IgA nephropathy (IgAN) is the most common and heterogeneous glomerular nephropathy. Several strategies have been used to determine the risk of progression to ESRD. We evaluate the prognostic significance and correlate the IgAN progression calculator (IgANPC) and the Oxford/MEST-C score in our population.
Material and methodsWe performed a retrospective study of biopsied patients with diagnosis of IgA nephropathy from 1990 to 2015. We classified the biopsies using MEST-C score and we correlated the score to clinical evolution. We also calculated the risk of progression with the online IgANPC at the time of the biopsy.
ResultsWe analyzed 48 biopsies, 83% of which were men with a mean age of 45 years at the time of the biopsy.
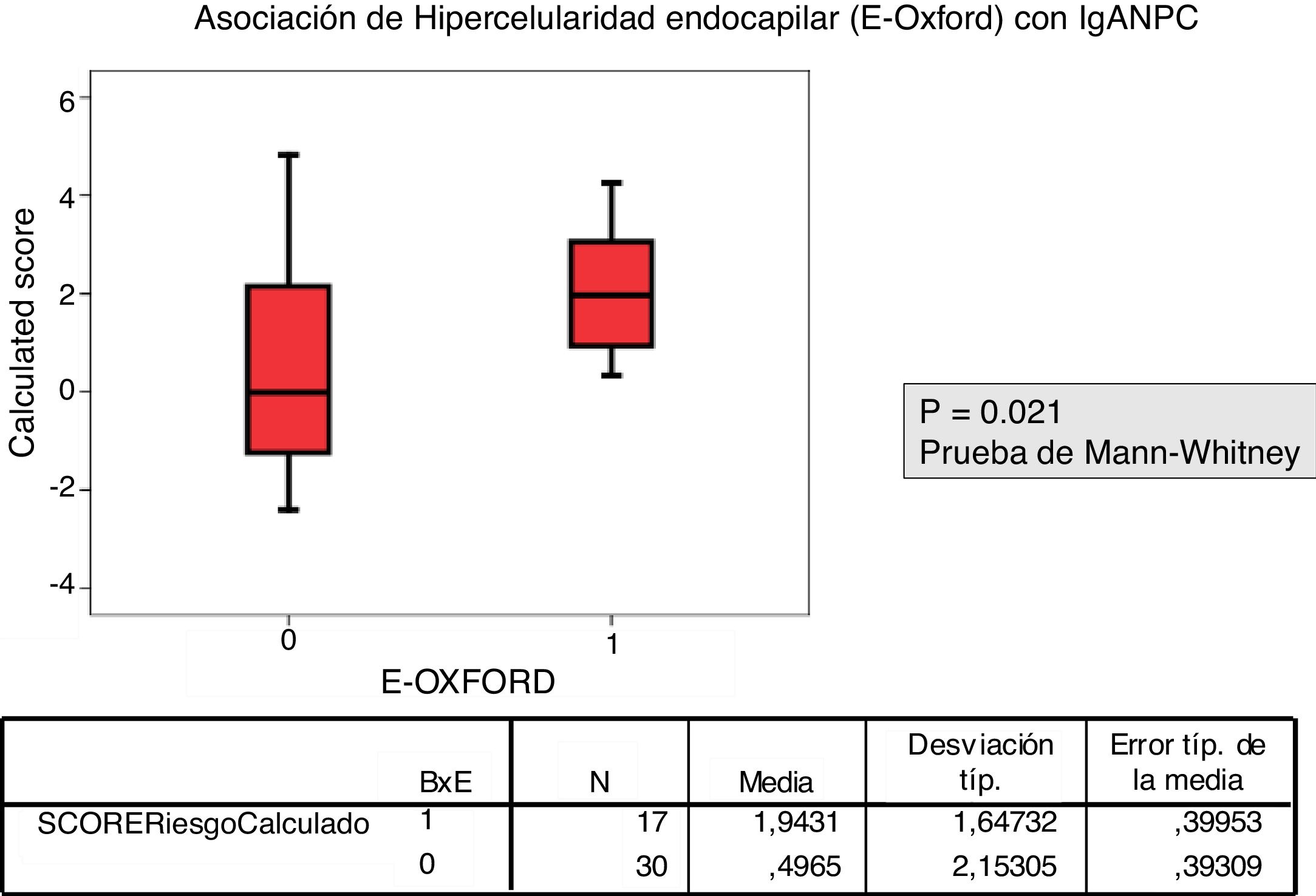
Patients with a biopsy E1 according to MEST-C score had a higher IgANPC score than those with E0 (p=.021).
