Functional and durable vascular access is needed for adequate hemodialysis. Arteriovenous fistula is preferred over prosthetic grafts or central venous catheters, but it is associated with high rates of primary failure and maturation failure.
Preoperative mapping of arm vessels with color Doppler ultrasound (CDU) has been shown to be helpful in achieving better short and long-term outcomes. Unfortunately, is more time-consuming than a physical examination and requires an experienced examiner and special equipment; some authors defend that CDU should not be part of the routine preoperative assessment.
We reported our experience in preoperative vessel mapping using color Doppler ultrasound to purpose a vascular access to the surgical team, surveillance of vascular access, and evaluation of main outcomes (primary failure, maturation failure, and patency).
MethodsThis is a single-center retrospective study that includes patients who attended a specific appointment for vascular access planning consultation between January 2019 and December 2021. A nephrologist performed the physical exam and vascular mapping and proposed to the vascular surgeon team a specific type and location of vascular access. Patients were followed until one month after the first hemodialysis through functioning vascular access.
ResultsIn this study, 167 patients were evaluated (114 incident patients – chronic kidney disease stage 4 or 5 – and 53 prevalent patients – under hemodialysis through central venous catheter). The vascular accesses proposed by nephrologist were radial-cephalic arteriovenous fistula in 70 patients (41.9%), brachio-cephalic arteriovenous fistula in 50 patients (29.9%), brachio-basilic arteriovenous fistula in 34 patients (20.4%), arteriovenous graft in 8 patients (4.8%) and central venous catheter in 2 patients (1.2%).
Vascular access was constructed in 141 patients: distal arteriovenous fistula in 57 patients (40.4%), brachio-cephalic arteriovenous fistula in 54 patients (38.3%), brachio-basilic AVF in 27 patients (19.1%), and arteriovenous graft in 3 patients (2.1%). The created access corresponds to the proposed access in 129 patients (91.5%).
Twenty-two (15.6%) primary failures were registered. Distal arteriovenous fistulas and diabetes mellitus were associated with a higher risk of primary failure (OR=3.929 (1.485–10.392), p=0.004; OR=3.867 (1.235–12.113), p=0.014, respectively).
The incidence of maturation failure at eight weeks was 4.8%.
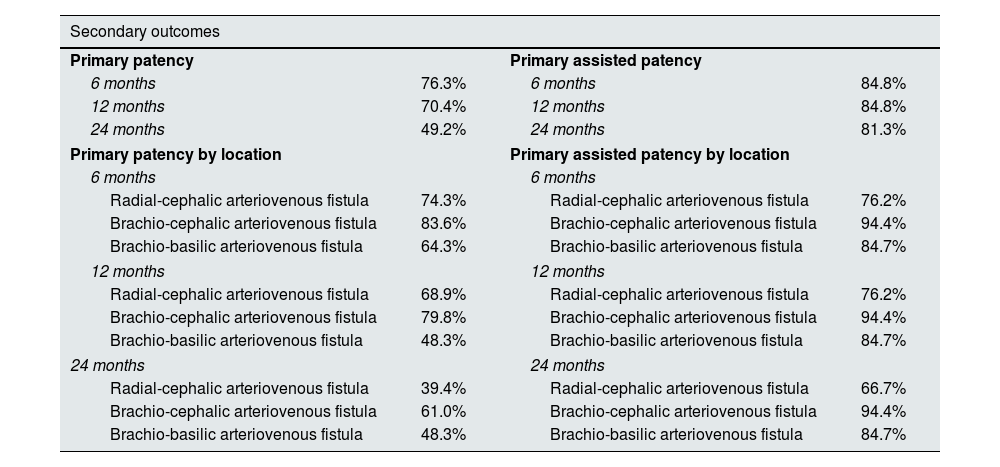
The primary patency at 6, 12 and 24 months was 76.3%, 70.4% and 49.2%. Primary assisted patency was 84.8% at 6 and 12 months and 81.3% at 24 months.
ConclusionsThis study demonstrates that the study of the entire vascular territory performed with color Doppler ultrasound, within a multidisciplinary team of nephrologists and vascular surgeons, is associated with high rates of autologous access and very low rates of primary failure and maturation failure (almost unprecedented in the literature).
Una adecuada hemodiálisis requiere un acceso vascular funcional y duradero. En general, se prefiere la fístula arteriovenosa a los injertos protésicos o a los catéteres venosos centrales, pero su uso se asocia con altas tasas de falla primaria y de maduración.
El mapeo preoperatorio de los vasos del brazo con ultrasonido Doppler color (UDC) ha demostrado ser útil para conseguir mejores resultados a corto y a largo plazo. Desafortunadamente, necesita más tiempo que un examen físico y requiere de un examinador experimentado y un equipo especial, por lo que algunos autores defienden que la UDC no debería formar parte de la valoración preoperatoria de rutina.
Informamos de nuestra experiencia en el mapeo preoperatorio de vasos usando UDC para proponer al equipo quirúrgico un acceso vascular, vigilancia del mismo, así como una evaluación de los resultados principales (fallo primario, fallo de maduración y permeabilidad).
MétodoEste es un estudio retrospectivo realizado en un solo centro que incluye pacientes que acudieron a una consulta a través de una cita específica de planificación del acceso vascular entre enero de 2019 y diciembre de 2021. Un nefrólogo realizó el examen físico y el mapeo vascular y propuso al equipo de cirujanos vasculares la ubicación y el tipo específico de acceso vascular. Se realizó un seguimiento de la evolución del acceso vascular en funcionamiento durante un mes después de la primera hemodiálisis.
ResultadosEn este estudio se evaluaron 167 pacientes, de los que 114 eran pacientes incidentes – enfermedad renal crónica (ERC) estadio 4 o 5 – y 53 eran pacientes prevalentes en hemodiálisis por catéter venoso central (CVC). Los accesos vasculares propuestos por el nefrólogo fueron fístula arteriovenosa radialcefálica en 70 pacientes (41,9%), fístula arteriovenosa braquiocefálica en 50 pacientes (29,9%), fístula arteriovenosa braquiobasílica en 34 pacientes (20,4%), injerto arteriovenoso protésico en 8 pacientes (4,8%) y catéter venoso central en 2 pacientes (1,2%).
Se construyó acceso vascular en 141 pacientes, de los que en 57 (40,4%) fue fístula arteriovenosa radialcefálica, en 54 pacientes (38,3%) fue fístula arteriovenosa braquiocefálica, en 27 pacientes (19,1%) fue fístula arteriovenosa braquiobasílica, mientras que 3 pacientes (2,1%) llevaban injerto arteriovenoso protésico. El acceso realizado se corresponde con el propuesto en 129 pacientes (91,5%).
