overload is possible with normal calcaemia.
14. Vascular calcifications have different consequences according to the type and location and they are a factor that contributes to cardiovascular morbidity and mortality.
15. Bone and vascular mineralisation occur by similar mechanisms.
16. The severity and progression of vascular calcifications, bone fractures and a lower bone mass are related to and associated with higher morbidity and mortality.
17. Modifiable cardiovascular risk factors (traditional and non-traditional) should be prevented and treated at an early stage.
18. The correction of vitamin D deficiency should be carried out at all stages of chronic kidney disease or dialysis and calcidiol levels should be 25-40ng/ml at all stages of CKD.
19. 1,25(OH2) D3 measurement does not seem to be useful from a clinical point of view.
20. In chronic kidney disease, the administration of vitamin D may have adverse effects both in the doses recommended by the guidelines and in high doses, and as such, there should be strict monitoring of calcium, phosphorus and creatinine.
21. The adverse effects of vitamin D supplementation for correcting deficiency/insufficiency are the same in any stage of CKD.
22. For calcitriol prescription in patients with chronic kidney disease not on dialysis, the initial dose should be 0.25 micrograms on alternate nights.
23. In all stages of chronic kidney disease, if a phosphate binder had to be added to the administration of vitamin D metabolites, those that contain calcium should be avoided.
24. In dialysis patients, treatment with native vitamin D or calcidiol added to treatment with active vitamin D favours hyperphosphataemia and hypercalcaemia, and this is why it is necessary to reduce the dose of both; in these cases, it seems preferable to use paricalcitol in order to reduce this risk.
25. In relation to vitamin D use, in peritoneal dialysis the same strategy should be employed as in haemodialysis.
26. It is important to control phosphorus levels before beginning therapies designed to act directly on the production and secretion of PTH, such as calcimimetics and/or vitamin D receptor (VDR) activators.
27. There is an agreement on the efficacy of combining calcimimetics with VDR activators in order to reduce PTH levels. However, the choice of calcimimetics and/or VDR activators is determined by serum phosphorus and calcium values.
28. Parathyroidectomies should be performed if PTH levels are higher than 1000pg/ml in spite of medical treatment.
29. A simple lateral dorsolumbar x-ray should be performed in all patients who receive a kidney transplant, in order to assess the presence of vertebral fracture and test mineral and bone density.
30. PTH and calcidiol should be tested every six months in kidney transplant patients.
31. Kidney transplant patients should be supplemented with vitamin D, as well as the rest of the general population, in order to maintain normal serum calcidiol values.
32. Treatment with active vitamin D or vitamin D analogues has been demonstrated to be effective in reducing bone mass loss immediately after transplantation.
33. The continuous administration of low doses of calcium (500mg/day) and some form of vitamin D is advisable for preserving bone mass in patients with steroid doses higher than 5mg/24h.
34. It is advisable for post-transplant hypercalcaemia secondary to persistent hyperparathyroidism to be treated with calcimimetics. If after a period of treatment with calcimimetics they are discontinued and hypercalcaemia reappears, the most appropriate attitude would be to recommence calcimimetic treatment.
35. Calcimimetic administration should probably be maintained in transplant patients in whom their use was necessary for controlling severe secondary hyperparathyroidism before transplantation.
36. There is no phosphate binder of choice for treating patients with a kidney transplant and deterioration of renal function who have hyperphosphataemia.
Statements in which no consensus was reached
The 18 items not agreed on by consensus can be summarised in the following 9 points:
DISCUSSION
To our knowledge, this is the first study designed to assess the perception of nephrologists, in this case Spanish nephrologists, in the clinical management of CKD-MBD. The study was carried out via the Internet and subsequently in a face-to-face meeting, using the modified Delphi method, which is a reliable remote consensus procedure already used in biomedical research,4,5 which avoids the difficulties and disadvantages of face-to-face discussion methods. These include travelling, bias of influence and loss of confidentiality. The main advantages offered by the Delphi method are controlled interaction between panellists, the opportunity to reflect and reconsider personal opinions without losing anonymity and statistical validation of the consensus reached.