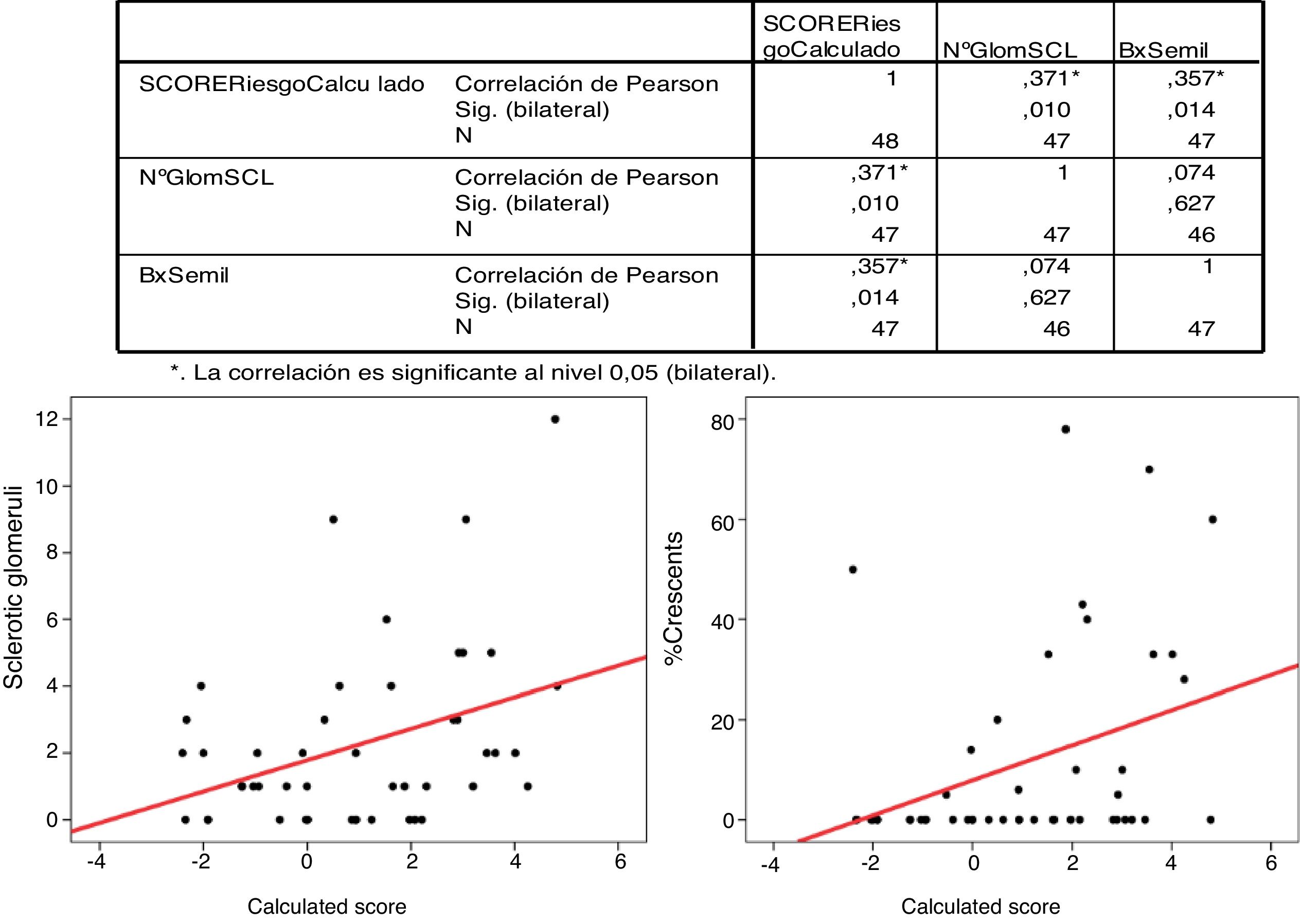
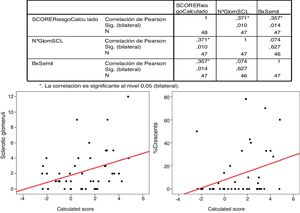
The Pearson's correlation for the percentage of crescents and the IgANPC risk score was statistically significant (p=.014) with r=0.357.