Se registraron 22 fallos primarios (15,6%). Según los resultados, el mayor riesgo de fracaso primario está asociado a las fístulas arteriovenosas distales (OR=3,929, con intervalo entre 1,485 y 10,392 y p=0,004) y a la diabetes mellitus (OR=3,867, con intervalo entre 1,235 y 12,113 y p=0,014).
La incidencia de fallo de maduración a las 8semanas fue del 4,8%.
La permeabilidad primaria a los 6, 12 y 24meses fue del 76,3%, del 70,4% y del 49,2%, respectivamente. La permeabilidad primaria asistida fue del 84,8% a los 6 y 12meses, mientras que a los 24meses llegó al 81,3%.
ConclusionesEste trabajo demuestra que el estudio realizado de todo el territorio vascular mediante ecografía Doppler por profesionales de un equipo multidisciplinar de nefrólogos y cirujanos vasculares se asocia a altas tasas de acceso autólogo y muy bajas tasas de fallo primario y de maduración (casi sin precedentes en la literatura).
The prevalence of end-stage chronic kidney disease (CKD) continues to increase worldwide, and hemodialysis (HD) is the most widespread renal replacement therapy.1 Vascular access is a sine qua non state for patients with chronic kidney disease (CKD) in treatment with HD.2 A functional and durable vascular access leads to a better long-term prognosis.3 Roca Tey points out several points that must be considered in order to optimize vascular access management, with special focus on the need for a dedicated team of vascular surgeons integrated into a multidisciplinary team and the performance of preoperative vascular mapping.2
Arteriovenous fistula (AVF) is preferred over prosthetic grafts or central venous catheters (CVC) because it is associated with lower mortality, infection risk, and cardiovascular events.3 Despite the proven benefits, the high rates of primary failure and maturation failure are problems to be overcome.4
Primary failure was described in more than 40% of cases in some series,5,6 and the absence of maturation is described in up to 53% of cases7 which increases the number of days of exposure to the CVC, increase the number of interventions and costs with vascular access and shortens its lifespan.
The proper functioning of AVF depends on the adequacy of vessels and the time allowed before use.8 In the past, the selection of vessels to be used for creation an AVF was based exclusively on physical examination. More recently, preoperative mapping of arm vessels with color Doppler ultrasound (CDU) before the creation of vascular access has been shown to be helpful in achieving a higher percentage of AVF, to determine the feasibility of creating the access and its best location, avoiding futile surgeries and improved long-term outcomes.4,9–11 Additionally, CDU allows postoperative surveillance, facilitating early diagnosis of complications.
Unfortunately, CDU is more time-consuming than a physical examination and requires an experienced examiner and special equipment; some authors defend that CDU should not be part of the routine preoperative assessment, used only when anomalies appear during the physical examination.12,13 Recent Spanish Guidelines recommend CDU use in all patients prior to the construction of vascular access.14
We reported our experience in preoperative vessel mapping using CDU to purpose a vascular access to the surgical team, surveillance of vascular access, and evaluation of main outcomes until hemodialysis begins.
MethodsStudy design and participantsThis is a single-center retrospective study that includes patients who attended a specific appointment for vascular access planning consultation at Oporto University Hospital Center between January 2019 and December 2021. Inclusion criteria were (a) age over 18 years and (b) the purpose of the consultation is vascular mapping for the creation of first vascular access. Patients may or may not have had previous hemodialysis sessions through a CVC: among the evaluated patients, there were 114 incident patients (CKD stage 4 or 5) and 53 prevalent patients (under hemodialysis through CVC).
In the vascular access consultation, a nephrologist evaluated the patient and performed the physical exam and vascular mapping through CDU. After patient evaluation, the nephrologist proposed to the vascular surgeon team (five surgeons and respective residents) a specific type and location of vascular access. The proposed access could be an AVF, arteriovenous graft (AVG) or CVC (we do not advocate vascular access planning in patients on peritoneal dialysis, waiting for a living donor kidney transplant in short term or when we do not expect to start dialysis within the next 6 months).
The following data were collected: demographic data, weight, height, medical history (diabetes mellitus – DM, peripheral arterial disease – PAD, ischemic coronary disease, previous stroke), and characterization of chronic kidney disease (etiology, proteinuria, serum creatinine and glomerular filtration rate on the date of the first consultation and the date of construction of the vascular access).
During the follow-up, the following data were recorded: date and type of access proposed by the nephrological team, date and type of vascular access constructed by the vascular surgery team, date of the beginning of hemodialysis, and access used in the first hemodialysis.
Patients were followed by the nephrologist in vascular access consultation until one month after the first hemodialysis through a functioning vascular access. During follow-up, vascular access complications, number and type of vascular interventions, and the need for subsequent vascular access creation were evaluated.
The investigation followed the principles outlined in the Declaration of Helsinki and was approved by the Institutional Review Board of the Oporto University Hospital Center.
Preoperative vascular evaluationAn ultrasound General Electric with high frequency (7–15MHz) linear probe was used for measurements.
Physical and DU assessment always began by non-dominant arm blood vessels; patients were in a supine position without angling the elbow joint to avoid vessels compression. Both the superficial and deep venous systems were examined from the wrist to the axillar vein and axillar artery, if technically possible.
When the venous anatomy was acceptable for an AVF, arterial examination was performed by palpating arterial pulses. If the radial artery was not suitable, the ulnar and brachial arteries were examined as alternative sources. Evaluation of the dominant arm was performed only when the non-dominant arm evaluation was unsatisfactory.
In our study, only vessels that met the minimum criteria were chosen – a venous luminal diameter of ≥2.5mm for distal AVF and ≥3.0mm for proximal AVF (using a tourniquet) and continuity with proximal veins in the arm; and arterial luminal diameter of ≥2.0mm. Vein compressibility and distensibility, distance from the skin surface, continuity with the deep venous system in the upper arm, and confirmation of the absence of ipsilateral central venous stenosis or occlusion were performed.
Vein distensibility was evaluated in a subjective way before and after a tourniquet placement, and we did not measure vein distensibility by DU. The presence of venous congestion and a reduced or absent respiratory variability in the axillar vein implied central stenosis exclusion by angiography before AV access creation.
The distensibility of the arterial wall was assessed by evaluation of the Doppler wave form in the radial artery during reactive hyperemia induced by reopening the first after it had been clenched for 2min (change from the high-resistance triphasic to low-resistance biphasic wave-form) and resistance index (RI) was measured and considered the target <0.7.
