The objective of this study was to evaluate the influence of ACE I/D gene polymorphisms on diabetic kidney disease (DKD) risk.
MethodsAll eligible investigations were identified, the number of various genotype in the case and control group were reviewed. The pooled analysis was performed using Stata software.
ResultsIn overall subjects, 24,321 participants with 12,961 cases and 11,360 controls were included. the pooled analysis showed a significant link between D allele, DD or II genotype and DKD risk (D versus I: OR=1.316, 95% CI: 1.213–1.427, P=0.000; DD versus ID+II: OR=1.414, 95% CI: 1.253–1.595, P=0.000; II versus DD+ID: OR=0.750, 95% CI: 0.647–0.869, P=0.000). The subgroup pooled analysis showed that ACE I/D gene polymorphism was correlated with DKD both in Asian and in Chinese population. In addition, ACE I/D gene polymorphism was correlated with type 2 DKD (D versus I: OR=1.361, 95% CI: 1.243–1.490, P=0.000; DD versus ID+II: OR=1.503, 95% CI: 1.310–1.726, P=0.000; II versus DD+ID: OR=0.738, 95% CI: 0.626 –0.870, P=0.000). However, there was no obvious correlation in Caucasian subjects and type 1 diabetic patients.
ConclusionACE I/D polymorphisms were correlated with DKD in Asian and type 2 diabetic populations. ACE D allele/DD genotype might be a risk factor, while ACE II genotype might be a protective factor for DKD.
El objetivo de este estudio fue evaluar la influencia de los polimorfismos del gen I/D de la ECA en el riesgo de enfermedad renal diabética (ERD).
MétodosSe identificaron todas las investigaciones elegibles, se revisó el número de varios genotipos en el grupo de casos y controles. El análisis combinado se realizó con el software Stata.
ResultadosEn el conjunto de los sujetos, se incluyeron 24.321 participantes con 12.961 casos y 11.360 controles. El análisis combinado mostró una relación significativa entre el alelo D, el genotipo DD o II y el riesgo de DKD (D frente a I: OR=1,316, IC del 95%: 1,213–1,427, P=0,000; DD frente a ID+II: OR=1,414, IC del 95%: 1,253-1,595, P=0,000; II frente a DD+ID: OR=0,750, 95% CI: 0,647-0,869, P=0,000). El análisis de subgrupos mostró que el polimorfismo del gen I/D de la ECA se correlacionaba con la DMD tanto en la población asiática como en la china. Además, el polimorfismo del gen I/D de la ECA se correlacionó con la DKD de tipo 2 (D frente a I: OR=1,361, IC del 95%: 1,243-1,490, P=0,000; DD frente a ID+II: OR=1,503, IC del 95%: 1,310-1,726, P=0,000; II frente a DD+ID: OR=0,738, 95% CI: 0,626 -0,870, P=0,000). Sin embargo, no hubo una correlación evidente en los sujetos caucásicos y en los pacientes diabéticos de tipo 1.
ConclusiónLos polimorfismos I/D de la ECA se correlacionaron con la DKD en poblaciones asiáticas y diabéticas de tipo 2. El alelo D de la ECA/genotipo DD podría ser un factor de riesgo, mientras que el genotipo II de la ECA podría ser un factor de protección para la DKD.
Diabetic kidney disease (DKD) is a severe and common complications in diabetic patients, it brings serious economic burden on society both in Western and Eastern countries.1 Recent studies indicated that chronic kidney disease (CKD) induced by diabetes was more common than primary glomerulonephritis in China.2 It has been demonstrated that albuminuria, elevated blood pressure, metabolic abnormalities, excessive oxidative stress and mitochondrial dysfunction were vital pathogenic factors in DKD.3,4 Unfortunately, the detailed pathogenesis of DKD is still not fully understood, and the mainstay of current treatment for DKD including controlling blood glucose and blood pressure are not fully effective. Hence a better understanding of the DKD pathogenesis is urgently needed.
