We report on a case of a 35-year-old Caucasian woman with primary Sjögren's Syndrome (pSS) diagnosed by salivary gland biopsy and IgM κ monoclonal gammopathy of undetermined significance (MGUS), treated with hydroxychloroquine 200mg daily since she was 22 year-old.
After 24 weeks into her first pregnancy, she developed marked asthenia with muscle pain and arthralgia, hypertension, oedema and palpable purpura of the lower limbs. Laboratorial testing revealed an increase in serum creatinine (sCr) (0.75→1.18mg/dL), anaemia (haemoglobin (Hb)=8.6g/dL), active urinary sediment and nephrotic-range proteinuria (3.8g/24h). She was diagnosed with preeclampsia with foetal distress and underwent a caesarean at 29 weeks of pregnancy. One week post-partum the patient was discharged home with sCr=0.84mg/dL, Hb=12g/dL, 24-hour proteinuria=1.4g and normotensive under nifedipine 30mg daily.
Three months post-partum, she was admitted to the emergency room presenting acute pulmonary oedema requiring invasive ventilatory support. Pulmonary auscultation showed fine crackles over both lung bases, she had moderate peripheral oedema and after urinary catheterization, oligoanuria was confirmed.
Laboratory tests revealed sCr=4.39mg/dL, K+=6.6mmol/L, brain natriuretic peptide=5013pg/mL, Hb=8.1g/dL, platelet count=248x109/L and negative blood cultures. Urinalysis showed 3+ proteinuria on a dipstick test. On echocardiogram there were no signs of endocarditis and she had a ventricular ejection fraction of 50%, renal ultrasound revealed normal kidneys and chest X-ray showed large bilateral pleural effusion.
Continuous haemodiafiltration was initiated and the patient was admitted to the intensive care unit, until she gained ventilatory autonomy and suspended haemodiafiltration, being transferred to the Nephrology Department three days later for continued care.
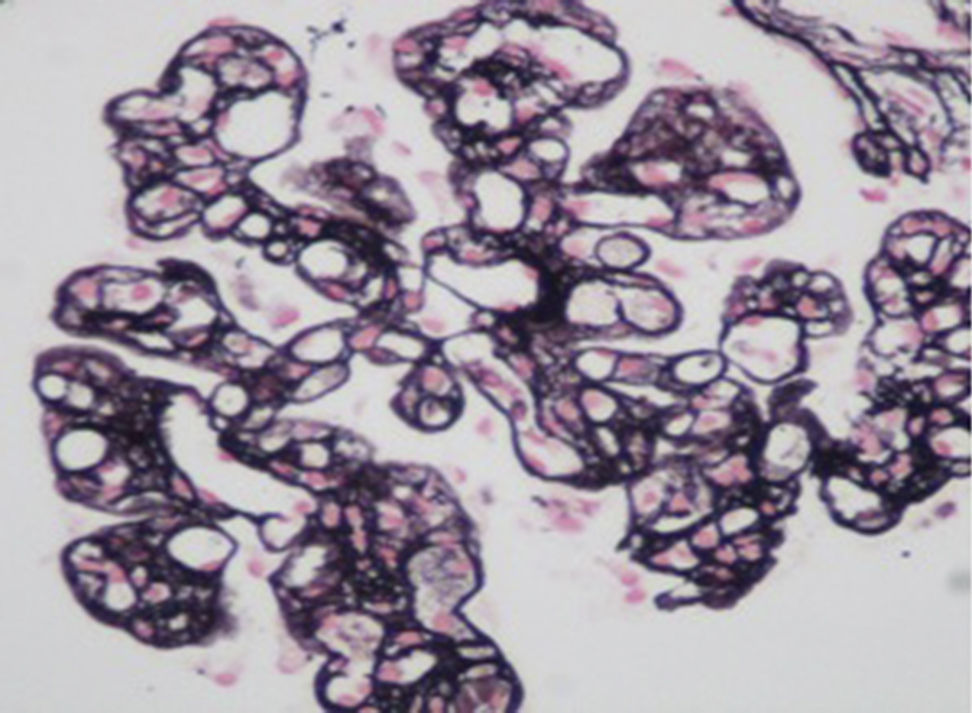
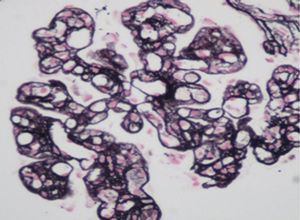
A kidney biopsy was performed revealing type I membranoproliferative glomerulonephritis with IgM (++), IgG (+) and C3 (++) deposits, glomerular capillary endotheliosis, focal and segmental thrombotic microangiopathy, tubulointerstitial nephritis and injuries of focal and discrete vasculitis. (Fig. 1).