Arterial inflow was also evaluated to exclude arterial stenosis. In this field, we did not propose AVF creation in three specific conditions. First, AVF was not created in the presence of calcifications of the feeding artery wall accompanied by a negative reactive hyperemia test in artery. Second, anastomosis was not created distal to a stenosis above 50% in radial artery. Third, we did not advocate AVF creation in the presence of cubital artery with stenosis, a diameter <1.5mm or absent associated with radial artery arteriopathy due to the increased risk of primary failure with distal AVF and of ischemia with proximal AVF. When high bifurcation of the brachial artery is found, resulting in deeper and larger ulnar artery and smaller and more superficial radial artery, the larger and deeper ulnar artery is recommended for anastomosis.
Forearm AVF location was preferred compared to upper-arm location. To make a final decision clinical data, risk factors associated with maturation failure, and CDU findings were considered. The aim was the creation of vascular access with more probability of success, reducing the time of dialysis CVC and the risk of maturation failure.
When patients did not have arteries and veins suitable for AVF, we immediately attempted an AVG. The criteria for good arterial inflow and venous outflow for AVG were brachial artery lumen diameter ≥3.0mm and axillar vein lumen diameter ≥4.0mm, respectively.
A postoperative surveillance scan was planned at 4–6 weeks after access creation to assess access maturation: patency, Qa, artery and vein diameter, presence of stenosis formation and measured flow in the fistula (ml/min), the vein diameter, and any abnormalities.
The AV access creation involved a multidisciplinary strategy.
The type and location of AV access proposal were made by the nephrologist, but surgeons have access to the CDU in the operating room, they can repeat the evaluation and they had the final word in relation to the choice of AV access.
Definition of variables and outcomesFive types of possible vascular access were recorded: radial-cephalic AVF (fed by radial artery), proximal AVF (brachio-cephalic or brachio-basilic, fed by brachial artery), AVG, and CVC.
The primary outcomes were the evaluation of primary failure (PF) and failure of vascular access maturation at eight weeks (MF). Secondary outcomes were the evaluation of primary patency (PP) and primary assisted patency (PAP) at 6, 12, and 24 months.
PF was defined as access failure due to early technical failures (intra-operative thrombosis or other complications or abandonment of the newly created AVF).
MF is defined as insufficient access flow to maintain dialysis or the inability to cannulate an AVF, if required, at eight weeks after surgery.
PP is the interval from the time of access placement until any intervention designed to maintain or reestablish patency, access thrombosis, or the time of measurement of patency. PAP is the interval from the time of access placement until access thrombosis or the time of measurement of patency, including surgical or endovascular interventions designed to maintain the functionality of a patent access.15
Statistical analysisCategorical variables were expressed as absolute counts and percentages. Continuous variables were presented as mean±standard deviation if normally distributed.
Survival evaluations were used to estimate PP and PAP with Kaplan–Meier curves. The association between the variables and each outcome was studied using Chi-square test and Fisher exact test, according to the sample size, in nominal variables. To continues variables, we used univariate logistic regression.
The log rank test was used to assess the differences between the groups in the Kaplan–Meir analysis. The inter-rater reliability between the nephrologist's proposal and the surgical team's decision was evaluated using the Cohen's kappa coefficient.
In all these tests, we obtained the odds ratio with a confidence interval of 95%; the significance level was 0.05. All statistical analyses were performed using SPSS-IBM.
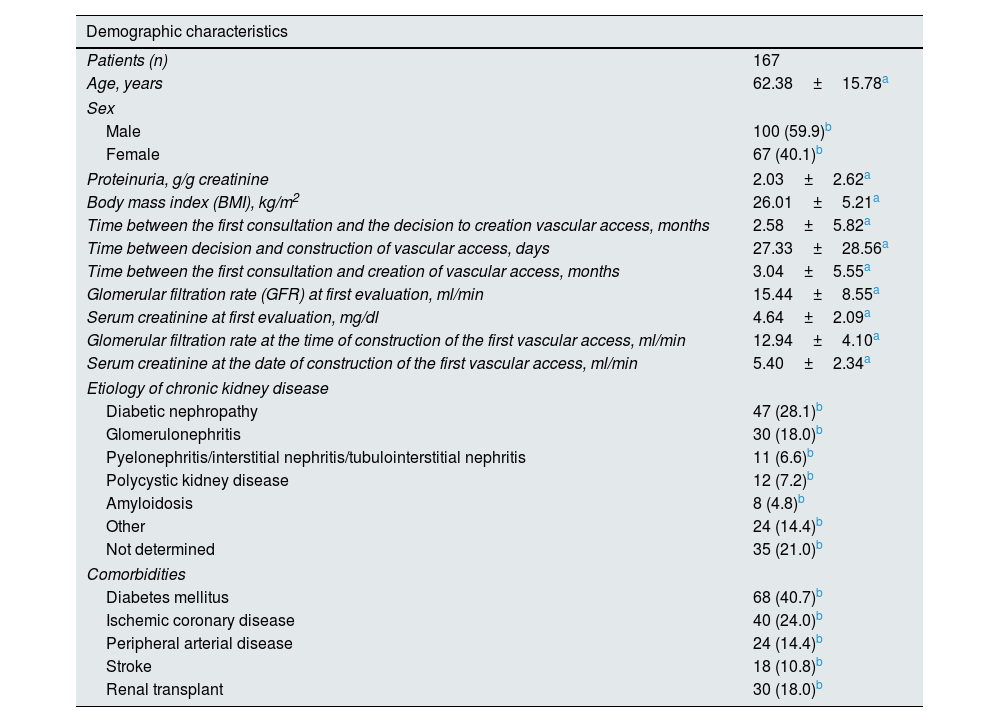
ResultsDemographic characteristicsIn this study, 167 patients (62.4±15.8 years) were evaluated in vascular access consultation with the aim of creating a vascular access for hemodialysis (Table 1). Gender distribution was: 100 men (59.9% of patients) and 67 women (40.1%). The comorbidities, in order of frequency, were: DM (40.7%), ischemic coronary disease (24%), PAD (14.4%), and previous stroke (10.8%).