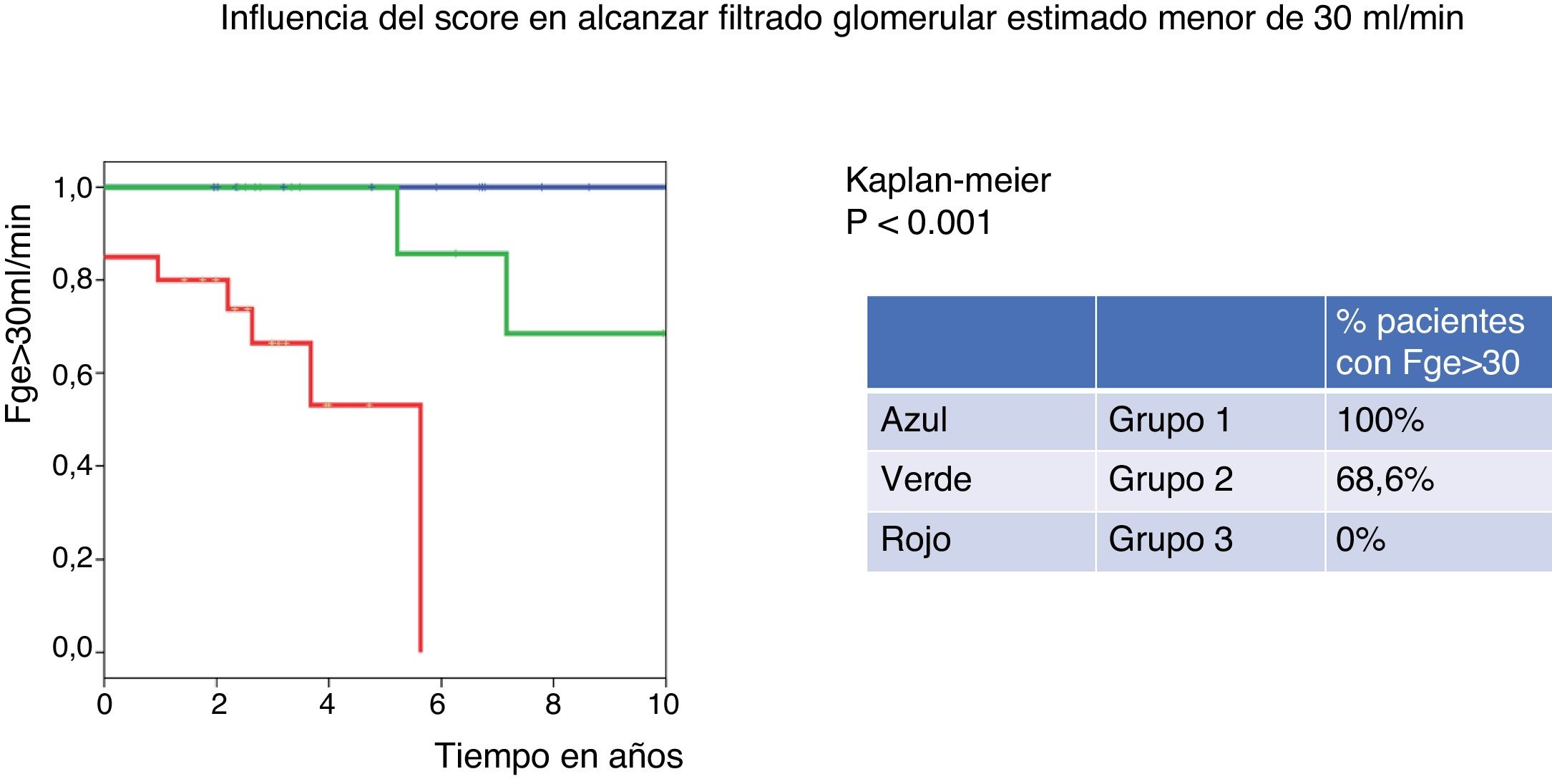
The percentage of patients with eGFR above 30ml/min at 10 years was 100% for the low-risk group (group 1 of IgANPC), and 0% for the high-risk group (group 3), log rank p=0.001.
The log rank comparison for variables of the MEST-C score, presented statistically significant results between E (0.036) and S (0.022) and the eGFR time<30ml/min.
A statistically significant relationship was also observed between T1 and eGFR<30ml/min.
The multivariate Cox regression analysis for IgANPC and eGFR<30ml/min demonstrated a strong correlation (p=.016) between the risk group and eGFR<30ml/min.
ConclusionIn our study population, the IgANPC predicts the time to eGFR<30ml/min, and adds information independent of the MEST.
The MEST-C classification and IgANPC are useful and independent ÿolos for prognostic prediction, but more studies are needed to validate its use in the general population.
La nefropatía IgA es la enfermedad glomerular más frecuente y heterogénea.
Hay estrategias histológicas y clínicas para determinar la progresión a ESRD.
Valoramos el significado pronóstico de la clasificación de Oxford/MEST-C y la calculadora de progresión de la NIgA (IgANPC) en nuestra población y relacionamos ambas herramientas.
Material y métodosRealizamos un estudio retrospectivo de biopsias NIgA de 1990 hasta 2015. Se realizó el MEST de las biopsias y se calculó el riesgo de progresión con IgANPC. Se relaciona con la evolución clínica.
ResultadosSe analizaron 48 biopsias, 83% varones de 45 años de media.
La correlación entre el MEST-C y el IgANPC score a la biopsia mostró una concordancia entre pacientes con un score IgANPC alto y E1 (p = 0,021).
La correlación de Pearson para el porcentaje de semilunas y el IgAPC es estadísticamente significativo (p = 0,014) con r: 0,357.
