La fibrosis sistémica nefrogénica es un trastorno fibrosante que afecta a pacientes con deterioro de la función renal y se asocia a la administración de medios de contraste basados en el gadolinio, empleados en la resonancia magnética. A pesar de tratarse de un grupo de fármacos que se consideraban seguros, la notificación de esta reacción adversa, potencialmente grave, supuso un punto de inflexión en las pautas de administración de estos medios de contraste. Se han intentado establecer parámetros de seguridad a fin de identificar a los pacientes con factores de riesgo por presentar insuficiencia renal. La estrecha farmacovigilancia y el rigor en la observación de las normativas actuales, con especial atención al valor del filtrado glomerular, han reducido los casos publicados relacionados con el uso de medios de contraste basados en el gadolinio. En un encuentro entre radiólogos y nefrólogos revisamos los aspectos más relevantes en la actualidad y las recomendaciones para su prevención.
Nephrogenic systemic fibrosis is a fibrosing disorder that affects patients with impaired renal function and is associated with the administration of gadolinium-based contrast media used in MRI. Despite being in a group of drugs that were considered safe, report about this potentially serious adverse reaction was a turning point in the administration guidelines of these contrast media. There has been an attempt to establish safety parameters to identify patients with risk factors of renal failure. The close pharmacovigilance and strict observation of current regulations, with special attention being paid to the value of glomerular filtration, have reduced the published cases involving the use of gadolinium-based contrast media. In a meeting between radiologists and nephrologists we reviewed the most relevant aspects currently and recommendations for its prevention.
INTRODUCTION
Since 1997, when it was reported by Cowper for the first time1, a condition called nephrogenic systemic fibrosis (NSF) has drawn the attention of nephrologists and radiologists from all over the world. It has been defined as a fibrosing disease that predominantly affects patients who have received gadolinium-based contrasts, with an estimated glomerular filtration rate (GFR) of less than 30ml/min/1.73m2 or those on haemodialysis2,3. In this document, we aim to summarise the clinical expression of NSF, the data known about different gadolinium-based contrasts, the possibilities of identifying patients at risk in order to prevent its onset and the types of treatment for this disease.
GADOLINIUM
Gadolinium-based contrast media (GBCM) are used in magnetic resonance imaging (MRI) studies due to their magnetic ability to change the position of the protons of water molecules in tissues, which is a change that improves the study’s diagnostic capacity. These contrast media act by shorting the T1 and T2 relaxation time of the tissues to which they are distributed, which fundamentally leads to an increased signal in T1-weighted sequences. However, if the GBCM concentration is high, T2 shortening is predominant, which causes a decrease in the signal. Nine agents have currently been approved and are available in Europe; their characteristics are summarised in Table 1.
Structure and pharmacokinetics
Gadolinium (Gd) is a heavy metal with a high paramagnetic capacity and which is not soluble in water. In its free form (Gd3) it is very toxic, and as such, it is necessary to chelate it with different organic ligands, creating gadolinium chelates4. There is a certain tendency for the ion to separate from the ligand in a process called chelation blocking5. If this process continues, there is transmetalation and this causes NSF6. Transmetalation is a chemical reaction whereby a secondary free metal with affinity for the chelate allows gadolinium release (Gd3). In renal failure patients, it decreases the renal elimination of GBCM; its half-life is extended, which increases the possibility of Gd3 dissociating from the chelate. This facilitates the recruitment of circulating fibrocytes, triggering the fibrosing reaction7,8. The structure of gadolinium chelates may be linear or macrocyclic, with the latter being that which shows higher thermodynamic stability constants. Being hydrophilic compounds, they can be classified9 as ionic and non-ionic, with the latter having lower osmolarity for the same concentration (Table 1). Of all the agents, non-ionic linear agents are the least stable and they increase the risk of transmetalation. As such, they are associated with a higher risk of NSF10,11.
In terms of the distribution after their intravenous administration (Table 1), GBCM are classified into three types: non-specific extracellular, mixed (hepatospecific extracellular and intracellular distribution with a variable percentage of biliary elimination) and intravascular (they remain in the intravascular space for longer). The vast majority of GBCM used in daily practice are from the first group12.