Serologies for human immunodeficiency virus, hepatitis B virus and hepatitis C virus (HCV) were negative, as well as HCV RNA testing. Complement study showed low C4 (C4<0,01) and normal C3 (C3=1,36) and high rheumatoid factor (195IU/mL; N=). Cryoglobulins were positive with polyclonal IgG and monoclonal IgM and kappa light chains.
The patient was treated with 1g methylprednisolone pulses on three consecutive days and subsequent oral prednisolone at a dosage of 1mg/kg/day.
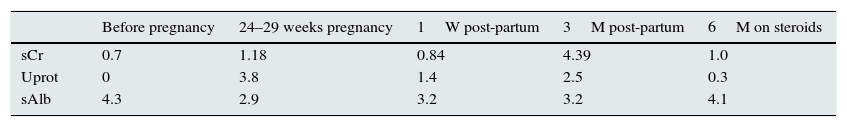
One year has passed and the patient has been weaned off steroid therapy maintaining clinical stability. Analytically, the values of sCr have stabilized at 1.0mg/dL, with proteinuria of 150mg/24h (Table 1).
The histological findings of our patients’ kidney biopsy revealed distinct injury patterns that could be inserted in various clinical pictures: glomerular capillary endotheliosis and focal and segmental thrombotic microangiopathy were expected in the context of previous preeclampsia1 while tubulointerstitial nephritis with injuries of vasculitis were compatible with pSS.2
Regarding the immune complex mediated MPGN, which might have been the cause of acute kidney injury, the differential diagnosis of its underlying cause was challenging and included autoimmune diseases, chronic infection and monoclonal gammopathy. MPGN associated with autoimmune diseases is rarely seen in patients with SS and we also excluded chronic infection. Considering monoclonal gammopathies, although the immunofluorescence microscopy on renal biopsy was not typical, our patient was diagnosed with IgM κ MGUS.3
A growing number of pathologic renal conditions are being attributed to a clonal plasma cell disorder that is less myeloma-like and more MGUS-like in terms of its bulk and proliferative rate and the term monoclonal gammopathy of renal significance (MGRS) was proposed to clarify the differences in respect to therapy.4,5 One of the conditions associated with MGRS is type II cryoglobulinaemia and there are recent reviews that show a close association between monoclonal gammopathy, cryoglobulinaemias and SS.6,7 In fact, in patients with SS and MGUS, mixed cryoglobulinaemia should always be investigated, especially if the monoclonal band is IgM k, as in the case we report.
In retrospect, there was several data favouring the diagnosis of cryoglobulinaemia in association with pSS, since our patient presented with Metlzer's triad and had laboratory findings compatible with this pathology.8
In summary, our patient had a MGRS associated with type II cryoglobulinaemia, presenting with MPGN, superimposed on SS.
Regarding treatment there are still many doubts about the best approach. Our patient had several poor prognostic indicators: a severe form of pSS and type II cryoglobulinaemia, both related to a higher propensity to develop a lymphoproliferative disorder, and MGUS, nowadays considered as a key marker of pSS activity.9,6,10 Considering this data, she could be a candidate for aggressive therapy with biological agents against B-cells.
For now, and after suspending steroid treatment, our patient has normal kidney function without significant proteinuria or complaints. Our approach is to maintain integrated inter-department follow up and in the future, rituximab will be present as a therapeutic option.