El 100% de los pacientes clasificados en el grupo 1 de IgANPC mantienen un FGe> 30ml/min a 10 años, mientras que ninguno de los del grupo 3 presenta un FGe> 30ml/min a 10 años (p = 0,001).
La comparación de log rank para variables del MEST-C score presenta resultados estadísticamente significativos entre E (0,036) y S (0,022), y el tiempo a FGe <30ml/min.
También se observa una relación estadísticamente significativa entre T1 y FGe <30ml/min.
El análisis multivariante con la regresión de Cox para IgANPC y FGe<30ml/min muestra una fuerte correlación (p = 0,016) entre el grupo de riesgo y FGe <30ml/min.
ConclusiónIgANP predice el tiempo hasta FGe <30ml/min y añade información independiente del MEST.
La clasificación de MEST-C score y el IgANPC score son útiles e independientes para la predicción pronóstica; queda validar su uso en la población general.
IgA glomerulonephritis, first described by Berger and Hinglais in 1968,1 was described as a very frequent glomerular disease with a benign course2; nowadays we know that it is not this is not totally true.
In IgA nephropathy, sometimes the clinical course may be indolent, and hematuria may be the only manifestation of the disease for many years and without progression over time. However a significant number of patients, up to 40% in some series, progress over the years toward a chronic kidney disease, eventually requiring renal replacement therapy after decades. Occasionally, the disease progresses more rapidly to end-stage renal disease in months or a few years.3,4
Thus, there is variability with respect to progression and prognosis of this disease, initially considered a benign entity but today we know that this is not the case, being the most frequent primary glomerular disease leading to dialysis.5,6
In recent years different prognostic tools have been developed to predict the risk of end-stage renal disease in patients diagnosed with IgA nephropathy.7 Those showing the greatest relationship with progression are based on histology, such as the Oxford/MEST score8 classification, completed in recent years by the association of crescents (to the score MEST, named MEST-C9). These are invasive techniques, requiring renal biopsy.
Also recently, non-invasive clinical tools to predict progression of the disease have been described. One of them is the IgA nephropathy progression calculator (IgANPC),10 only validated in the Chinese population, which includes 4 clinical and analytical parameters at the diagnosis of the disease. However, a validated tool to predict the progression of this entity is not yet available in the general population.
In the present study we analyze the prediction capacity of the IgANPC in our population, as well as its link with the MEST-C classification, relating the different MEST-C variables with this calculator.
Material and methodsDuring the last 25 years we have performed 866 kidney biopsies in patients from our center. The reference area of our hospital for renal biopsies currently includes the entire province of Cantabria and Río Carrión Palencia Hospital Complex; years ago it also included the Bierzo hospital in León, so in our study there are also some patients from these regions.
This is a retrospective study using all patients with kidney biopsy from 1990 to 2015. Of these, 108 patients were diagnosed of IgA glomerulonephritis. Analytical, clinical and demographic data was collected. Patients not included in the analysis were those with incomplete follow-up (n=17), less than 18 years old (n=11) and those with incomplete data in their records (n=32 patients of the hospital El Bierzo and Río Carrión). For patients of Palencia that did have a correct follow-up, we had the collaboration of the Nephrology Service of the Rio Carrión Hospital. A total of 48 patients were analyzed.
The following demographic,clinical and biochemical parameters in blood and urine were collected: age, height, weight, systolic blood pressure (SBP) and diastolic blood pressure, presence or absence of macroscopic hematuria, creatinine, CKD-EPI, serum albumin, uric acid, hemoglobin, 24h proteinuria, protein/creatinine ratio in an urine sample, hemoglobinuria and hematuria in the urinary sediment. All this information was obtained at the time of the kidney biopsy, 2 years after and at the end of the follow-up or initiation of renal replacement therapy (RRT). The time at which the glomerular filtration rate (GFR) fell below 30ml/min or the initial value of serum creatinine doubled were also collected.
Regarding the histology data, the following parameters were collected: the number of glomeruli, the number of sclerosed glomeruli and the variables of the MEST, the percentage of crescents and the presence of C4d and C3 by immunofluorescence.
All biopsies were reviewed and reclassified according to the Oxford/MEST-C criteria with the help of our Pathology Department.
In addition, the risk of progression was calculated using the online calculator IgANPC (http://www.columbiamedicine.org/divisions/gharavi/calc_progression.php). This calculator is based on 4 parameters, both clinical and biochemical, obtained at the time of the kidney biopsy. The parameters on which it is based are: GFR, serum hemoglobin (g/dl), serum albumin (g/dl) and systolic blood pressure (mmHg). Depending on the values obtained, patients are classified as low risk (<−0.887), medium (between −0.887 and 0.993) or high (>0.993), and the result of this calculator was recorded.
Statistic analysisContinuous variables are shown as mean±standard deviation, and qualitative variables are expressed as frequency and percentage.
