The objective of this study was to share our hospital's experience regarding non-conventional vascular access: translumbar catheters (TLCs) and transhepatic catheters (THCs) in haemodialysis (HD) patients.
A retrospective study was conducted during the period 2009–2015, and a total of 40 catheters were inserted in 24 HD patients: 26 TLCs and 14 THCs. All patients had previously been diagnosed with stenosis of the superior vena cava and femoral arteries by fluoroscopy. Tunnelled catheters were used for the TLC (Medcomp® 14F×55cm) and THC (Medcomp® 14F×28cm). This study was reviewed and approved by the Ethics Committee of the Hospital Nacional Alberto Sabogal and authorised by the Instituto de Evaluación de Tecnologías Sanitarias e Investigación [Institute for Evaluation of Health Technologies and Research] (IETSI) of EsSalud. The survival analysis was performed in June 2016.
Data analysis was performed using the StataCorp Stata/SE 10 program.
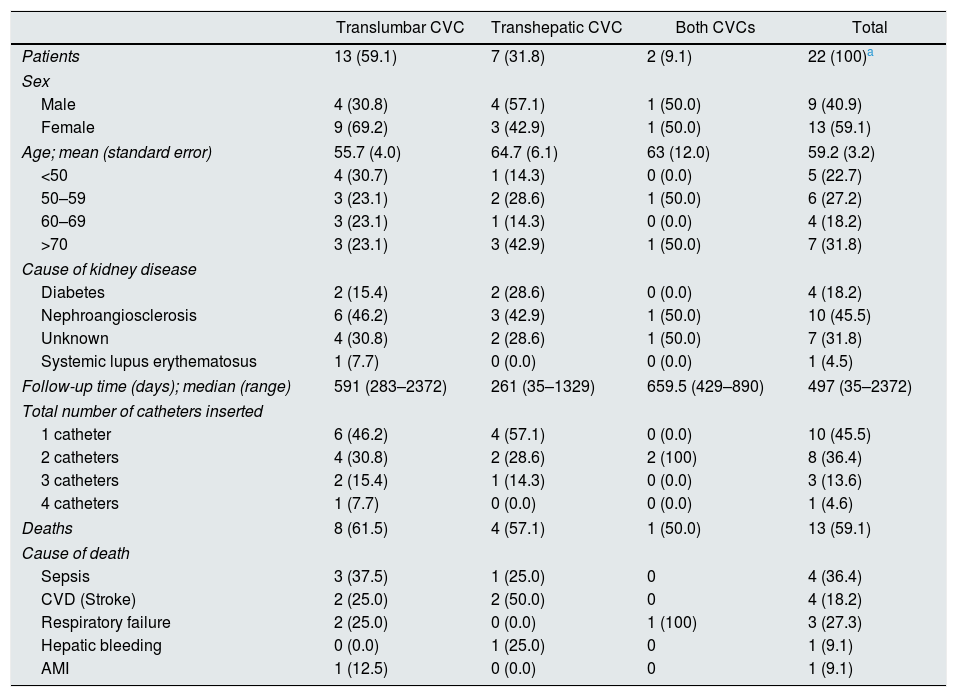
Twenty-four patients were assessed. In two patients the placement of the catheter was unsuccessful. Therefore, they were not included in the analysis (Table 1). Thirteen patients (59.1%) were female. The average age of these patients was 59.2 (range: 30–83). In 13 patients (56.5%) only a TLC was inserted, in 8 (34.8%) only a THC and in 2 (8.7%) both types of catheter were inserted. The median follow-up time for these patients was 591 days (283–2372) in the TLC group, 235.5 days (35–1329) in the THC group and 659.5 days (429–890) in the group with both types of catheters. The most common cause of chronic kidney disease (CKD) was nephroangiosclerosis (43.5%), followed by an unknown cause (34.8%) and diabetes mellitus (17.4%). There was only one case of lupus nephritis as a cause of CKD. In total, 13 patients died during the study period, 8/15 (61.5%) in the TLC group, 4/8 (50.0%) in the THC group and 1/2 (50%) in the group which received both catheters. There were no significant differences in the follow-up times for these three groups. No patient died due to the direct cause of catheter placement. However, one patient died 30 days after placement of a THC as he presented with a hepatic haematoma. This was the only death in the placement of a non-conventional venous access.
Patients with a TLC, THC or both.