GD chelates have a molecular weight that ranges between 500 and 1,000Da, they are not bound to plasma proteins and are not lipophilic, which means that after their intravenous administration, there is a distribution and balance within the extracellular space. All of these characteristics help to create the good glomerular filtration capacity of GD chelates6. They are small molecules that leave the vascular space quickly, with a half-life in plasma of around 15-30 minutes. They do not cross the blood-brain barrier or the cell membrane, and as such, after leaving the vascular space, they are distributed around the interstitial space. They are eliminated, without being metabolised, through glomerular filtration. In patients with normal renal function, 98% of Gd is eliminated in urine in the first 24 hours13, and it is not eliminated from or reabsorbed into the renal tubule14. Pharmacokinetic studies have demonstrated its elimination by glomerular filtration, extending the contrast’s half-life by more than 30 hours but without side effects of nephrotoxicity. In renal failure patients, peritoneal clearance of GBCM was 3.8ml/minute/1.73m2 with a T1/2 of 52.7 hours, which is not surprising, given the slow clearance of peritoneal dialysis techniques. 75% of doses administered were eliminated by peritoneal dialysis after 5 days and as such, peritoneal dialysis is not an effective technique for eliminating contrast. After two haemodialysis sessions, 95% of the gadolinium dose administered was eliminated but there were no tests of its efficacy in the removing the risk of NSF. However, we recommend that patients on dialysis undergo haemodialysis less than two hours after administration and another haemodialysis session the next day. It is not routinely recommended in non-dialysis patients6.
Dose and administration range
As a gadolinium atom modifies the relaxation times of many neighbouring hydrogen nuclei, the contrast dose used is low, significantly lower than the quantity of iodine administered for computerised tomography studies15. The most used commercial preparations have a concentration of 0.5 molar (0.5M), and as such, the standard administration dose is 0.1mmol/kg of weight, equivalent to 0.2ml/kg of contrast4. High doses and increases in the accumulated dose increase the risk of NSF6.
NEPHROGENIC SYSTEMIC FIBROSIS
NSF is an acquired fibrosing disorder that has been observed in patients with severely impaired renal function. Although the term “nephrogenic systemic fibrosis” was adopted in 2005, it was recognised for the first time in 1997 and reported in the year 2000 by Cowper as a scleromyxedema-like illness in dialysis patients1. In our country, Rodríquez Jornet et al. published the first case in 2009, with a detailed pathological review of the patient, and the macroscopic and microscopic images are available at: http://www.revistanefrologia.com/modules.php?name=articulos&idarticulo=129&idlangart=ES16. Table 2 displays the chronology and evolution of the term.
Epidemiology
NSF affects most cases of patients with impaired renal function, particularly those with an estimated glomerular filtration rate of less than 30ml/min/1.73m2 independently of the origin of renal damage (acute, chronic or haemodialysis patients)2,3, who are administered GBCM. According to Zou et al., the two most affected groups are patients with chronic renal failure (CRF) on dialysis (85% of cases) and those with acute renal failure17. Another patient group that may be affected are those with liver failure who have acute hepatorenal syndrome18,19. It is well-known that not all risk patients exposed to GBCM have a disease6.
NSF is more common in middle-aged patients (50-60 years of age)20, although it may affect children and the elderly21,22. There are no differences according to race or sex, or any relationship with the cause or duration of CRF20.
Although various authors have reported different prevalences in accordance with the population selected, it is currently estimated that there is a mean incidence of 0%-18% in the risk population23. There is a clear relationship between the dose of GBCM used and the risk of NSF, with there being a NSF incidence close to 0 after an exposure to a standard dose15,24. Differences were also reported in the incidence of NSF according to the characteristics of the molecule, with a greater number of cases of NSF having been recorded after exposure to non-ionic linear compounds. As we have mentioned before, it seems that there is a greater risk of incidence in the peritoneal dialysis patient group25.
Thanks to the knowledge of risk factors and the better use of GBCM, the number of cases of NSF has decreased significantly26. Since 2008, there have been no cases of any CM being reported without these CM being replaced27. Many hospitals have continued to use the same GBCM but have changed the patterns of use.
Aetiopathogenesis
Although the exact pathogenesis of NSF continues to be unknown, the only solid association identified in all patients with NSF is renal failure, both in its chronic and acute forms, and its presence is a sine qua non condition for the diagnosis of the disease28. However, only a small percentage of the risk population exposed to GBCM develops NSF, and cases of NSF have also been reported without exposure to GBCM29.