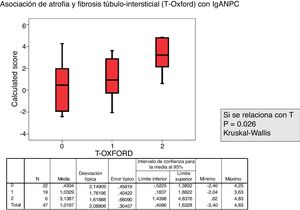
The Mann–Whitney U test was used for comparison of quantitative variables of the MEST-C and the IgANPC scores. The Kruskal–Wallis test was used in the case of the variable time T (T0, T1 and T2 to define the degree of fibrosis and tubulo-interstitial atrophy).
The IgANPC score as a continuous variable was correlated with the percentage of crescents and the number of sclerosed glomeruli using Pearson correlation test.
The log rank comparison test was applied for variables of the MEST-C score and the time elapsed to reach end stage renal disease (ESRD).
Cox regression analysis was used to relate the different variables of the MEST-C score with the time elapsed to ESRD.
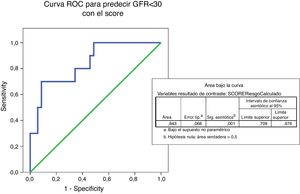
Kaplan–Meier curves were performed to determine the influence of the score on the progression to advanced chronic renal disease (estimated GFR<30ml/min).
The SPSS for Windows version 15.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
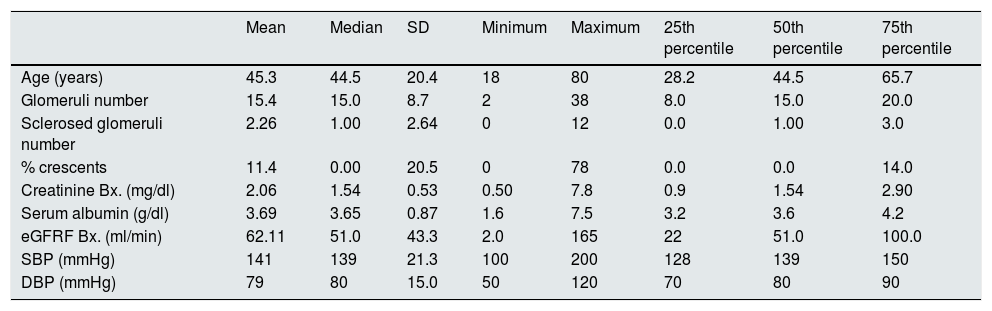
ResultsIn our renal biopsies, IgA constitutes 12% of the diagnosis. The percent of males and female was 83% and 17%, respectively.The average age at the time of the biopsy was 45.3 years, with a standard deviation of 20.8 years. The mean serum creatinine was 2mg/dl with eGFR of 62.1±43.3ml/min (Table 1).
Description of the patients characteristics.
| Mean | Median | SD | Minimum | Maximum | 25th percentile | 50th percentile | 75th percentile | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 45.3 | 44.5 | 20.4 | 18 | 80 | 28.2 | 44.5 | 65.7 |
| Glomeruli number | 15.4 | 15.0 | 8.7 | 2 | 38 | 8.0 | 15.0 | 20.0 |
| Sclerosed glomeruli number | 2.26 | 1.00 | 2.64 | 0 | 12 | 0.0 | 1.00 | 3.0 |
| % crescents | 11.4 | 0.00 | 20.5 | 0 | 78 | 0.0 | 0.0 | 14.0 |
| Creatinine Bx. (mg/dl) | 2.06 | 1.54 | 0.53 | 0.50 | 7.8 | 0.9 | 1.54 | 2.90 |
| Serum albumin (g/dl) | 3.69 | 3.65 | 0.87 | 1.6 | 7.5 | 3.2 | 3.6 | 4.2 |
| eGFRF Bx. (ml/min) | 62.11 | 51.0 | 43.3 | 2.0 | 165 | 22 | 51.0 | 100.0 |
| SBP (mmHg) | 141 | 139 | 21.3 | 100 | 200 | 128 | 139 | 150 |
| DBP (mmHg) | 79 | 80 | 15.0 | 50 | 120 | 70 | 80 | 90 |
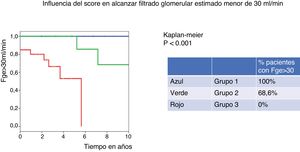
Based on the score obtained with the IgANPC, patients were classified into 3-risk groups. In the low risk group there were 25% of patients, in the intermediate and high risk groups 27.1% and 47.9% respectively. After 10 years, all patients classified in the low-risk group (group 1) of IgANPC maintain a eGFR>30ml/min while in the medium risk group (group 2) only 68.6% had eGFR>30ml/min and none of the patients in the high risk group (group 3) have a eGFR>30ml/min at 10 years (p=0.001) (Fig. 1).
After reclassifying the biopsies using the MEST-C, the percent of patients in M1 was 83%, being 35% in E1, 39.6% in S1 and T0 47.9%, T1 39.6% and T2 12.5%.
