The aim of this study was to evaluate the trough concentrations (Cptrough) and the tacrolimus dosage regimen after the conversion of Prograf or Advagraf to Envarsus (new pharmaceutical form with MeltDose technology that improves the absorption of fat-soluble drugs) in patients with stable renal transplantation, and their renal function.
We selected stable renal transplant patients who were converted to Envarsus. Two periods were defined: Baseline and Conversion (Envarsus) and they were stratified according to the pharmaceutical form used in the Baseline period. Sixty-one patients were included (24 with Advagraf and 37 with Prograf), with an average age of 52years. The mean post-transplant time at the time of conversion to Envarsus was 76.3months and the mean follow-up in the Baseline and Conversion period was 10.1months and 11.6months, respectively.
In the Prograf and Envarsus group, the Cptrough medians were 6.6 vs 6.4ng/ml (p=.636), with a mean daily dose that decreased significantly from 3mg to 2mg (p<.001), respectively, maintaining the filtration rate.
The median Cptrough values in the Advagraf and Envarsus groups were 5.7ng/ml and 6.3ng/ml (p=.07), with a median daily dose of 7mg and 4mg (p<.001), respectively, and the same renal function.
In stable renal transplant patients, the conversion from Advagraf to Envarsus has allowed the dose of tacrolimus to be reduced by 42.9% and, in the case of Prograf, by 33.3%, maintaining similar Cptrough values, without renal function being altered.
El objetivo del presente estudio fue evaluar las concentraciones valle (Cpvalle) y la pauta posológica de tacrolimus tras la conversión de Prograf o Advagraf a Envarsus (nueva forma farmacéutica con tecnología Meltdose que mejora la absorción de fármacos liposolubles) en pacientes con trasplante renal estable, y su función renal.
Se seleccionaron los pacientes trasplantados renales estables que fueron convertidos a Envarsus. Se definieron dos periodos: basal y conversión (Envarsus), y se estratificaron en función de la forma farmacéutica utilizada en el periodo basal. Se incluyeron 61 pacientes (24 con Advagraf y 37 con Prograf), con una edad media de 52años. El tiempo medio postrasplante en el momento de la conversión a Envarsus fue de 76,3meses y el seguimiento medio en el periodo basal y conversión fue de 10,1 y 11,6meses, respectivamente.
En el grupo Prograf y Envarsus las medianas Cpvalle fueron 6,6 vs 6,4ng/ml (p=0,636), con una dosis diaria media que disminuyó significativamente de 3 a 2mg (p<0,001), respectivamente, manteniendo el filtrado renal.
Las medianas Cpvalle en los grupos Advagraf y Envarsus fueron 5,7 y 6,3ng/ml (p=0,07), con una mediana de dosis diaria de 7 y 4mg (p<0,001), respectivamente, e igual función renal.
En pacientes trasplantados renales estables la conversión de Advagraf a Envarsus ha permitido reducir la dosis de tacrolimus un 42,9% y la de Prograf un 33,3% para mantener unas Cpvalle similares, sin que se altere la función renal.
Most of the immunosuppressive therapy regimens used in renal transplantation currently include tacrolimus.1 The effectiveness of the twice-daily-dose formulation of tacrolimus (Prograf) has been proven in multiple studies.2 The conversion of the immediate-release pharmaceutical form to a prolonged-release formulation (Advagraf) has also been studied in depth; in the Spanish study of conversion between the two formulations, Guirado et al.3 proved its efficacy and safety in 96% of patients, with a conversion base of 1–1mg. The efficacy and safety of these two formulations have been verified in numerous non-inferiority studies and found to be similar.4
Tacrolimus is characterised by having a narrow therapeutic margin and a large inter- and intra-individual pharmacokinetic variability which requires close pharmacokinetic monitoring. The strong correlation between the area under the curve and the trough level allows personalisation of the dose only by monitoring the trough concentration.5–7
As a result of the combination of poor water solubility, metabolisation of tacrolimus in the intestinal tract and the activity of the P-glycoprotein pump of the intestinal enterocytes, Prograf has low bioavailability of tacrolimus, about 17% in renal transplant recipients.8
Lack of adherence to treatment is a common cause of graft loss,9 and the evidence that adherence improves significantly when switching from twice to once daily administration of the drug is another factor in favour of single-daily-dose formulations.10–12 In addition to better adherence, the Spanish study showed that after conversion, patients clearly expressed their preference for prolonged-release tacrolimus, which can be taken once a day.3
A new pharmaceutical form of extended-release tacrolimus has been marketed recently, Envarsus, which could provide a combination of improved adherence and bioavailability; as, Advagraf, it is administered once a day, and its formulation is based on the Meltdose release system,13 designed to increase the bioavailability of drugs that are poorly soluble in water.