Given that exposure to GBCM does not explain all cases of NSF, other coadjuvant risk factors have been studied that may contribute to its development, many of them associated with situations of renal failure. Pro-inflammatory factors: vessel injury, surgery, thrombosis, procoagulant stages, severe infection, chronic hepatitis C, chronic liver disease and liver transplantation, hyperparathyroidism and hypothyroidism. Biochemical factors: acidosis, intravenous iron, erythropoietin, calcium and phosphorus30.
Pathophysiological mechanisms
The two forms, free Gd ions and the chelate-Gd complex may cause the release of cytokines, stimulating skin macrophages (Gd-free ions) or peripheral blood monocytes (chelate-Gd complexes). All of these processes (macrophage activation, pro-inflammatory cytokine release, differentiation of fibrocytes in blood, activation of fibroblasts, TGF-β pathways, metallothionein, FGF-23 and Klotho protein) stimulate fibroblasts30, a response that creates collagen deposits and fibrosis by increasing transforming growth factor beta 1 levels31. The presence of renal failure contributes to the release of free GD3 by increasing transmetalation in a uraemic environment and decreasing the glomerular filtration rate32. A complete diagram with pathophysiological mechanisms published by Chopra et al. is available at http://www.hindawi.com/journals/ijn/2012/912189/fig1/30.
Diagnosis
It presents clinically as a thickening and hardening of the skin, associated with pain, muscle weakness, bone pain and joint contractures, which causes severe disability3. Over time there may be loss of flexibility, limited mobility and joint contractures2,34. Lesions may appear in the form of plaques (58%) with irregular edges and papules (32%), nodules (17%), macules, vesicles, blisters, bullae and ulcers2,21,35,36. It typically affects the legs, but may be found anywhere apart from the face in most cases35. These skin lesions progress over time to fibrotic skin surrounded by wrinkles, also known as “orange peel”37. Most lesions are hyperpigmented and erythematous (39%), but their colour can vary (purple, brown, yellow, pink, orange-red, grey-brown)35,38. Sometimes these symptoms can be confused and wrongly treated as cellulitis6. Kroshinsky et al. published the case of a 46-year-old woman with CRF, oedema in her legs and skin changes, who was examined and a differential diagnosis was carried out, with macroscopic and microscopic images of the dermis being created39. At the time this condition was first reported, scientists thought that it was just a skin disorder, but it is nowadays well-known that it affects joints, the muscular system, the testicles, the kidney, the heart and the dura mater31,40,41. Another sign of interest is that it has similar symptoms to conjunctivitis in 75% of cases6.
The onset of symptoms is variable; it generally occurs between two weeks and two months after exposure to GBCM. However, delayed onset has also been reported, years after exposure17.
The histological diagnosis is based on a skin biopsy where skin fibrosis is observed, with thickened collagen bundles and a variable quantity of elastic fibres and mucin. The mediating cell is the circulating fibrocyte (CD34 and positive procollagen I in the immunohistochemistry stain)28,42. In most cases, the inflammatory cells are not present and on some occasions, perivascular mononuclear infiltrate has been observed43. Sanyal et al. carried out a histological review of a clinical case with an electron microscope and energy dispersive x-ray fluorescence44.
With regard to its association with GBCM, the first publications are from 200645,46, with the presence of gadolinium in tissues being demonstrated only one year later47,48. Under normal conditions, GBCM are eliminated by glomerular filtration in 1-2 days.
Prognosis
The natural outcome of NSF is not fully known. It has been reported that in up to 5% of cases, it may have a fulminant course20. A third will have a mild course without functional limitation17. There is increased mortality after 24 months of skin manifestations of NSF49. The true mortality rate is unknown and is difficult to determine, given the high prevalence of other comorbidities34.
Treatment
There is no evidence of effective treatment and only in transplant patients has an improvement or a detention in the progression of renal disease been achieved in the case of acute renal failure50.
As mentioned above, GBCM molecular weight allows glomerular filtration6 and given these characteristics, there is the possibility of elimination with haemodialysis51. Several authors have carried out studies that confirm the elimination of various types of GBCM with three haemodialysis sessions of three hours each. Based on these results, the European Society of Radiology recommends carrying out nine hours of haemodialysis over three sessions. However, gadofosveset is an agent that is difficult to eliminate by haemodialysis due to a large proportion of it being bound to serum albumin6. Broome et al. presented a series with three patients who developed NSF despite undergoing the previously indicated haemodialysis sessions52. To present, no studies have been carried out on continuous haemofiltration or continuous venovenous haemodiafiltration.