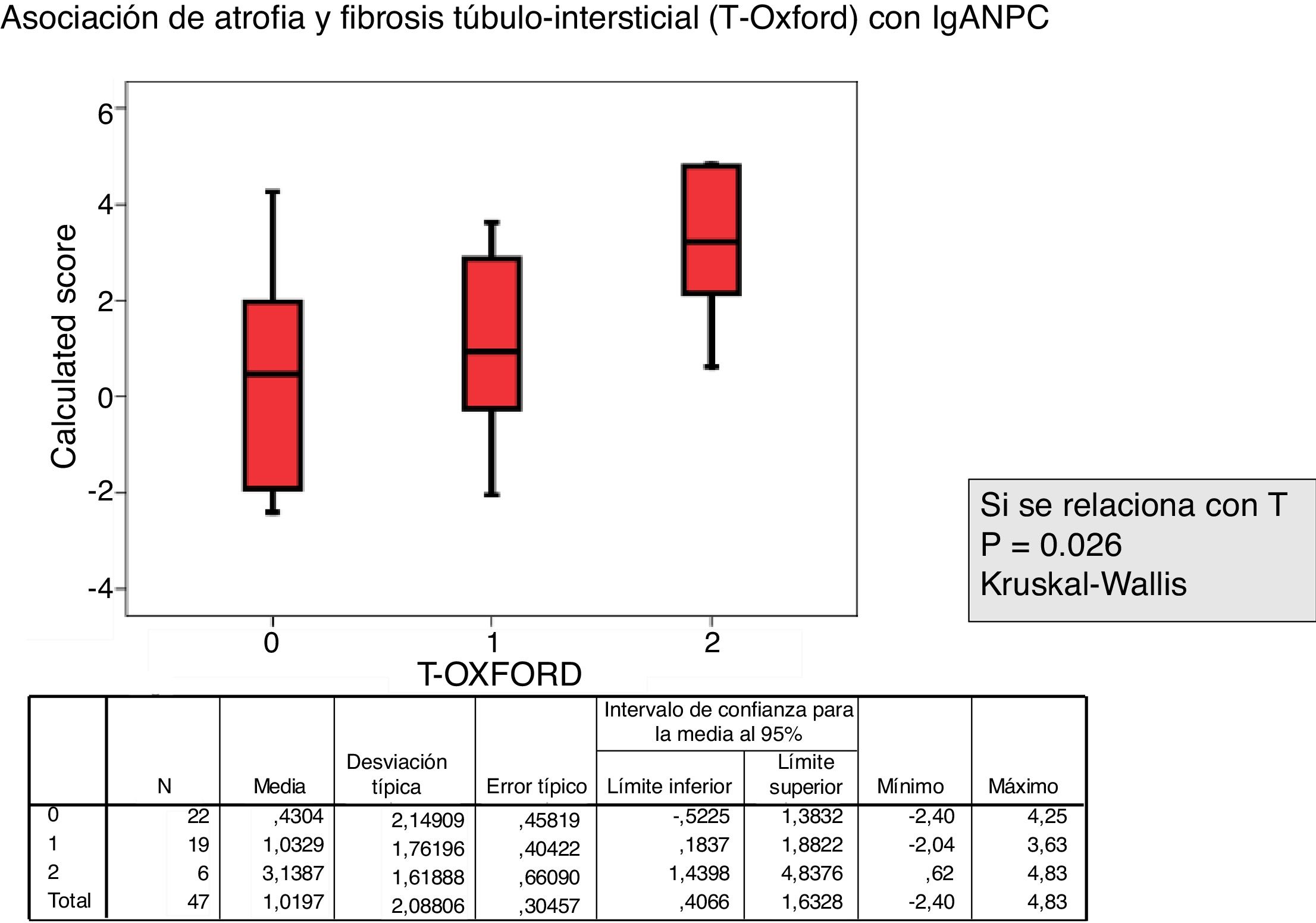
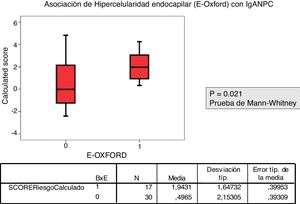
The relationship between the value of each MEST-C variable and the probability of progression calculated using the IgANPC was analyzed. It was observed a concordance between patients with a high IgANPC score and E1 (p=0.021). Likewise, we found a relationship between the score and T (p=0.026); the higher the score, the greater the tubulo-interstitial atrophy (Figs. 2 and 3). The rest of the MEST-C variables were not statistically related to the IgANPC score.
There was a significant correlation (Pearson's correlation) between the percentage of crescents and the IgANPC (r=0.375, p=0.014). No significant correlations were found between the other variables (Fig. 4).
By log rank test the period of time elapsed until reaching ESRD was significantly related to MEST-C score variables E (p<0.036) and S (p<0.022).