Studies of conversion from both Prograf and Advagraf to Envarsus have been carried showing that bioavailability Envarsus is superior. Moreover, the Envarsus datasheet summary states that “Patients who are recipients of an allogeneic transplant, maintained with two daily doses of Prograf (immediate release) or Advagraf (once a day) and who require conversion to one daily dose of Envarsus, should have their total daily dose changed at a ratio of 1:0.7 (mg:mg); the maintenance dose of Envarsus should therefore be 30% lower than the dose of Prograf or Advagraf”.14 However, so far there have been no studies using data obtained from routine practice analysing conversion of the different formulations of tacrolimus to Envarsus.
The aim of this study was to analyse the tacrolimus trough concentrations and dosage regimen after conversion from Prograf or Advagraf to Envarsus in patients with stable renal transplantation, and to assess the impact on renal function.
Material and methodsThis was a retrospective, observational study. We selected renal transplant patients from Hospital General Universitario de Alicante who were converted from Prograf or Advagraf to Envarsus from January 2015 to April 2017. Two periods were defined: baseline (up to the day before the change to Envarsus) and conversion (from the change to Envarsus on). Patients were then stratified according to the pharmaceutical form used during the baseline period, immediate-release (Prograf) or prolonged-release (Advagraf). The maximum follow-up time in both periods was limited to one year. The general immunosuppressive regimen at the time of renal transplantation included tacrolimus (initial dose: Prograf 0.1mg/kg/12h p.o. or Advagraf 0.2mg/kg/day p.o.; subsequent doses were adjusted to maintain a tacrolimus trough concentration of 8–10ng/ml for the first month and 6–8ng/ml thereafter, mycophenolate mofetil 1g/12h p.o. or mycophenolic acid 360mg/12h p.o., basiliximab or thymoglobulin in high-risk patients and corticosteroids in tapering doses.
Inclusion criteria were: adult patients with more than 6 months after receiving a kidney from a cadaver donor with stable renal function who were converted to Envarsus to improve adherence to treatment (in the case of Prograf) or for suspected toxicity or low bioavailability, with at least three tacrolimus trough concentration values at steady state were available in both study periods.
Blood samples were taken in the trough period (prior to the morning dose). The tacrolimus concentration was determined in whole blood by enzyme immunoassay (Thermo TAC – DRI) (range: 1.2–30ng/ml).
The variables were collected retrospectively from the pharmacokinetic reports of the Pharmacy Department's Clinical Pharmacokinetics Unit. The maximum follow-up time in both periods was one year. For each patient we calculated the mean tacrolimus trough concentration, the mean dose, the mean dose-normalised concentration (ratio between the tacrolimus trough concentration and the dose) and mean renal function, assessed by glomerular filtration rate calculated using the CKD-EPI equation. Additionally, intra-individual variability was quantified from the coefficient of variation of the tacrolimus trough concentrations for the two periods and the two formulations.
The quantitative variables are described by mean and 95% confidence interval (95% CI) or median (25th–75th percentile (p25–p75)) and the comparison of the quantitative variables was analysed using a one-way ANOVA or the Mann–Whitney U test depending on the type of distribution of the variables. The level of statistical significance was set as alpha<0.05. The statistical and graph analysis used the R statistics software program (http://www.R-project.org).