Clinical characteristics of the study population.
| Demographic characteristics | |
|---|---|
| Patients (n) | 167 |
| Age, years | 62.38±15.78a |
| Sex | |
| Male | 100 (59.9)b |
| Female | 67 (40.1)b |
| Proteinuria, g/g creatinine | 2.03±2.62a |
| Body mass index (BMI), kg/m2 | 26.01±5.21a |
| Time between the first consultation and the decision to creation vascular access, months | 2.58±5.82a |
| Time between decision and construction of vascular access, days | 27.33±28.56a |
| Time between the first consultation and creation of vascular access, months | 3.04±5.55a |
| Glomerular filtration rate (GFR) at first evaluation, ml/min | 15.44±8.55a |
| Serum creatinine at first evaluation, mg/dl | 4.64±2.09a |
| Glomerular filtration rate at the time of construction of the first vascular access, ml/min | 12.94±4.10a |
| Serum creatinine at the date of construction of the first vascular access, ml/min | 5.40±2.34a |
| Etiology of chronic kidney disease | |
| Diabetic nephropathy | 47 (28.1)b |
| Glomerulonephritis | 30 (18.0)b |
| Pyelonephritis/interstitial nephritis/tubulointerstitial nephritis | 11 (6.6)b |
| Polycystic kidney disease | 12 (7.2)b |
| Amyloidosis | 8 (4.8)b |
| Other | 24 (14.4)b |
| Not determined | 35 (21.0)b |
| Comorbidities | |
| Diabetes mellitus | 68 (40.7)b |
| Ischemic coronary disease | 40 (24.0)b |
| Peripheral arterial disease | 24 (14.4)b |
| Stroke | 18 (10.8)b |
| Renal transplant | 30 (18.0)b |
The main cause of chronic kidney disease was diabetic nephropathy (28.1%) followed by glomerulonephritis (18.0%) and polycystic kidney disease (7.2%); 14.4% of patients have a multifactorial etiology and 21.0% of patients had no determined etiology.
The time between the first evaluation and the decision to create a vascular access was 2.6±5.8 months. The time between the decision to the creation and the surgical construction of vascular access was 27.3±28.6 days.
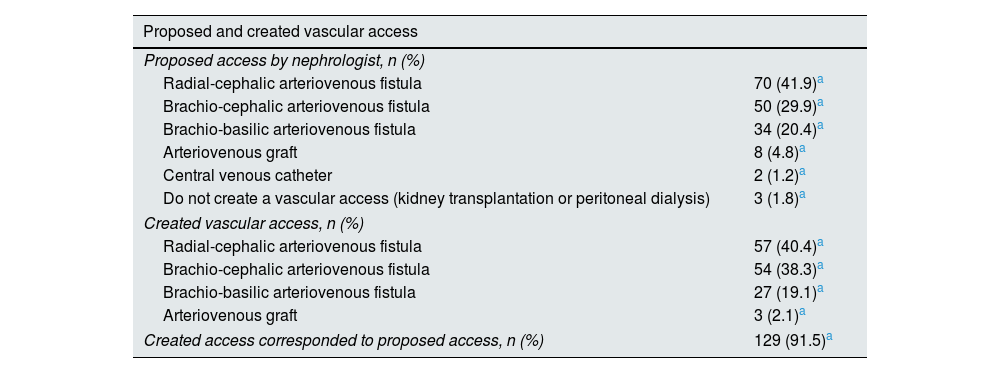
Type of vascular access: proposed and creationThe vascular accesses proposed by nephrologist were radial-cephalic AVF in 70 patients (41.9%), brachio-cephalic AVF in 50 patients (29.9%), brachio-basilic AVF in 34 patients (20.4%), AVG in 8 patients (4.8%) and CVC in 2 patients (1.2%) (Table 2). In three patients (1.8%) a construction of vascular access was not proposed because a short-term living donor kidney transplant is predictable or because they chose peritoneal dialysis.
Description of the vascular access proposed by the nephrological team and the first created vascular access.
| Proposed and created vascular access | |
|---|---|
| Proposed access by nephrologist, n (%) | |
| Radial-cephalic arteriovenous fistula | 70 (41.9)a |
| Brachio-cephalic arteriovenous fistula | 50 (29.9)a |
| Brachio-basilic arteriovenous fistula | 34 (20.4)a |
| Arteriovenous graft | 8 (4.8)a |
| Central venous catheter | 2 (1.2)a |
| Do not create a vascular access (kidney transplantation or peritoneal dialysis) | 3 (1.8)a |
| Created vascular access, n (%) | |
| Radial-cephalic arteriovenous fistula | 57 (40.4)a |
| Brachio-cephalic arteriovenous fistula | 54 (38.3)a |
| Brachio-basilic arteriovenous fistula | 27 (19.1)a |
| Arteriovenous graft | 3 (2.1)a |
| Created access corresponded to proposed access, n (%) | 129 (91.5)a |
A vascular access was constructed in 141 patients: radial-cephalic AVF in 57 patients (40.4%), brachio-cephalic AVF in 54 patients (38.3%), brachio-basilic AVF in 27 patients (19.1%), and AVF graft in 3 patients (2.1%).
The created access corresponds to the proposed access in 124 patients (87.9%). Twenty-six patients did not create vascular access for some reasons: death, refusal, or modification of option.
Primary failureAmong the 141 vascular access created, 22 (15.6%) primary failures were registered (Table 3). Sex was not a statistically significant determinant for the occurrence of primary failure (OR=1.775 (0.710–4.435), p=0.216).
Relationship between clinical characteristics and patients’ vascular access and outcomes (primary failure and maturation failure).