The Delphi method was developed in the 1950s by scientists of the Rand Corporation as a method for making informed decisions based on expert opinions.8 Since then, it has been used to assess behaviour and decision-making in various sectors,9-12 and also recently in areas of nephrology.13-19 Despite having undergone some changes, it continues to be a viable approach for compiling expert opinions through a structured iterative process in which a consensus is developed.20,21
This process involves several interactions with participants who, in general, create two or more rounds of responses within a reasonable period of time, in our case two months. The results of first round of answers can be modified in the second round and it is even possible to propose other items based on comments from all participants.22 Furthermore, the Delphi technique offers a series of specific advantages and this is particularly useful because it avoids obstacles commonly observed in other discussion groups, such as interpersonal influence and time constraints.22-24
With this technique, those surveyed do not know the identity of the other panellists and therefore, they are freer, with fewer personal and social limitations.21 Furthermore, they can complete the questionnaire at their own leisure and not simultaneously with the other participants.25 The Delphi method has the advantage that various techniques can be employed for its statistical analysis.26
In our study, we achieved a high degree of consensus in the first round and observations were made that were very useful for improving or modifying some statements in the second round. At the end of the study, a consensus was achieved in most items considered; in fact, it was higher than 80%, which shows high homogeneity in the management of CKD-MBD disorders by nephrologists.
It is important to underscore that, although some members of the scientific committee in this study and authors of this article participated in the development of the S.E.N.3 and KDIGO2 clinical practice guidelines, what they report in this study does not represent their personal opinion or their interpretation of the 86 statements issued in the survey. Their role was to objectively summarise (without influencing the final result) the consensus reached by the 59 nephrologists surveyed, following the Delphi methodology in relation to what these nephrologists, who are particularly interested in the subject, believe about managing mineral and bone metabolism. These opinions may or may not be consistent with that recommended by the clinical practice guidelines,2,3 but they are useful for communicating their degree of agreement, compliance, application and implementation in clinical practice. Therefore, the points of agreement and disagreement are a representation of the real situation and are the basis for the comments and thoughts described below in the discussion of these results.
There was agreement on the low usefulness of serum phosphorus and calcium levels for assessing their metabolism and that as renal function deteriorated there would be an overload of phosphorus and calcium. Nevertheless, at least with phosphorus, the panel considered that phosphate binders should not be used unless serum values of the latter are above the normal range. This widespread opinion of accepting that, despite a potential phosphorus overload there is still not sufficient evidence to start treatment with phosphate binders (statements 1 to 3), indicates a prudent attitude from nephrologists towards a subject that undoubtedly requires more scientific evidence before we aim for new indications of phosphate binders.
Furthermore, this attitude is consistent with recent results that have sparked controversy and suggest that, although in stages 3 and 4 chronic kidney disease there is a tendency towards phosphorus overload, thanks to the known compensatory phosphaturic mechanisms, mainly through FGF-23 and PTH, there would still not be significant phosphorus overload, but there would be an obvious calcium overload due to the kidney’s inability to regulate the removal of the latter as renal function decreases.27,28 Therefore, in stages 3 and 4 chronic kidney disease, the use of phosphate binders would not be indicated without hyperphosphataemia, and less still if calcium-containing binders were used, given that by avoiding a theoretical overload of phosphorus, we could aggravate rather than improve the situation, exposing patients to an unnecessary overload of calcium.28,29 In line with this last concept, we observed the importance, given in the nephrologists’ responses, to calcium overload, an aspect in which there was directly and indirectly agreement and homogeneity (statements 6 to 8 and 13 to 16) in considering it as a determinant of morbidity and mortality.
In the two sections related to other very important aspects in the management of CKD-MBD, such as the use of nutritional vitamin D, active forms of vitamin D, calcimimetics and when it would be necessary to replace medical treatment with parathyroidectomy, in general, there were more points of agreement than of disagreement, but there are still many gaps to be filled. Amongst them, due to their involvement in the routine management of patients, it is necessary to highlight the lack of consensus on PTH values that should be used for starting pharmacological treatment and on whether there is justification for combining the use of native vitamin D with active forms of vitamin D. This lack of agreement is not surprising, given that it reflects the uncertainty (due to lack of scientific evidence) in the recommendations of some clinical practice guidelines, such as the 2009 K/DIGO CKD-MBD guidelines.2
By contrast, there was a high degree of agreement in relation to the levels of PTH at which we should consider that there is therapeutic failure and perform a parathyroidectomy. The figure considered as the threshold was 1000pg/ml. This is consistent with that recently published by the COSMOS study, in which it was observed that Mediterranean countries consider this level to be the most appropriate, while Scandinavian countries would perform surgery with lower PTH levels of around 700pg/ml.30 If we take into account the 2009 K/DIGO CKD-MBD recommendations and the recent preliminary results of the COSMOS study (consistent with previous studies), presented by the European Renal Association-European Dialysis and Transplant Association31 on what PTH values should be considered acceptable, serum PTH of 700pg/ml would be a low value for indicating parathyroidectomy.