ResultsThe study included 61 patients, 37 males and 24 females, all were Caucasian and transplanted at Hospital General Universitario de Alicante, as they were under the health care covered by the hospital; the mean age of the patients was 52.6 (95% CI: 48.7–54.5) and the mean body weight 69.9kg (95% CI: 66.8–72.9). The mean time after-transplantation when converted to Envarsus was 76.3months (95% CI: 21.8–104.2). The number of tacrolimus trough concentrations included in the baseline and conversion periods was 217 and 298, respectively. The mean follow-up of the baseline and conversion periods was 10.1months (95% CI: 9.0–11.1) and 11.6months (95% CI: 10.8–12.4), respectively.
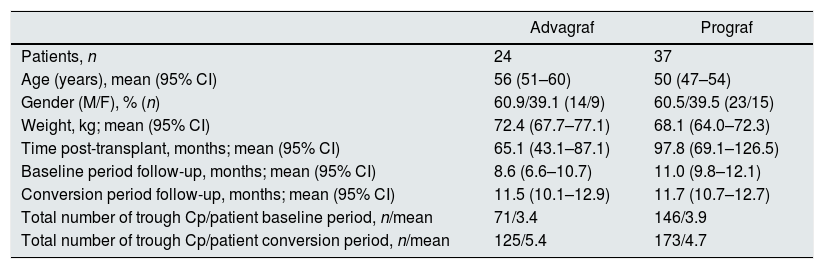
Twenty four out of the 61 patients included, were treated with Advagraf and 37 with Prograf. The demographic data stratified by pharmaceutical form (Prograf/Advagraf) are shown in Table 1.
Baseline demographic data of patients converted to Envarsus stratified according to the pharmaceutical form used during the baseline period (Prograf or Advagraf).