| Primary outcomes | |
|---|---|
| Primary failure, n (%) | 22 (15.6) |
| Maturation failure, n (%) | 8 (4.8) |
| Primary failure | Maturation failure | |||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0.983 (0.956–1.010) | 0.209* | 0.994 (0.951–1.038) | 0.784* |
| Sex | 1.775 (0.710–4.435) | 0.216+ | 0.867 (0.197–3.811) | 1.000 |
| Proteinuria | 0.853 (0.629–1.158) | 0.309* | 0.803 (0.496–1.300) | 0.372* |
| Body mass index (BMI) | 1.019 (0.931–1.116) | 0.682* | 0.878 (0.721–1.069) | 0.194* |
| Time between the first consultation and the decision to create vascular access | 0.986 (0.906–1.074) | 0.753* | 1.007 (0.899–1.129) | 0.902* |
| Time between decision and construction of vascular access | 0.997 (0.979–1.015) | 0.716* | 1.015 (0.999–1.031) | 0.062* |
| Time between the first consultation and the creation of vascular access | 0.987 (0.905–1.077) | 0.771* | 1.038 (0.935–1.153) | 0.481* |
| Glomerular filtration rate (GFR) at first evaluation | 0.926 (0.814–1.053) | 0.241* | 1.029 (0.978–1.083) | 0.273* |
| Serum creatinine at first evaluation | 1.096 (0.887–1.356) | 0.395* | 1.122 (0.830–1.517) | 0.454* |
| GFR at the date of construction of the first vascular access | 0.968 (0.834–1.124) | 0.672* | 1.059 (0.923–1.216) | 0.412* |
| Serum creatinine at time of construction of the first vascular access | 0.983 (0.776–1.246) | 0.887* | 1.223 (0.947–1.579) | 0.123* |
| Etiology of chronic kidney disease | ||||
| Diabetic nephropathy | 0.198 (0.044–0.887) | 0.021+ | 0.632 (0.122–3.282) | 0.715 |
| Glomerulonephritis | 2.800 (0.996–7.872) | 0.062 | 13.611 (2.884–64.244) | 0.001 |
| Pyelonephritis/interstitial nephritis/TIN | 0.582 (0.070–4.839) | 1.000 | 0.000 (0.000–0.000) | 1.000 |
| Polycystic kidney disease | 1.090 (0.222–5.352) | 1.000 | 0.000 (0.000–0.000) | 1.000 |
| Amyloidosis | 0.000 (0.000–0.000) | 0.59 | 0.000 (0.000–0.000) | 1.000 |
| Multifactorial or other | 3.344 (1.100–10.166) | 0.038 | 0.000 (0.000–0.000) | 1.000 |
| Unknown | 0.836 (0.259–2.691) | 1.000 | 0.512 (0.060–4.367) | 1.000 |
| Comorbidities | ||||
| Diabetes mellitus | 3.867 (1.235–12.113) | 0.014+ | 1.441 (0.328–6.326) | 0.725 |
| Ischemic coronary disease | 2.041 (0.563–7.396) | 0.407 | 0.490 (0.109–2.195) | 0.392 |
| Peripheral arterial disease | 2.020 (0.437–9.338) | 0.527 | 1.462 (0.170–12.583) | 1.000 |
| Stroke | 1.226 (0.257–5.857) | 1.000 | 0.000 (0.000–0.000) | 1.000 |
| Renal transplant | 0.646 (0.213–1.963) | 0.441 | 0.548 (0.102–2.948) | 0.613 |
| Vascular access | ||||
| Radial-cephalic arteriovenous fistula | 3.929 (1.485–10.392) | 0.004+ | 1.895 (0.449–8.001) | 0.453 |
| Brachio-cephalic arteriovenous fistula | 0.211 (0.059–0.750) | 0.010+ | 1.391 (0.331–5.853) | 0.721 |
| Brachio-basilic arteriovenous fistula | 0.928 (0.286–3.004) | 1.000 | 0.000 (0.000–0.000) | 1.000 |
| Arteriovenous graft | 0.000 (0.000–0.000) | 1.000 | 0.000 (0.000–0.000) | 1.000 |
TIN: tubulointerstitial nephritis.
The mean age of patients with PF was 58.3±15.7 years; age was not significantly associated with the occurrence of primary failure (OR=0.983 (0.956–1.010), p=0.209).
All PF occurred in AVF, with the following locations: 15 radial-cephalic AVF (PF 26.3%), three brachio-cephalic AVF (PF 5.6%), and four brachio-basilic AVF (PF 14.8%).
Radial-cephalic AVFs were associated with a higher risk of PF in this study (OR=3.929 (1.485–10.392), p=0.004). Brachio-cephalic AVFs have a lower risk of PF in this study (OR=0.211 (0.059–0.750), p=0.010). Brachio-basilic AVFs and AVG were not statistically significantly correlated with the occurrence of PF.
In this study, DM was associated with a higher risk of PF (OR=3.867 (1.235–12.113), p=0.014), while previous stroke, PAD, and ischemic coronary disease did not significantly influence this event. However, in evaluation by the etiology of chronic kidney disease, diabetic nephropathy appeared to be a protective factor against PF (OR=0.198 (0.044–0.887), p=0.021).
The glomerular filtration rate at first evaluation and at the time of the creation of vascular access did not significantly influence the occurrence of PF.
Maturation failureIn this study, the incidence of maturation failure at eight weeks was 4.8% for AVF (Table 3); all patients required surgical or endovascular intervention to allow access maturation.
The mean age was 61.5±26.7 years; three patients had DM (37.5%), and one patient had PAD (12.5%). The cause identified for MF was inflow stenosis in 75% of cases, outflow stenosis in 12.5%, and central stenosis in 12.5%.
Four maturation failures were identified in radial-cephalic AVF and four in brachio-cephalic AVFs.
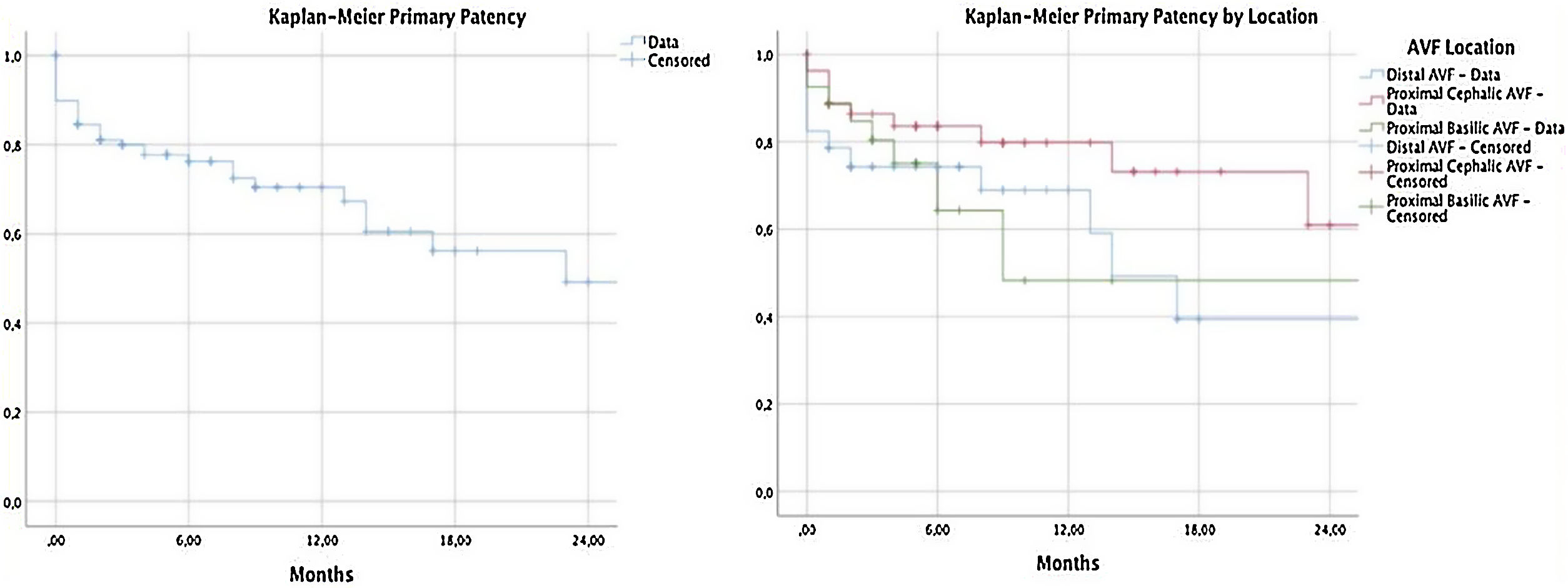
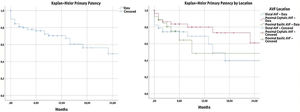
Primary patency and primary assisted patencyIn our population, the PP at 6, 12 and 24 months was 76.3%, 70.4% and 49.2%. The assessment of PP according to location is described in Table 4. In the assessment by location, no statistically significant differences between the groups were found (p=0.128) (Fig. 1).