Lastly, there were also points of agreement and disagreement in relation to CKD-MBD, renal transplantation and calcimimetics. There was a consensus on the need for post-transplant calcimimetics to be related to the severity of pre-transplant hyperparathyroidism, but not on the need for it in the management of post renal transplantation hypophosphataemia.
To summarise, we believe that the information obtained through the Delphi consensus is practically useful, given that it describes the current situation of CKD-MBD in Spain and we have an insight into the thoughts and likely actions of Spanish nephrologists who are most closely related to the area of mineral and bone metabolism in the regular management of patients. As can be observed in the responses, this is not always consistent with that recommended by the guidelines,2,3 but it does not necessarily represent inadequate clinical practice. In some cases, it probably does, but in others, it may be a basis for reconsidering some of the recommendations and, therefore, there is a need to regularly revise the guidelines with the objective of improving them and updating them in accordance with the new evidence available.
However, it is necessary to recognise that the consensus reached is in the context of a very specific setting and, as such, it has various limitations. Amongst them, we must highlight that it was restricted only to our country and to the group surveyed (empirically classified a priori as experts), due to their special interest in the subject, but who do not represent the overall opinion of nephrologists or of many others who did not participate because of their lesser relationship with this subject, but who also participate in the management of CKD-MBD disorders.
Accepting the study’s limitations, we considered that the items in which there was a consensus reinforce some CKD-MBD concepts with their impact on daily clinical practice and allow the degree of homogeneity that we could expect in this area to be assessed. As already mentioned, the items in which there was not a consensus help us to know the areas of uncertainty and are very useful for specifying in which aspects there is a greater need for further understanding and for carrying out prospective studies that allow the management of CKD-MBD disorders to be improved.
Acknowledgements
This study was possible thanks to the logistical support of Shire Pharmaceutical Ibérica and the backing of the Spanish Society of Nephrology. The technical team responsible for implementing the method and statistical analysis was Luzan 5.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
ADDENDUM
Scientific committee
Jorge Cannata (Coordinator)
Hospital Central de Oviedo
Jordi Bover
Fundación Puigvert. Barcelona
Secundino Cigarrán
Hospital da Costa Burela
Elvira Fernández
Hospital Arnau de Vilanova. Lérida
Emilio González Parra
Hospital Fundación Jiménez Díaz. Madrid
Víctor Lorenzo
Hospital Universitario de Canarias. La Laguna. Tenerife
Isabel Martínez
Hospital de Galdakano.
Juan Navarro
Hospital La Candelaria. Tenerife
Mariano Rodríguez
Hospital Universitario Reina Sofía. Córdoba
Jose-Vicente Torregrosa
Hospital Clinic. Barcelona
Expert panel
Francisco José Ahijado Hormigos
Hospital Virgen de la Salud
Jaume Almirall
Parc Taulí Sabadell
Rafael Alonso Valente
Complejo Hospitalario Universitario de Santiago de Compostela
M.ª Luisa Amoedo Rivera
Hospital General de Elche
Francisco José Ariza Fuentes
Centro de Diálisis Fresenius Cabra (Córdoba)
Eduardo Baamonde Laborda
Centro Diálisis Avericum
Guillermina Barril
Hospital de la Princesa
Sergio Bea
Hospital La Fe
Josep Calpe
Diaverum Rotellar
Antonio Crespo Navarro
Fresenius Gandia
Alfonso Cubas
Hospital de Getafe
Antonio de Paula
Hospital Universitario Río Ortega
Patricia de Sequera Ortiz
Hospital Infanta Leonor
Rodrigo Delgado Zamora
Hospital Vírgen del Rocío
Gloria Del Peso
Hospital de la Paz de Madrid
Rafael Díaz-Tejeiro Izquierdo
Hospital Virgen de la Salud
Amparo Fernández- F
resenius Rambla marina
Adreu Foraster-
Diaverum Baix Llobregat
Antonio Galán
Hospital General Universitario Valencia
César García Cantón
Hospital Insular de las Palmas
Gorka García Erauzquin
Hospital de Cruces. Bilbao.