Recent studies showed that genetic factors damage was involved in the onset of DKD.5 Additionally, the susceptibility of DKD was associated with some single genes polymorphism (e.g. methylenetetrahydrofolate reductase and angiotensin converting enzyme).6 Angiotensin converting enzyme (ACE) gene contained 21kb base, it was located on 17q23 including 26 exons and 25 introns. Single nucleotide polymorphisms (SNPs) frequently occurs in the ACE gene, it has been identified 6 polymorphism markers of ACE, and Alu insertion/deletion (I/D) fragment in the 16th intron is the most investigated, ACE gene polymorphism could be divided into DD, ID, II genotype based on this I/D polymorphic marker locus.7 Some previous studies has found that ACE I/D polymorphism could influence the occurrence of diabetes-related renal damage.8 In addition, some pooled analysis as regards the impacting of ACE I/D gene polymorphism on DKD susceptibility has been completed.9,10 However, the pooled results were controversial and inconsistent. In this study, we further assess the potential impact of ACE I/D gene polymorphism on DKD through analyzing much more trials.
MethodsSearch strategyThe eligible trials were carefully searched form various databases (e.g. PubMed, Cochrane databases, Embase and China National Knowledge Infrastructure Database). Various search terms were used as follows: angiotensin-converting enzyme, ACE, ACE insertion/deletion, ACE I/D, diabetic nephropathy, diabetic kidney disease, DN, DKD, diabetes mellitus, kidney, renal, gene, gene polymorphism.
Study inclusion criteriaThe inclusion criteria were used as follows: (1) the study including two comparison group (DKD patients vs control patients), (2) the association between ACE I/D gene polymorphism and DKD has been reported, (3) the detailed number of ACE genotypes has been provided, (4) the ACE I/D genotype distributions of control group was conformed to Hardy–Weinberg equilibrium (HWE) testing.
Data extraction and analysisEach study characteristics was extracted, the pooled analysis was performed using the Stata software (version 12.0). An odds ratio (OR) with a 95% confidence interval (CI) was calculated. It was considered statistically significant for the pooled OR when a P-value<0.05. The impact of ACE I/D gene polymorphism on DKD risk was analyzed using different four models: Method 1, D allele versus I allele; Method 2, DD genotype versus ID genotype+II genotype; Method 3, II genotype versus DD genotype+ID genotype; Method 4, ID genotype versus DD genotype+II genotype. The heterogeneity was assessed using Q and I2 statistics. In addition, Begg's adjusted rank correction test was performed to evaluate the publication bias, there was potential publication bias when a P value<0.05.11
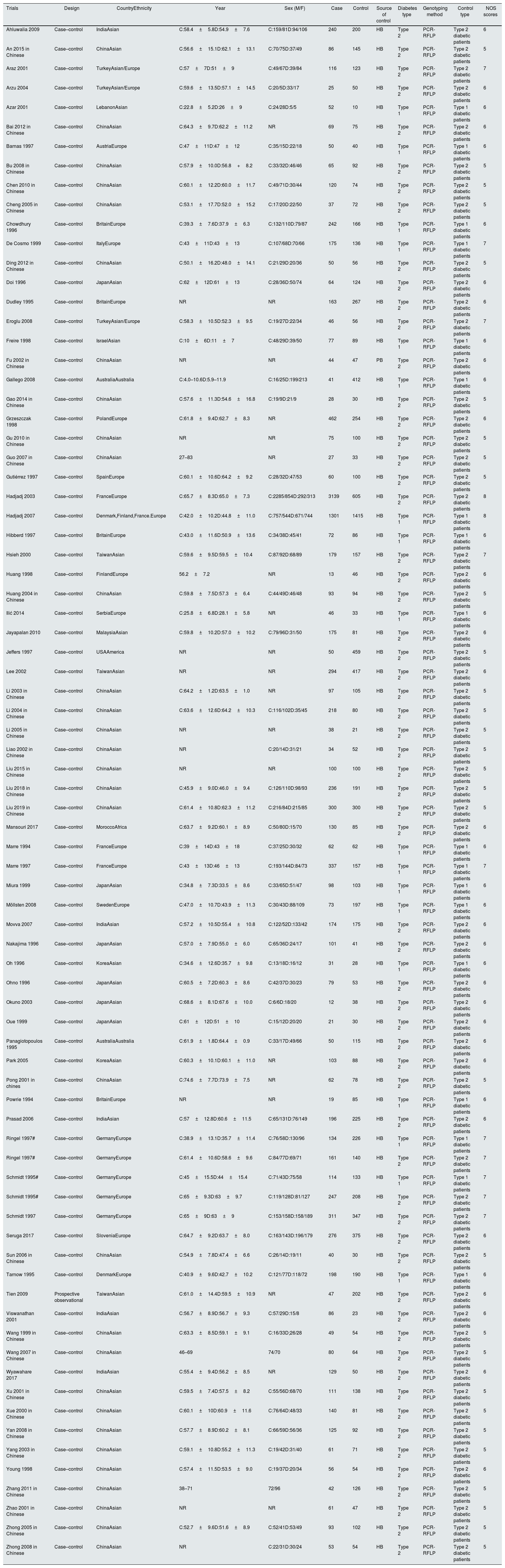