| Advagraf | Prograf | |
|---|---|---|
| Patients, n | 24 | 37 |
| Age (years), mean (95% CI) | 56 (51–60) | 50 (47–54) |
| Gender (M/F), % (n) | 60.9/39.1 (14/9) | 60.5/39.5 (23/15) |
| Weight, kg; mean (95% CI) | 72.4 (67.7–77.1) | 68.1 (64.0–72.3) |
| Time post-transplant, months; mean (95% CI) | 65.1 (43.1–87.1) | 97.8 (69.1–126.5) |
| Baseline period follow-up, months; mean (95% CI) | 8.6 (6.6–10.7) | 11.0 (9.8–12.1) |
| Conversion period follow-up, months; mean (95% CI) | 11.5 (10.1–12.9) | 11.7 (10.7–12.7) |
| Total number of trough Cp/patient baseline period, n/mean | 71/3.4 | 146/3.9 |
| Total number of trough Cp/patient conversion period, n/mean | 125/5.4 | 173/4.7 |
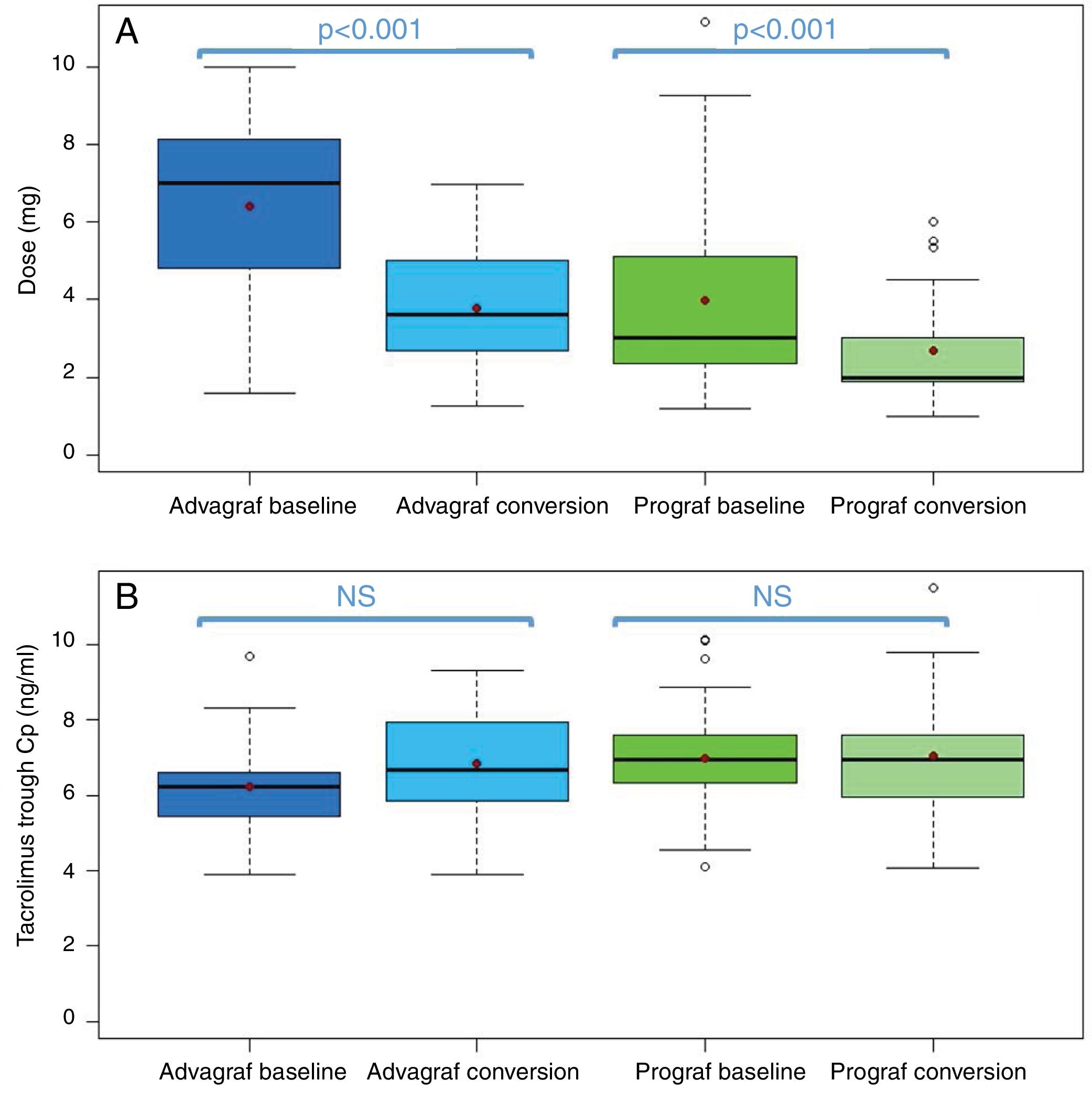
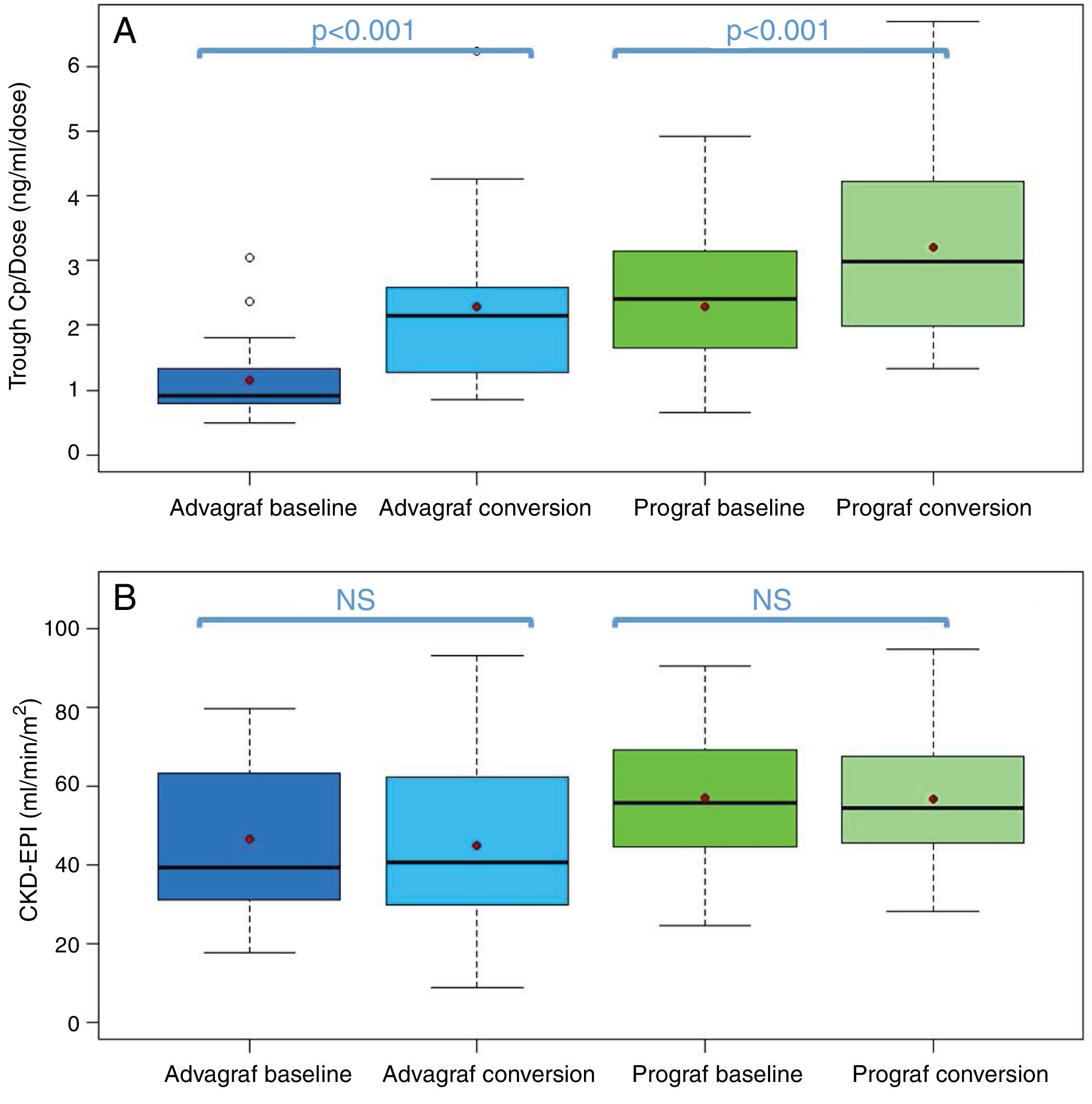
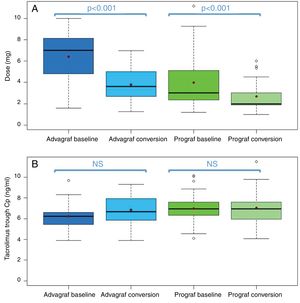
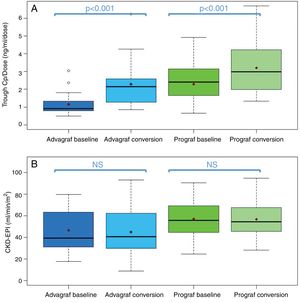
In the Prograf and Envarsus groups, the median tacrolimus trough concentrations were similar: 6.6ng/ml (p25–p75: 5.2–7.7) vs 6.4ng/ml (p25–p75: 4.7–8.2) (p=0.636), with a daily dose which decreased significantly from 3mg (p25–p75: 2–4.5) to 2mg (p25–p75: 1.75–3.0) (p<0.001), while the glomerular filtration rate remained stable with values of 53.7ml/min/m2 (p25–p75: 38.7–66.2) and 50.0ml/min/m2 (p25–p75: 34.4–62.2) (p=0.06), respectively. The median of the trough concentration normalised for dose of tacrolimus was 35% higher in the conversion period (baseline: 2.3ng/ml/mg [p25–p75: 1.4–3.1] vs conversion: 3.1ng/ml/mg [p25–p75: 1.9–4.6]) (p<0.001) (Figs. 1 and 2). The intra-individual variability was 21.4% during the baseline period and 15.1% during the conversion period.
Boxplot of: (A) tacrolimus trough concentration; and (B) tacrolimus trough concentration in the baseline and conversion periods stratified according to the pharmaceutical form used during the baseline period (Prograf or Advagraf). The red dot corresponds to the mean. NS: not significant.