| Translumbar CVC | Transhepatic CVC | Both CVCs | Total | |
|---|---|---|---|---|
| Patients | 13 (59.1) | 7 (31.8) | 2 (9.1) | 22 (100)a |
| Sex | ||||
| Male | 4 (30.8) | 4 (57.1) | 1 (50.0) | 9 (40.9) |
| Female | 9 (69.2) | 3 (42.9) | 1 (50.0) | 13 (59.1) |
| Age; mean (standard error) | 55.7 (4.0) | 64.7 (6.1) | 63 (12.0) | 59.2 (3.2) |
| <50 | 4 (30.7) | 1 (14.3) | 0 (0.0) | 5 (22.7) |
| 50–59 | 3 (23.1) | 2 (28.6) | 1 (50.0) | 6 (27.2) |
| 60–69 | 3 (23.1) | 1 (14.3) | 0 (0.0) | 4 (18.2) |
| >70 | 3 (23.1) | 3 (42.9) | 1 (50.0) | 7 (31.8) |
| Cause of kidney disease | ||||
| Diabetes | 2 (15.4) | 2 (28.6) | 0 (0.0) | 4 (18.2) |
| Nephroangiosclerosis | 6 (46.2) | 3 (42.9) | 1 (50.0) | 10 (45.5) |
| Unknown | 4 (30.8) | 2 (28.6) | 1 (50.0) | 7 (31.8) |
| Systemic lupus erythematosus | 1 (7.7) | 0 (0.0) | 0 (0.0) | 1 (4.5) |
| Follow-up time (days); median (range) | 591 (283–2372) | 261 (35–1329) | 659.5 (429–890) | 497 (35–2372) |
| Total number of catheters inserted | ||||
| 1 catheter | 6 (46.2) | 4 (57.1) | 0 (0.0) | 10 (45.5) |
| 2 catheters | 4 (30.8) | 2 (28.6) | 2 (100) | 8 (36.4) |
| 3 catheters | 2 (15.4) | 1 (14.3) | 0 (0.0) | 3 (13.6) |
| 4 catheters | 1 (7.7) | 0 (0.0) | 0 (0.0) | 1 (4.6) |
| Deaths | 8 (61.5) | 4 (57.1) | 1 (50.0) | 13 (59.1) |
| Cause of death | ||||
| Sepsis | 3 (37.5) | 1 (25.0) | 0 | 4 (36.4) |
| CVD (Stroke) | 2 (25.0) | 2 (50.0) | 0 | 4 (18.2) |
| Respiratory failure | 2 (25.0) | 0 (0.0) | 1 (100) | 3 (27.3) |
| Hepatic bleeding | 0 (0.0) | 1 (25.0) | 0 | 1 (9.1) |
| AMI | 1 (12.5) | 0 (0.0) | 0 | 1 (9.1) |
AMI: acute myocardial infarction; CVC: central venous catheter; CVD: cerebrovascular disease; THC: transhepatic catheter; TLC: translumbar catheter.
At the end of follow-up, nine patients were still alive, seven of whom had a functioning catheter at that time, and two went on to undergo peritoneal dialysis. In the group of those who died, eight died with the catheter functioning, three with the catheter not functioning and two had gone on to receive peritoneal dialysis. The cause of death was cardiovascular in five patients, followed by sepsis in four, respiratory failure in three and a case of hepatic bleeding in one patient with a THC (Table 1).
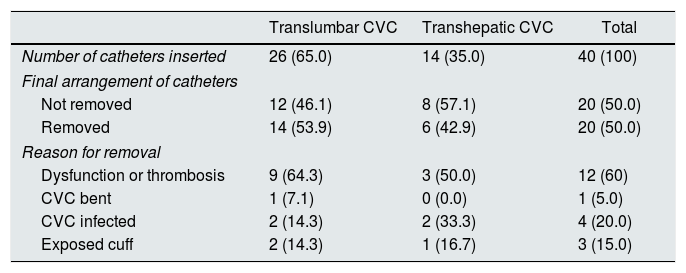
As regards the catheters, a total of 40 were inserted in the 22 patients included in this study (Table 2). The TLC site was used on 26 occasions (65%), while the THC site was used on 14 occasions (35%). Regarding the reasons to remove the catheter, we can indicate that the first reason was dysfunction or thrombosis (60%), followed by infection (20%) and exposed cuff (15%).
Features of the TLCs and THCs.
| Translumbar CVC | Transhepatic CVC | Total | |
|---|---|---|---|
| Number of catheters inserted | 26 (65.0) | 14 (35.0) | 40 (100) |
| Final arrangement of catheters | |||
| Not removed | 12 (46.1) | 8 (57.1) | 20 (50.0) |
| Removed | 14 (53.9) | 6 (42.9) | 20 (50.0) |
| Reason for removal | |||
| Dysfunction or thrombosis | 9 (64.3) | 3 (50.0) | 12 (60) |
| CVC bent | 1 (7.1) | 0 (0.0) | 1 (5.0) |
| CVC infected | 2 (14.3) | 2 (33.3) | 4 (20.0) |
| Exposed cuff | 2 (14.3) | 1 (16.7) | 3 (15.0) |
CVC: central venous catheter; THC: transhepatic catheter; TLC: translumbar catheter.