Most treatments proposed are still being researched and they are currently yielding suboptimal results (oral steroids, extracorporeal photopheresis, plasmapheresis, thalidomide, cyclophosphamide, pentoxifylline, intravenous immunoglobulin, interferon alpha and vitamin D, ultraviolet radiation and etanercept)20. Recently, combined treatments with imatinib and extracorporeal photopheresis have been attempted53,54. The efficacy of treatment with alefacept was also confirmed in three patients with NSF55. The improvement in renal function (transplantation and resolution of acute renal failure) may slow down and even reverse the process20. However, in reality, no treatment has shown to be effective; therefore, prevention is important.
PREVENTION OF NEPHROGENIC SYSTEMIC FIBROSIS
Identification of patients with chronic kidney disease
The classification of chronic kidney disease (CKD) followed the initial publication of the National Kidney Foundation through the Kidney Disease Outcomes Quality Initiative (K-DOQI) guidelines56. The definition of CKD by K-DOQI is as follows:
Renal damage for at least three months, defined by structural or functional abnormalities of the kidney or without a decrease in the GFR and shown by pathological changes or renal damage markers (changes in the composition of blood or urine or changes in images of the kidney).
GFR <60ml/min/1.73m2 for more than three months, with or without renal damage.
It is common in consultations for renal function to be studied simply by measuring serum creatinine (SCr). However, and although it is true that SCr is a good follow-up parameter of the evolution of filtration, it is not always equivalent to glomerular filtration. SCr also depends on factors other than the GFR, such as tubular elimination and the generation and extrarenal elimination of creatinine, which explains the wide range for SCr in healthy individuals. Some studies57 show a high percentage of males and particularly of females who have reductions in the GFR with normal SCr. Even with creatinine ranges between 1.3 and 2.5mg/dl, there are significant percentages of very severe renal failure (GFR below 30ml/min/1.73m2). Therefore, the real prevalence of individuals with renal failure appears to be higher than that which can be determined by studying SCr. The results of these observations are important. This “hidden” renal failure may easily worsen due to the large amount of medications, particularly in glomerular haemodynamics, such as non-steroidal anti-inflammatory drugs, angiotensin-converting-enzyme inhibitors and other types of drugs. Likewise, patients often undergo x-ray examinations when there is an inadequate evaluation of renal function, based only on plasma creatinine.
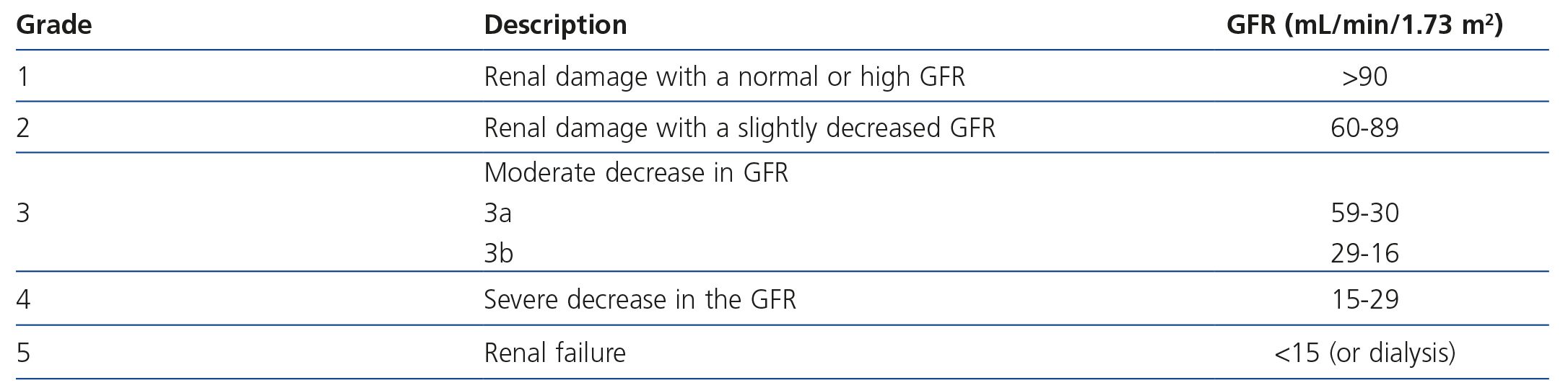
The international organisation KDIGO (Kidney Disease Global Outcomes; http://www.kdigo.org/) recommends using prediction equations to calculate the GFR based on SCr. In adults, the formulas most used are those of the Modification of Diet in Renal Disease (MDRD) study and that of Cockcroft and Gault58. There are certain circumstances in which the first is not validated (Table 3) and in order to estimate the GFR, 24-hour urine should be collected or studies of creatinine clearance in 24-hour urine, iothalamate, iohexol or insulin should be carried out. In any case, the estimation of GFR using the MDRD formula is more accurate that SCr, and considering these limitations, the doctor may obtain valid information about renal function. Recently, KDIGO recommended a new formula for calculating renal function, called CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration), which is more accurate than MDRD for values close to 60ml/min59. Likewise, it updated the CKD classification by incorporating the CGA concept: C: cause of CKD, G: GFR incorporating groups 3a and 3b, and A: albuminuria with three subgroups: A1 (<30mg/g of creatinine), A2 (30-300) and A3 (>300)60 (Table 4).