Cox regression analysis shows that ESRD is significantly related with IgANPC (p=0.028) (HR=1.864 (95% CI, 1.127–3.083). ESRD is also related with T1 (HR=4.465; 95% CI, 1.179–16.905). Multivariate analysis shows a strong correlation between IgANPC with eGFR<30ml/min and the risk group (p=0.016) (HR=13.701; CI 95%, 1.644–114.209).
Patients with the highest histological variables of E and T (the MEST groups of E1 and T2 and T3) showed a higher risk of reaching eGFR<30ml/min (p=0.016 and p=0.001, respectively).
The multivariate analysis showed that the IgANPC score is independently related with a higher risk of developing a eGFR<30ml/min (HR=13.701; 95% CI, 644–114.209; p=0.016).
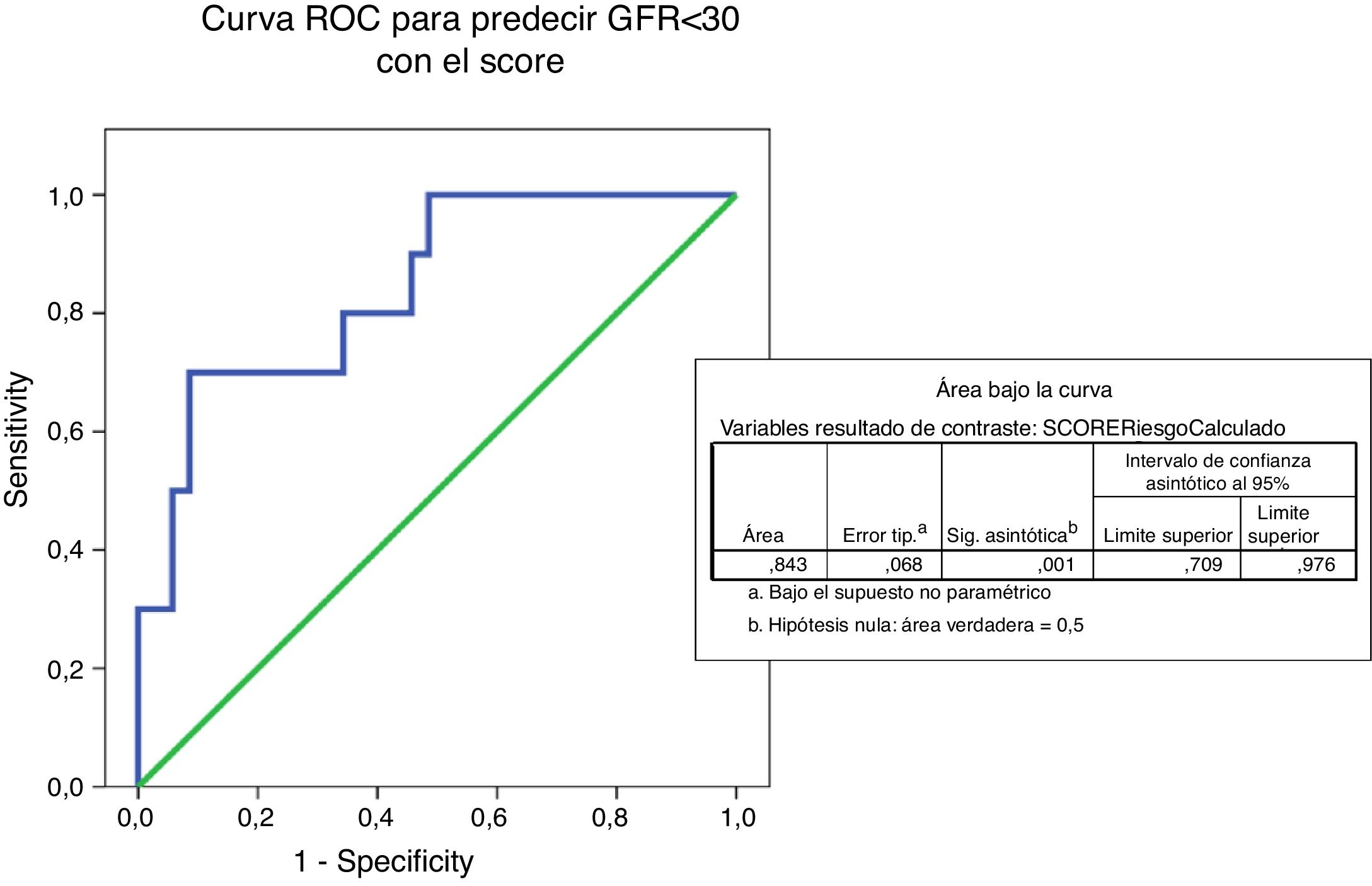
The ROC curve predicting eGFR<30ml/mim by IgANPC score shows an area under the curve of 0.843 which indicates that the test is a good predictor (between 0.75 and 0.90) of progression to advanced renal disease (Fig. 5).