Sagrario García Rebollo
Hospital Universitario de Canarias
Remedios Garofanos López
Hospital de Torrecardenas
José Manuel Gil Junquero
Hospital General Ciudad de Jaén
Miguel Ángel González
Hospital Clínico Valencia
Enrique Gruss
Hospital de Alcorcón
José Antonio Herrero Calvo
Hospital Clínico de San Carlos
M.ª Victoria Hidalgo Barquero
Hospital de Zafra
Emma Huarte
Hospital San Pedro. Logroño
María Jesús Izquierdo
Complejo Asistencial. Universitario de Burgos
José Lacueva
Alcer Liria
Luz Lozano
Hospital de Fuenlabrada
Joaquín Manrique
Complejo Hospitalario de Navarra
Rosario Moreno López
H. Militar Zaragoza
M.ª Antonia Munar
Hospital Son Espasses Mallorca
Agustín Ortega
Asyter Albacete
Magdalena Palomares Bayo
Hospital Carlos Haya
María Parallé Alcalde
CMD Montequinto
Jesús Pérez
H. Miguel Servet
Miguel Pérez Fontán
Complejo Hospitalario Universitario de A Coruña
Celestino Piñera
Hospital Universitario Marqués de Valdecilla (Santander)
José Luis Pizarro
Centro de Diálisis Diaverum Málaga