There are occasionally no data on renal function. In patients who have unknown renal function and who require an x-ray examination with gadolinium, a series of parameters should be considered, such as renal failure risk factors, which will mean that the examination must be delayed until their exact renal function is known (Table 5). The study of risk factors must be part of the routine before using GBCM in any hospital.
IMMEDIATE ADVERSE REACTIONS TO GADOLINIUM-BASED CONTRAST MEDIA
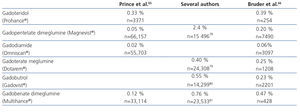
GBCM are very safe drugs, with a low immediate adverse reaction (IAR) rate of 0.07%-2.4%61-63, mostly of a mild nature, mainly nausea or headaches at the time of injection.
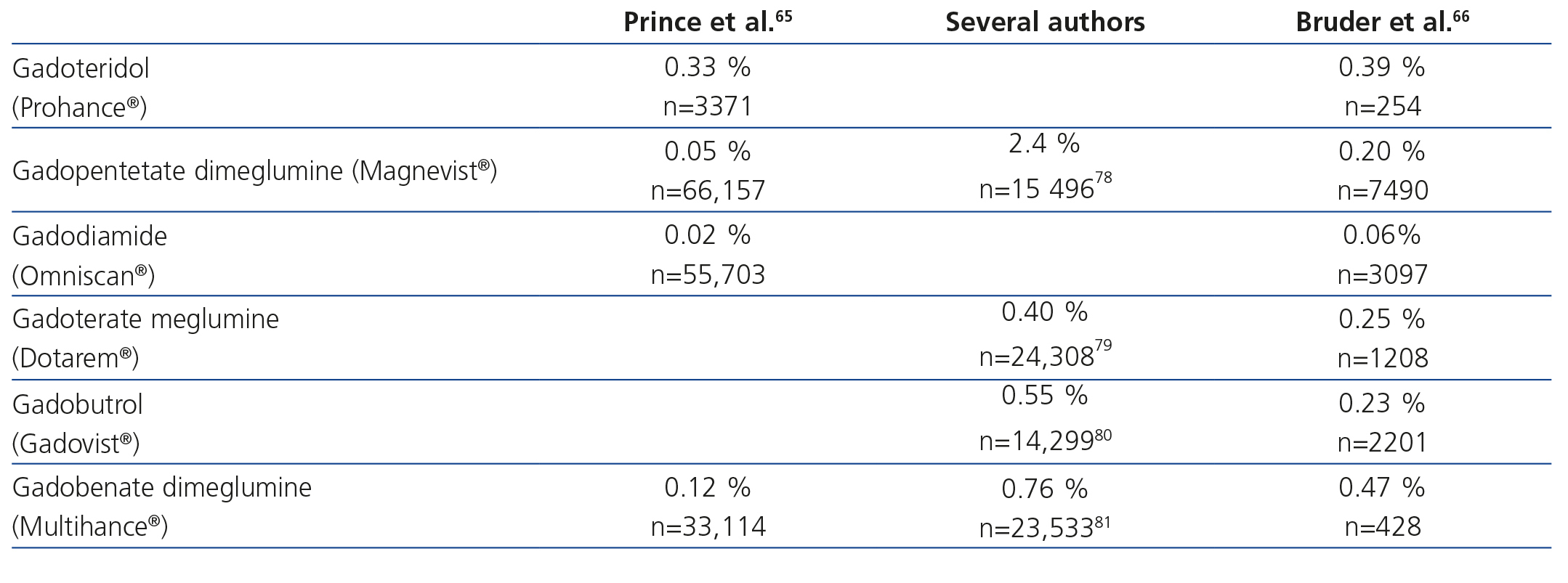
Although all GBCM show quite a similar IAR incidence64, there are differences in their occurrence that cannot seem to be explained by their physicochemical characteristics65,66 (Table 6).