Discussion and conclusionsThe IgA glomerulonephritis is underdiagnosed and its evolution is heterogeneous. Factors strongly related to the progression to ESRD are the presence of persistent proteinuria 1.000mg/24h, hypertension (BP>140/90mmHg) and elevated serum creatinine11–14; the patients who combine the high creatinine and proteinuria have the greatest risk of progression, presenting ESRD in 15–25% after 10 years, and 20–30% after 20 years of follow-up.15–18 The persistence of hematuria has been also associated with a poor prognosis in different studies.19–23
There is evidence that many patients with IgA glomerulonephritis have a deficit in the glycosylation of the IgA1 molecule and this abnormality may be an important factor in the genesis of this disease.24–31
Yanagawa et al. demonstrated that galactosyl-deficient anti-IgA IgG present an area under the ROC curve of 0.813 to discriminate IgA nephropathy from other autoimmune causes of chronic renal diseases.32 Recently, galactosyl-deficient IgA has also been related to renal prognosis in patients with IgA nephropathy.33–35
Different groups have used these serological markers to assess disease activity and their response to different treatments. Berthelot et al. demonstrated that levels of IgA1 galacto sil-deficient, anti-IgA galactosyl-deficient IgG and the soluble CD89-IgA complex predicts recurrence after renal transplantation.36 Other groups have observed that steroid treatment reduces levels of galactosyl-deficient IgA1, while the use of rituximab does not decrease levels of galactosyl-deficient IgA1 and galactosyl-deficient IgA IgG, which could explain its lack of efficacy. to treat IgA nephropathy.37,38 The possibility of having a future treatment of IgA nephropathy, as observed in some animal models, using of recombinant IgA1 protease makes these serological markers available of maximum interest39 to monitor this nephropathy.
Given the frequency of IgA nephropathy, it is necessary to have tools that allow us to know in the best no invasive way, the probability of progression to ESRD to help the clinician to select patients susceptible to treatment and also give the most accurate information about the prognosis at the time of diagnosis. The prognostic tools available today are clearly insufficient and all input is welcome.
In the present study, we have not directly analyzed the classic parameters that have been related to progression, although creatinine and hypertension are included in the IgANPC, since the calculation is made based on eGFR, Systolic blood pressure, albumin and serum hemoglobin.
Regarding proteinuria, persistent hematuria and other markers that have been classically associated with prognosis and MEST,40–42 it has not been the subject of analysis in this work.
In 2014, a work by the VALIGA group of the ERA-EDTA by Coppo et al. in Kidney International, different variables of the MEST are related with the prognosis of IgA nephropathy. In this work, a greater value of the variables M, S and T is related to a worse prognosis, and this association is independent of other variables. When the histological changes of the MEST are related to clinical variables such as proteinuria, the prognostic capacity of the test increases significantly in the group of untreated patients.41
We have demonstrated in our group of patients that the IgANPC is an adequate tool to predict the period of time to reach FGe<30ml/min, and adds prognostic information independent of the MEST-C. In addition, this is a non-invasive tool that, unlike MEST-C, does not require a renal biopsy for its calculation. It also allows us to give concrete prognostic figures regarding the risk of developing ERCT or the need for renal replacement therapy, by expressing its result in a percentage. The latter allows the clinician to inform the patient about the prognosis in a clear and understandable way based not only in professional experience but also on a standardized tool.
In recent years, the MEST classification has been optimized by adding the percentage of crescents to this score.9 Our study confirms that this is correct, we found that the group with high-risk of progression of the disease had a higher percentage of lesions with extracapillary proliferation.
We can conclude that the classification of MEST-C score and the IgANPC score are useful and independent tools for prognostic prediction, it is necessary to validate their use in the general population and relate them to the available serological markers.
Limitations of the studyFirst, this study is a retrospective analysis, with the biases inherent to this type of analysis. Nevertheless, it is the first study to evaluate and match IgANPC and MEST-C score in our population. Second, the number of patients analyzed is low, so the power of statistical results obtained is limited. Third, our data refers to the population of Cantabria and Palencia in Spain, therefore, they cannot be completely extrapolated to other geographical areas. Fourth, the clinical follow-up of the patients was very uneven in time (with a dispersion of 2 years the least follow-up, and 22 years the longest), which may be related to the absence of differences in the variables of clinical assessment between the study groups.
Conflict of interestsThe authors declare no conflict of interest.
Please cite this article as: Martín-Penagos L, Benito A, Oviedo MV, López del Moral Cuesta C, Martín López J, Gómez Román J, et al. ¿Es posible predecir la evolución de la nefropatia IgA? Validamos la calculadora de progresión de nefropatia IgA y su relación con Oxford score en nuestra población. Nefrología. 2019;39:523–530.