Dolores Prados Garrido
Hospital San Cecilio
Mario Prieto
Hospital de León (León)
Constantino Fernández Rivera
Complejo Hospitalario Universitario de A Coruña
Minerva Rodríguez García
Hospital Universitario Central de Asturias (Oviedo)
Esther Romero Ramírez
Hospital Virgen de las Nieves
Emilio Sánchez
Hospital Universitario Central de Asturias (Oviedo)
Dolores Sánchez de la Nieta García
Hospital General de Ciudad Real
Olga Sánchez García
Hospital San Pedro de Alcántara
Ramón Saracho
Hospital Santiago. Vitoria
Rafael Selgas
Hospital de la Paz
Jordi Soler
Fresenius Reus
Daniel Torán Montserrat
Hospital General de Jeréz
Fernando Tornero Molina
Hospital de Arganda
Martí Vallés
Hospital Josep Trueta Girona
Nicanor Vega Díaz
Hospital Doctor Negrín
Borja Zalduendo
Hospital Donostia
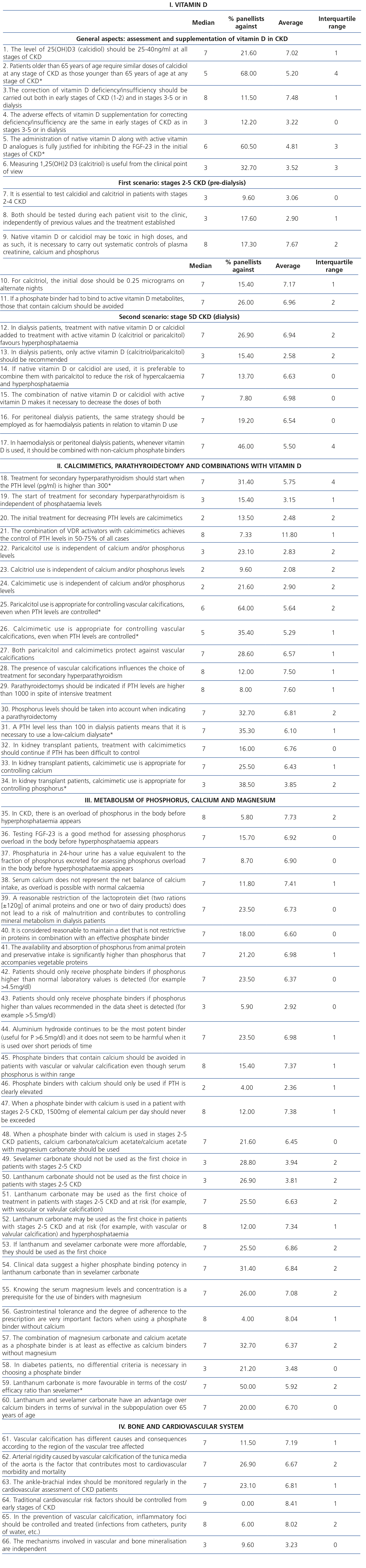
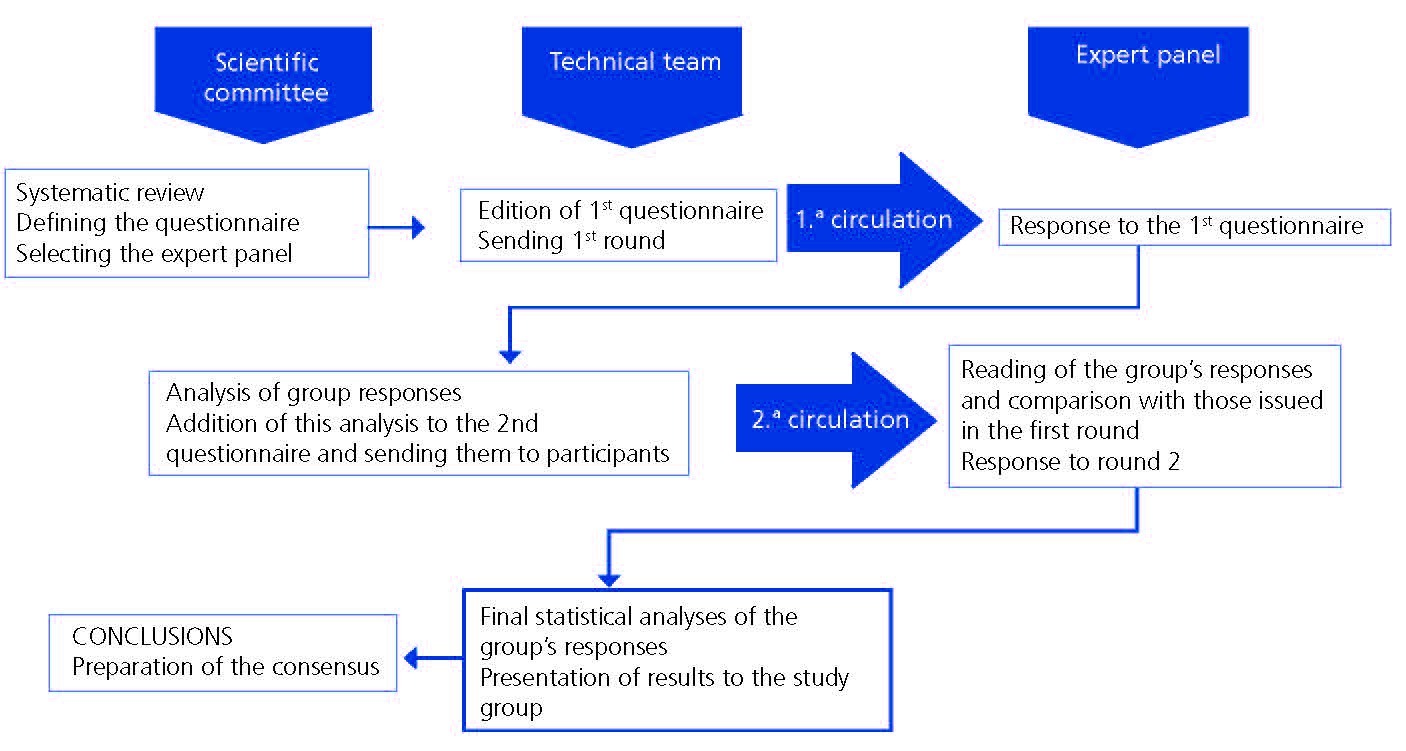
Table 1. Statistical results for the 86 statements.
Figure 1. Delphi method flowchart. Study development diagram.