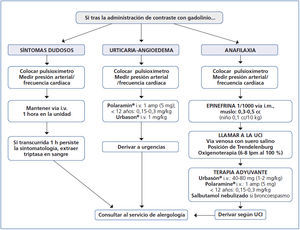
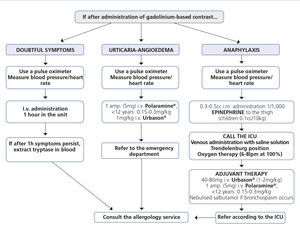
Among IAR to GBCM, we must highlight allergic reactions, due to their relevance, which are defined as a type of adverse reaction measured immunologically by antibodies or lymphocytes, characterised by being specific and recurrent if the patient is exposed to the drug again67. Two types of allergic reaction to x-ray contrast media are distinguished depending on the moment of presentation: immediate and non-immediate or delayed68. Immediate allergic reactions are measured by immunoglobin E; if a systemic allergic reaction develops, there is anaphylaxis. This is caused by the release of histamines and other mediators, causing symptoms that may put the life of the patients at risk: laryngeal oedema, angioedema, upper airway obstruction, urticarial, nausea, vomiting, low blood pressure and/or shock.
The occurrence of allergic reactions to GBCM is unpredictable, although it is known that its incidence increases in asthmatic patients and in those with food allergies and/or medication allergies58,69.