Boxplot of: (A) tacrolimus trough concentration – daily dose ratio; and (B) glomerular filtration rate (expressed as CKD-EPI) in the baseline and conversion periods stratified according to the pharmaceutical form used during the baseline period (Prograf or Advagraf). The red dot corresponds to the mean. NS: not significant.
Likewise, tacrolimus trough concentrations in the Advagraf and Envarsus groups were not significantly different (median: 5.7ng/ml [p25–p75: 4.8–7.0] vs 6.3ng/ml [p25–p75: 5.1–7.7]) (p=0.07), with a median daily dose of 7mg (p25–p75: 4–8) and 4mg (p25–p75: 2.75–5.0) (p<0.001), and a glomerular filtration rate which remained stable in both periods with values of 36.4ml/min/m2 (p25–p75: 28.8–33.65) and 33.7ml/min/m2 (p25–p75: 25.9–59.5) (p=0.248), respectively. During the conversion period, the median of the trough concentration normalised for the dose of tacrolimus was 83.3% higher than in the baseline period: 0.9ng/ml/mg (p25–p75: 0.74–1.43) vs 1.65ng/ml/mg (p25–p75: 1.28–2.85), respectively (p<0.001) (Figs. 1 and 2). As with Prograf, intra-individual variability in the baseline period was higher than that found in the conversion period (25.7% vs 17.4%, respectively).
Switching pharmaceutical form of tacrolimus resulted in a reduction of the dose of tacrolimus of 42.9% after changing from Advagraf to Envarsus and 33.3% from Prograf to Envarsus, maintaining similar tacrolimus trough concentrations.
DiscussionIn the population of patients analysed in the present study to maintain stable tacrolimus trough concentrations, the dose of tacrolimus had to be reduced by 42.9% when changing from Advagraf to Envarsus and 33.3% if changing from Prograf to Envarsus. Envarsus is a recently marketed pharmaceutical form with MeltDose technology. MeltDose, developed by Veloxis Pharmaceuticals increases the bioavailability and control the release of fat-soluble drugs in delayed-release pharmaceutical forms.13 There are therefore two pharmaceutical forms of delayed-release tacrolimus currently available, Advagraf and Envarsus, which are administered once daily. The switch from Prograf or Advagraf to Envarsus has been analysed in a number of different studies.7,15–18 However, to our knowledge, this is the first clinical study of conversion of patients treated with immediate- or prolonged-release tacrolimus to Envarsus. Our results are similar to those obtained by other authors. In a study of conversion from an immediate-release form (Prograf) to Envarsus in stable renal transplant recipients, Gaber et al.7 demonstrated that with a reduction of 30% from the Prograf dose, a similar area under the curve and similar trough concentrations were obtained with Envarsus, but with a significantly lower peak, percentage of fluctuation (change in peak-to-trough concentrations with respect to the mean concentration) and swing (the change in peak-to-trough concentrations with respect to the minimum concentration). The increase in the trough concentration tacrolimus normalised for the dose- or trough concentration/dose ratio is also an indicator of increased bioavailability. In our series concentration/dose ratio was increased by 35% from Prograf to Envarsus and 83.3% from Advagraf to Envarsus. Rostaing et al.15 found an increase in the aforementioned ratio two years after transplant.
Similar results have been found in the conversion between the two delayed-release forms (Advagraf to Envarsus). In the ASTCOFF pharmacokinetic study of conversion with two sequences (Prograf-Envarsus-Advagraf and Prograf-Advagraf-Envarsus), Tremblay et al.16 concluded that to achieve the same degree of exposure the dose of Advagraf had to be increased by 8% with respect to Prograf, and when switching from Advagraf or Prograf to Envarsus the dose had to be reduced by 36% and 30% respectively. These results are very similar to those obtained in our study.
In a cohort study, Niioka et al.17 concluded that to maintain the same area under the curve, a larger dose of Advagraf is necessary than of Prograf, as both the area under the curve and the trough concentration were approximately 25% lower with Advagraf than Prograf, showing that Advagraf had worse bioavailability. In our study, we also showed that the bioavailability of Advagraf is slightly lower than that of Prograf, since the dose reduction necessary to maintain the same levels was 9.6% higher.