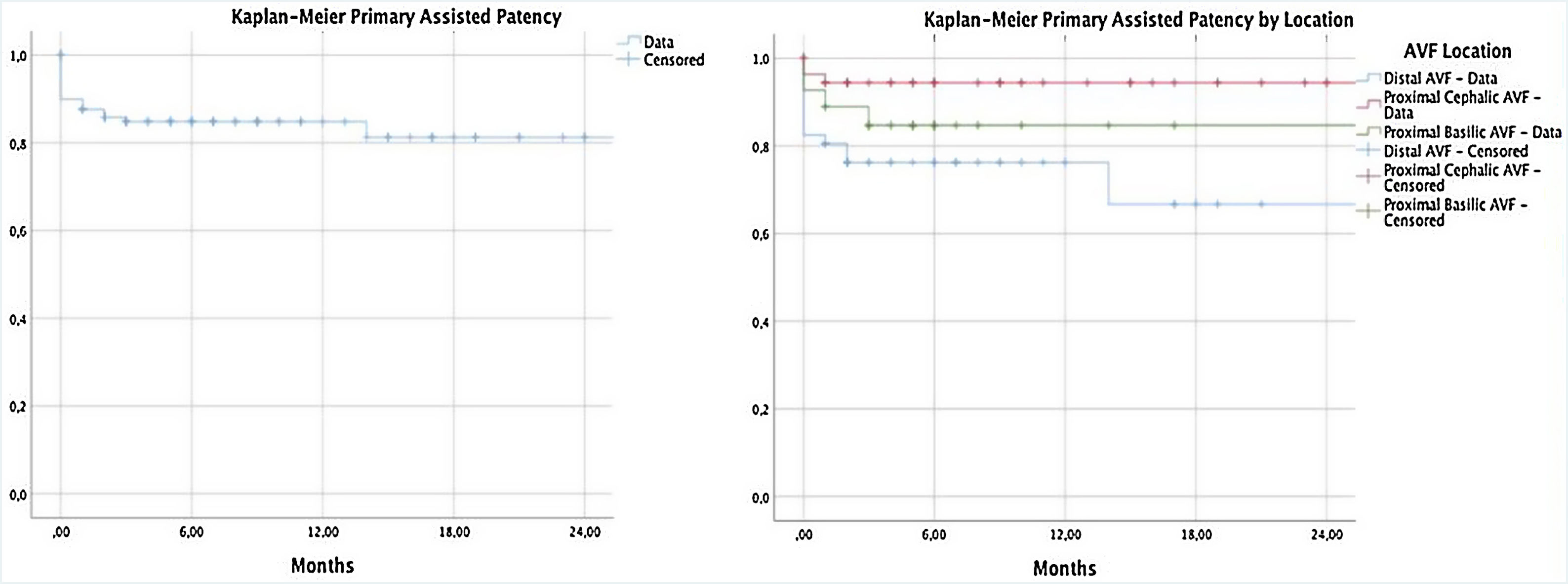
Description of primary patency and primary assisted patency at 6, 12, and 24 months.
| Secondary outcomes | |||
|---|---|---|---|
| Primary patency | Primary assisted patency | ||
| 6 months | 76.3% | 6 months | 84.8% |
| 12 months | 70.4% | 12 months | 84.8% |
| 24 months | 49.2% | 24 months | 81.3% |
| Primary patency by location | Primary assisted patency by location | ||
| 6 months | 6 months | ||
| Radial-cephalic arteriovenous fistula | 74.3% | Radial-cephalic arteriovenous fistula | 76.2% |
| Brachio-cephalic arteriovenous fistula | 83.6% | Brachio-cephalic arteriovenous fistula | 94.4% |
| Brachio-basilic arteriovenous fistula | 64.3% | Brachio-basilic arteriovenous fistula | 84.7% |
| 12 months | 12 months | ||
| Radial-cephalic arteriovenous fistula | 68.9% | Radial-cephalic arteriovenous fistula | 76.2% |
| Brachio-cephalic arteriovenous fistula | 79.8% | Brachio-cephalic arteriovenous fistula | 94.4% |
| Brachio-basilic arteriovenous fistula | 48.3% | Brachio-basilic arteriovenous fistula | 84.7% |
| 24 months | 24 months | ||
| Radial-cephalic arteriovenous fistula | 39.4% | Radial-cephalic arteriovenous fistula | 66.7% |
| Brachio-cephalic arteriovenous fistula | 61.0% | Brachio-cephalic arteriovenous fistula | 94.4% |
| Brachio-basilic arteriovenous fistula | 48.3% | Brachio-basilic arteriovenous fistula | 84.7% |
PP excluding PF was 89.7% at six months, 82.5% at 12 months, and 58.6% at 18 months.
Primary assisted patency was 84.8% at 6 and 12 months and 81.3% at 24 months (Table 4). PAP was different according to the location of vascular access (p=0.009) (Fig. 2).