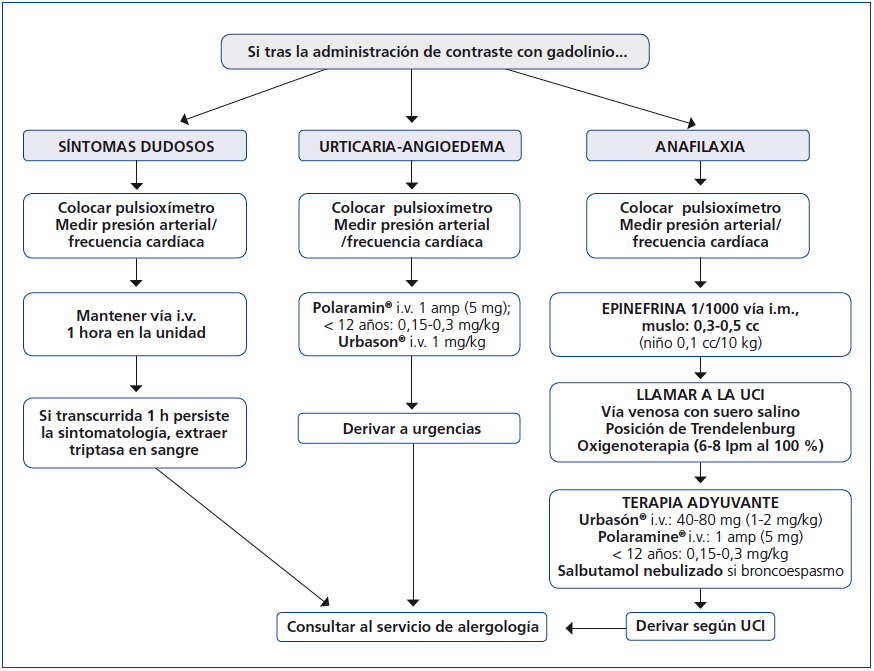
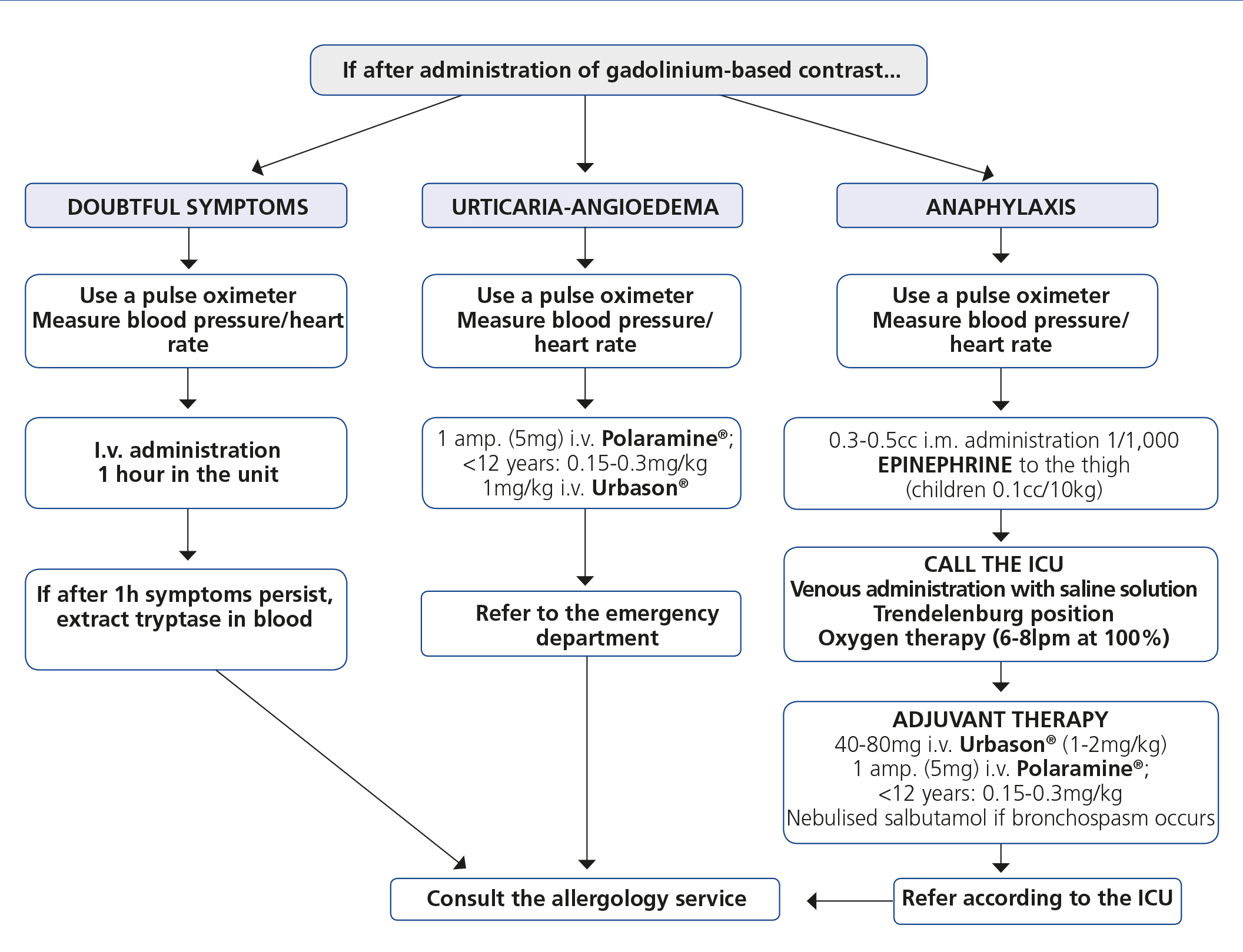
With regards to how to act against an allergic reaction to a GBCM, Figure 1 displays an algorithm, which schematically shows how to manage these emergency situations in the x-ray diagnosis department.
LEARN FROM EVIDENCE
Clinical use and abuse
Since the introduction of GBCM in MRI, its applications have been increasing daily, and it is now used in all organs of the body. During the first few years, a false sense of security was created, which led to an overenthusiastic use of GBCM, which were often used as replacements for iodinated contrasts in computerised tomography or conventional angiography studies in patients who were allergic to iodinated contrasts or in those with renal failure and even in MRI, at doses much higher than those recommended.
This use of GBCM, before NSF was reported, was carried out without any type of control in terms of dose or administration times and without taking any precautions in relation to the renal function of patients.