This dose reduction would not only be necessary in conversion, but also in induction. As shown in the Budde et al. study,19 which compared recipients with Envarsus versus Prograf from the time of the transplant to the end of one year of follow-up. The Envarsus group reached the target levels faster than the Prograf group, although it should be noted that they received a higher initial dose (0.17mg/kg compared to 0.1mg/kg), and at 3weeks the dose needed to reach the same levels was lower. These data reinforce the idea of a greater initial absorption of Envarsus than Advagraf. In other studies it has been demonstrated the inferiority of Advagraf vs Prograf in achieving levels during the immediate postoperative period.18,20 Wlodarczyk et al.18 showed that using the same starting dose, 0.2mg/kg/24h, the area under curve for tacrolimus on day1 was 30% lower for Advagraf than for Prograf (232 and 361ngh/ml, respectively), and that it was comparable on day 14, but using higher doses of Advagraf. The KDIGO guidelines specifically conclude that the sooner therapeutic levels of calcineurin inhibitors are reached, the more effective will be in preventing acute rejection21; this illustrate the importance of choosing the pharmaceutical presentation of the drug that will most quickly reach therapeutic levels in the immediate postoperative period.
The better bioavailability and/or reduction in apparent clearance varies over the time, as the recipients in the study by Budde et al.19 received a 14% lower dose of tacrolimus at one year. In the extension of that study, Rostaing et al.15 showed that the dose of Envarsus was 24% lower than that of Prograf at 24months, these are similar data to the obtained in our study. Bunnapradist et al.22 randomised a group of recipients with stable renal transplant to Prograf or Envarsus and observed a progressive reduction of up to 25% in the administered dose of Envarsus, with no differences in trough levels.
It is noteworthy that the aforementioned studies include a not-insignificant number of African-American recipients,7,15,17,22 a population that has been shown to require up to twice the dose15 as compared with the Caucasian population to reach the same trough levels.23 Our study population is homogeneous, including only Caucasian patients and coming from a very limited geographical area, which would eliminate possible confounding factors.
The fact that lack of adherence is a common cause of graft loss9 and that adherence significantly improves if the medication is changed from twice to a single daily dose,10 the use of a once-daily tacrolimus formulation should be an absolute requirement, especially when the evening absorption of Prograf is lower than the morning absorption.24,25 In addition, the use of Advagraf reduces the intra-individual coefficient of variation to 40% compared to Prograf, which reinforces the idea of using a once-a-day pharmaceutical form of administration compared to two administrations.26–28 In our study, intra-individual variability also decreased when converting patients from Prograf or Advagraf to Envarsus (CV: 21.4% vs 15.1% and 25.7% vs 17.4%, respectively).
Due to the narrow therapeutic margin of tacrolimus and given the different pharmacokinetics of Prograf, Advagraf and Envarsus, these drugs are not bioequivalent and interchangeable, so changes in the formulations could lead to significant adverse effects. Therefore, close pharmacokinetic monitoring is recommended when switching formulations29; hence the high number of trough concentration determinations made in our study after conversion to Envarsus (baseline period: 217 vs conversion period: 298).
One of the most important limitations of our study is its retrospective observational nature, and the fact that trough tacrolimus determinations were carried out within a routine clinical practice in an outpatient setting. Therefore, in the two periods the follow-up were not exactly the same and there was large variability in the time from transplant to conversion for both formulations (Table 1). However, as said, our results are comparable to previous studies, although the percentage dose reduction in the Advagraf group is higher than that described in other studies.
In conclusion, our study shows that Envarsus is a pharmaceutical form of tacrolimus that may provide benefits for immunosuppression in renal transplantation, besides being a single daily dose formulation, the improved bioavailability enables a significant reduction in the dose tacrolimus achieving a similar trough concentration, without variations in renal function.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Franco A, Más-Serrano P, Balibrea N, Rodriguez D, Javaloyes A, Díaz M, et al. Envarsus, una novedad para los nefrólogos del trasplante: estudio observacional retrospectivo. Nefrología. 2019;39:506–512.