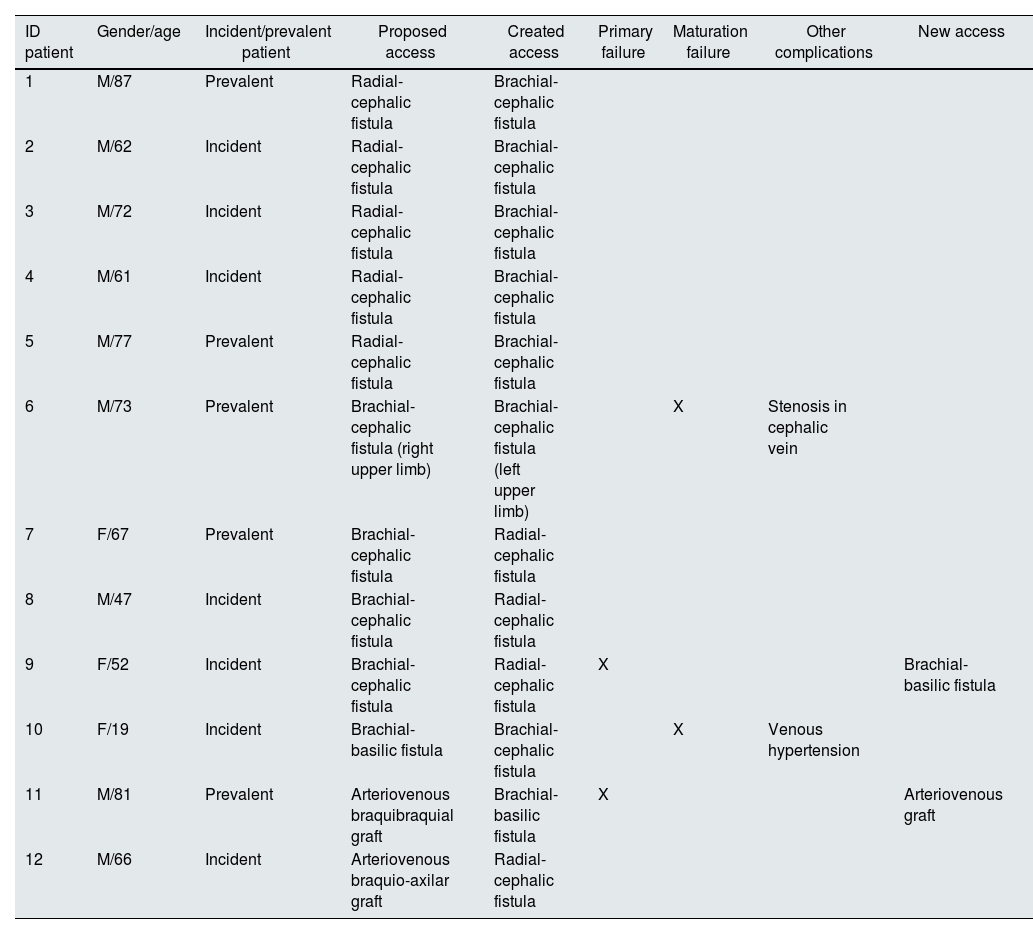
Relationship between nephrologist and surgical team decisionIn 12 patients (8.5%) there was a discrepancy between the nephrologist's proposal and the vascular surgeon's final decision in the operating room (Table 5). High reliability was documented between the nephrologist and surgical team assessments (k=0.874; p<0.01; concordance=91.5%).
Description of cases in which there was discrepancy in the assessment between the nephrologist and the surgical team.
| ID patient | Gender/age | Incident/prevalent patient | Proposed access | Created access | Primary failure | Maturation failure | Other complications | New access |
|---|---|---|---|---|---|---|---|---|
| 1 | M/87 | Prevalent | Radial-cephalic fistula | Brachial-cephalic fistula | ||||
| 2 | M/62 | Incident | Radial-cephalic fistula | Brachial-cephalic fistula | ||||
| 3 | M/72 | Incident | Radial-cephalic fistula | Brachial-cephalic fistula | ||||
| 4 | M/61 | Incident | Radial-cephalic fistula | Brachial-cephalic fistula | ||||
| 5 | M/77 | Prevalent | Radial-cephalic fistula | Brachial-cephalic fistula | ||||
| 6 | M/73 | Prevalent | Brachial-cephalic fistula (right upper limb) | Brachial-cephalic fistula (left upper limb) | X | Stenosis in cephalic vein | ||
| 7 | F/67 | Prevalent | Brachial-cephalic fistula | Radial-cephalic fistula | ||||
| 8 | M/47 | Incident | Brachial-cephalic fistula | Radial-cephalic fistula | ||||
| 9 | F/52 | Incident | Brachial-cephalic fistula | Radial-cephalic fistula | X | Brachial-basilic fistula | ||
| 10 | F/19 | Incident | Brachial-basilic fistula | Brachial-cephalic fistula | X | Venous hypertension | ||
| 11 | M/81 | Prevalent | Arteriovenous braquibraquial graft | Brachial-basilic fistula | X | Arteriovenous graft | ||
| 12 | M/66 | Incident | Arteriovenous braquio-axilar graft | Radial-cephalic fistula |
In this group, most patients were male (n=9; 75%) and seven were incident patients (58.3%). The rate of primary failure and maturation failure in this group was 16.6%.
In five patients, the nephrologist proposed the construction of a radial-cephalic fistula, and the surgical team opted for constructing a brachio-cephalic fistula; in this group, no further complications were identified in the development of vascular access.
In three patients with proposed brachio-cephalic fistula, the surgical team opted to construct a radial-cephalic fistula; primary failure was documented in one of them.
In one patient, there was disagreement regarding the laterality of vascular access: a brachio-cephalic fistula was proposed in the right upper limb and was created in the left upper limb; the fistula had maturation failure and need for endovascular intervention due to recurrent stenosis of the cephalic vein.
In two patients, the construction of a vascular graft was proposed by nephrologist: in one patient, the surgical team initially constructed a brachio-basilic fistula, which had a primary failure, and subsequently, a vascular graft was constructed; in another patient, a radial-cephalic fistula was successfully constructed in the contralateral limb.
DiscussionAlthough European Best Practice guidelines recommend routine DUS mapping before the creation of AVF, KDOQI suggests selective preoperative (PE) ultrasound in patients at high risk of AV access failure rather than routine vascular mapping in all patients.16,17 This aspect reflects the lack of consensus that favors its use by routine in patients with suitable veins on physical examination. Wong et al., in a recent meta-analysis, showed an increase in the number of AVFs but did not show a clear benefit in general outcomes in patients undergoing routine PE-CDU. However, early primary failures were not included in the analysis.7 More recently, a meta-analysis of 5 randomized clinical trials concluded that preoperative clinical examination should always be complemented with vascular mapping with DUS, avoiding negative surgical explorations and significantly reducing the immediate AVF failure rate.7
Successful AVF function significantly impacts patients requiring hemodialysis; creating the first access without complications reduces the cost of managing failed fistulas, which may need reoperation or endovascular procedures.4
In our study, we evaluated the outcomes of 141 vascular accesses. A primary failure of 15.6% was documented. Although comparison of studies is often difficult due to the high degree of heterogeneity in the definitions used, this percentage of primary failure was substantially lower than in most studies.