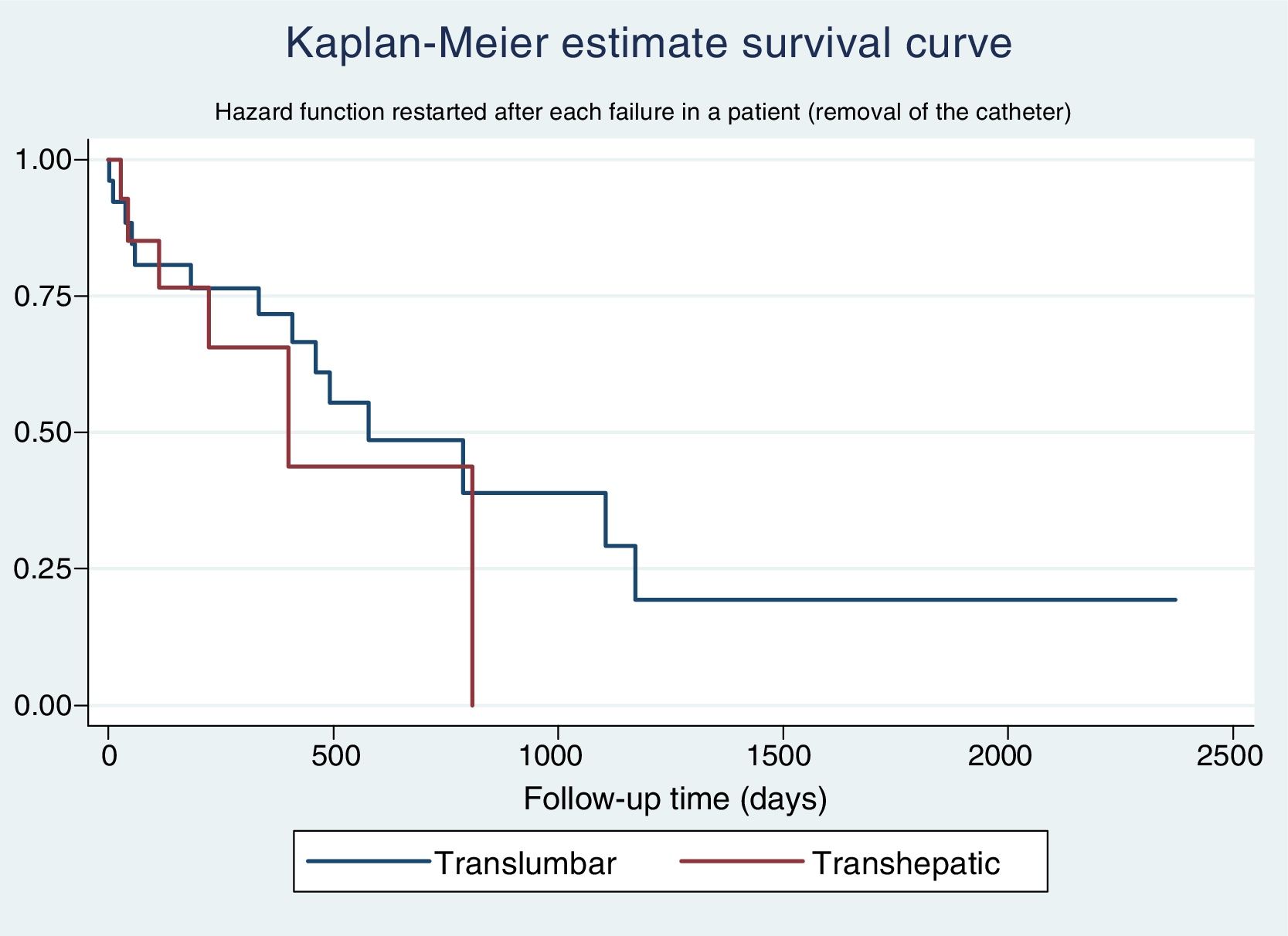
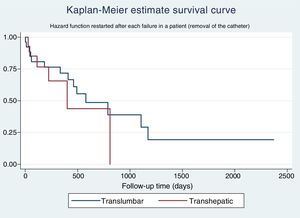
The Kaplan–Meier estimate survival curve for catheter survival shows a slightly greater survival for TLCs compared to THCs, but this difference is not significant (Fig. 1).
The univariate Cox regression analysis for catheter survival shows an HR of 1.50 (0.67–3.39) for THCs compared to TLCs. When performing the multivariate analysis, the final model delivered a marginally statistically significant HR for the removal of the catheter for a THC 1.86 (0.88–3.88) compared to a TLC.
The overall results of our study have similarities with the median follow-up results, as well as those for complications.1–9
TLCs have a greater survival and a lower HR for removal risk than THCs, and, despite the fact that these findings are not statistically significant, we recommend using a TLC as the first choice.
The main limitation of the study is that the sample size is very small, which does not allow us to find any associations that might exist. Furthermore, the information obtained for this study was very limited, and some variables which could be considered for a better understanding of the catheters, such as CKD duration, weight or body mass index at the time of catheter placement, among others, were omitted.
The use of TLCs or THCs is a viable and safe option for our patients. They allow a viable venous access in patients with few options for dialysis while they wait for a kidney transplant or a change to peritoneal dialysis.
To Dr Juan Vicente Guanira of the Instituto de Evaluación de Tecnologías en Salud e Investigación (IETSI) - EsSalud.
Please cite this article as: Mori JR, Alguiar JR, Oviedo CP, Munares PM, Sanchez ML, Linares MC, et al. Catéteres translumbares y transhepáticos para hemodiálisis: una opción viable. Nefrologia. 2019;39:88–90.