The reporting of this delayed and potentially serious adverse reaction marked a turning point that forced x-ray departments to establish new guidelines aimed at protecting patients. Although the initial information may have been confusing, some evidence was clear and shed light with regard to the measures to adopt to prevent disease: it was only reported in patients with severe renal failure (GFR<30), its incidence was related to the administration of high doses of gadolinium and it was more common in patients with pro-inflammatory symptoms.
Clinical limitations in the use of gadolinium-based contrast media
The main limitation with regard to the use of GBCM in MRI is the difficulty of knowing the GFR of patients, particularly outpatients. In this regard, collaboration between the x-ray department, which would have to routinely record renal failure risk factors (Table 4) before carrying out the GBCM study, and the doctor who requests the test, who must provide information about the patient’s renal function and assess the risk/benefit of the test requested for the patient. If any of the risk factors of renal failure are confirmed or the GFR of the patient cannot be excluded or assessed, it would be preferable to postpone it until MDRD or CKD-EPI are determined in another test.
Since gadolinium has been considered an agent the potentially causes NSF, restrictive guidelines have been designed for its administration (Table 7), with the most important aspects being the possession of recent GFR data and the adjustment of doses used in accordance with the latter (Table 8).
In 2010, the U.S. Food and Drug Administration (FDA) established general precautions on the use of GBCM and limited Magnevist®, Omniscan® and Optimark® GBCM in patients with acute renal failure and high-risk severe CRF43. Two years after the recommendations carried out by the FDA, a 71% decrease was observed in MRI in patients with MDRD 30ml/min/1.73m2 and a 99% increase was observed in requests for SCr a month before carrying out MRI70. A year before, the European Medicines Agency also contraindicated the use of the aforementioned GBCM in patients with severe renal failure, infants and those awaiting liver transplantation43. According to Bennet et al., in Denmark since 2007 and in the United States since 2009, no new cases of NSF have been published71.
The possibility of this adverse reaction occurring should not limit clinical action. It is essential to find a balance between the guarantee of patient safety and the carrying out of the tests necessary for correct clinical management. As such, the need for a test and its effectiveness will be discussed clinically, and other diagnostic options will be taken into account, as well as alternative contrasts. In short, the risk/benefit will be weighed up.
CONCLUSIONS
GBCM are a group of drugs with differentiated physiochemical characteristics that are increasingly being used in diagnosis by MRI.
Due to the fact that they were initially used without taking patients’ renal function into account, and without an exact knowledge of the toxic doses permitted, a series of adverse effects appeared, and in particular, the predominantly dermatological multiple organ fibrosing disorder subsequently known as NSF, which discredited its use.
The reporting of this delayed and potentially severe adverse reaction marked a turning point, since it made it compulsory to establish consensuses to protect patients by assessing the GFR and risk factors. All of this along with dose adjustment have decreased the number of adverse reactions significantly and in the last five years there have hardly been any published cases with the use of gadolinium.
As for IAR, all GBCM have a low and similar incidence, although there are some differences between them, with gadodiamide having the lowest incidence63,64,67. These reactions, although they are generally mild, can occasionally be severe and even fatal.
Patient protection is key when GBCM are used in x-rays. The identification and selection of patients at risk, the assessment of the risk and benefit and informing the patient about the adverse effects are essential.
In most cases, it is necessary to assess the patient, make a multidisciplinary decision, and in particular, treat every case individually.
Conflicts of interest
The content of this review is based on an update of Working Session presentations sponsored by GE Healthcare entitled What happened to NSF? The current situation, which took place at the XXXI SERAM Conference (Granada, 2012). The publication of this review was carried out independently of the Working Session sponsor.
Table 1. Classification of the different gadolinium-based contrasts according to their distribution
Table 2. Chronology and evolution of the term &
Table 3. Circumstances in which the MDRD (Modification of Diet in Renal Disease) equation is not valid for calculating the glomerular filtration rate
Table 4. Classification of chronic kidney disease (57)
Table 5. Chronic kidney disease risk factors
Table 6. Percentage of immediate adverse reactions to gadolinium-based contrast media
Table 7. Measures to avoid nephrogenic systemic fibrosis development
Table 8. Administration of gadolinium adjusted to renal function
Figure 1. Protocol for treating adverse reactions to gadolinium.