A previous study comparing immediate surgical failure between patients with preoperative evaluation by CDU and patients without CDU evaluation showed an immediate failure of 5.6% in the first group and 25% in the second group.18 A randomized study designed to assess the outcomes of AVFs concerning preoperative CDU use showed that CDU group had a lower rate of immediate failure (4% versus 11%, p<0.028); however, this study did not find differences in access survival after 1 year.19
Gjorgjievski et al. recently presented similar results to those observed in our study (primary failure of 16.3%) in patients with a preoperative evaluation with CDU,20 and a meta-analysis of five randomized controlled trials that included 574 patients significantly showed lower rates of immediate AVF failure in patients with CDU evaluation before surgery.21 Previously, our group presented similar results to this work using an identical methodology (AVF primary failure of 15%).4 A recent randomized clinical study compared a group of patients undergoing a vessel mapping with PE-CDU and a control group (only physical examination) show a significantly higher rate of primary failure in the control group.22
Certain patient-related factors, namely female gender, advanced age, and forearm fistula, have been associated with poor vascular access prognosis, despite routine venous mapping.6,23
We documented a significantly higher PF rates in radial-cephalic AVFs and lower in brachio-cephalic AVFs, which is in line with what is described in the literature. Surprisingly, we did not document differences in rates of primary failure and maturation failure in female patients; the exact mechanism of different AVF outcomes between genders is unclear but several factors have already been considered: differences in vascular diameter, reactivity, and differences in the ability of venous dilatation to arterial pressure.24 In this work, we did not record the diameter of the vessels used for surgery, which could help understand this result. Still, we note that our strategy and surgical proposal are independent of the patient's gender. We believe that our systematic preoperative evaluation with CDU and the technical skills of the surgical team justify this result.
DM is a recognized poor prognostic factor for vascular access in terms of primary failure25 and overall access survival, as a recent meta-analysis shows.26 In this study, the presence of DM tripled the risk of primary failure. However, diabetic nephropathy appeared to be a protective factor against primary failure. This result is not in line with what is described in the literature and cannot be easily explained by the authors; a possible explanation may be the eventual misdiagnosis in the etiology of kidney disease (diagnosis is primarily clinical rather than histological).
Despite being carried out in a tertiary hospital center, this study showed a reduced prevalence of AVG (2.1%). This results from the policy of primacy of autologous access, the long experience with basilic veins, and the routine use of CDU. A previous prospective study that evaluated the effect of preoperative CDU showed similar results, with an impressive increase in the percentage of AVF (34–64%).27
In this work, we document a very low MF at eight weeks (4.8%) compared to what is described in the literature. The most recent studies with preoperative vascular mapping showed rates of MF between 6.3% and 33%.4,20 Hossain et al. documented the impact of preoperative vascular assessment on AVF maturation, with an increase in the rate of MF in the group in which ultrasound was not performed (18 and 47%, respectively).11 The different temporal cut-offs to define the outcome limit an extended comparison with our study.
A recent single-center retrospective study with preoperative CDU evaluation by three vascular surgeons showed a maturation rate (defined as a palpable thrill and/or successful cannulation of the fistula with the ability to deliver a flow rate of 400ml/min) of only 67%28; the prevalence of radial-cephalic AVFs was similar to our study (41% and 40.4%), however, in this study, during the vascular evaluation, the tourniquet was not routinely applied (contrary to what happened in our work).
El Khoury et al. documented that obese patients have a higher risk of MF.28 In obese patients, the importance of the CDU is even more pronounced: a recent study that compared outcomes related to vascular access in patients who underwent physical examination only and patients who underwent CDU showed a reduction in maturation failure from 45% to 9%.11 Our study did not find differences in maturation failure related to body mass index. Surprisingly, we found an increased risk of MF in patients with glomerulonephritis, which is not described in the literature.
Our results are the consequence of a multidisciplinary approach, a good relationship between the medical team (nephrologists) and the surgical team (vascular surgery), and the equality of criteria related to vascular access. This last aspect becomes even clearer considering the high agreement between nephrologists and the vascular team (91.5%) and the absence of statistical differences in inter-rater reliability (k=0.874; p<0.01). It should be noted that the main difference in approach results in the creation of brachial-cephalic fistulas in patients with a proposal for distal fistulas (probably a result of the preoperative evaluation dictating the lack of technical feasibility in distal vessels).
This small group of patients had a maturation failure rate almost three times higher than the rest of the population; these results are probably explained by the vessel complexity of this group of patients, which led to discrepancies between evaluators. However, future studies are needed to clarify these findings. The primary failure rate was similar to the observed in the entire population.
In our practice, we are rigorous in the complete evaluation of the vessels to be proposed; whenever possible, when a vascular alteration that predicts maturation difficulties is identified (namely focal vein stenosis or significant lesions as a result of previous puncture), this vein is not proposed for use in surgery. This may explain, in part, our reduced rate of maturation failure (almost unprecedented in the literature) - and may help to explain the increased rate of maturation failure in patients in whom there was a discrepancy between nephrologists and vascular surgeons. However, this rigor does not make proximal accesses preferred in our study, as can be seen by the high number of proposed radial-cephalic AVFs (41.9%).
Our study showed high PP and PAP rates compared to recent studies using preoperative CDU.21,29 The PP rate in our population at six months was 70.4% (82.5%, excluding PF), and PAP was 84.8% at 6 and 12 months. Secondary patency has been achieved at the expense of endovascular interventions that increase the cost of access and are unpleasant for patients. Silva et al. compared access outcomes in 172 patients undergoing preoperative venous mapping with historical controls. The PP at one year increased from 48 to 83%.30 More recently, Torres et al. evaluated a prospective cohort that created AVF after CDU and compared the outcomes of accesses with previous cohorts without preoperative assessment. PP in the first year increased with CDU (59.5–71.9%), and PAP at 1 and 2 years was significantly higher in CDU group (63.2–80.7% and 58.1–70.2%, respectively).29
Certainly, several factors contributed to the positive results observed in this study: the existence of a multidisciplinary team in permanent contact, the evaluation by only one experienced nephrologist (guaranteeing a complete uniformity of criteria), and the high experience of the surgical team in the creation of vascular access for hemodialysis.
The main limitations of this study are its retrospective and unicentric design. The lack of data on vascular access diameters may limit comparison with other studies.
ConclusionThe authors defend that the study of the entire vascular territory performed with CDU, within a multidisciplinary team of nephrologists and vascular surgeons, is associated with high rates of autologous access and very low rates of primary failure and maturation failure (almost unprecedented in the literature).
FundingNone declared.
Conflict of interestNone declared.