ResultsStudy characteristicsAfter carefully searching and checking in various databases, we finally included 77 studies in this research.12–88 The principal characteristics of included trials are described in Table 1. 24,321 participants with 12,961 cases and 11,360 controls were included, 31 studies were published in Chinese and 46 in English, from a total 22 countries. In this studies, both type 1 and type 2 diabetic patients were analyzed. The average age of participants ranged from 4 to 74 years. According to the Newcastle-Ottawa Scale (NOS), the quality of included studies was generally at the medium level. As shown in Table 2, 10 studies were not included in this pooled analysis due to they failing to meet the HWE testing.24,39,56,57,64,65,69,74,82,85 In addition, we have extracted the number of various genotype in the case and control group (Table 2).
Characteristics of studies included in the meta-analysis.
| Trials | Design | CountryEthnicity | Year | Sex (M/F) | Case | Control | Source of control | Diabetes type | Genotyping method | Control type | NOS scores |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahluwalia 2009 | Case–control | IndiaAsian | C:58.4±5.8D:54.9±7.6 | C:159/81D:94/106 | 240 | 200 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| An 2015 in Chinese | Case–control | ChinaAsian | C:56.6±15.1D:62.1±13.1 | C:70/75D:37/49 | 86 | 145 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Araz 2001 | Case–control | TurkeyAsian/Europe | C:57±7D:51±9 | C:49/67D:39/84 | 116 | 123 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 7 |
| Arzu 2004 | Case–control | TurkeyAsian/Europe | C:59.6±13.5D:57.1±14.5 | C:20/5D:33/17 | 25 | 50 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Azar 2001 | Case–control | LebanonAsian | C:22.8±5.2D:26±9 | C:24/28D:5/5 | 52 | 10 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 6 |
| Bai 2012 in Chinese | Case–control | ChinaAsian | C:64.3±9.7D:62.2±11.2 | NR | 69 | 75 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Barnas 1997 | Case–control | AustriaEurope | C:47±11D:47±12 | C:35/15D:22/18 | 50 | 40 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 6 |
| Bu 2008 in Chinese | Case–control | ChinaAsian | C:57.9±10.0D:56.8+8.2 | C:33/32D:46/46 | 65 | 92 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Chen 2010 in Chinese | Case–control | ChinaAsian | C:60.1±12.2D:60.0±11.7 | C:49/71D:30/44 | 120 | 74 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Cheng 2005 in Chinese | Case–control | ChinaAsian | C:53.1±17.7D:52.0±15.2 | C:17/20D:22/50 | 37 | 72 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Chowdhury 1996 | Case–control | BritainEurope | C:39.3±7.6D:37.9±6.3 | C:132/110D:79/87 | 242 | 166 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 6 |
| De Cosmo 1999 | Case–control | ItalyEurope | C:43±11D:43±13 | C:107/68D:70/66 | 175 | 136 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 7 |
| Ding 2012 in Chinese | Case–control | ChinaAsian | C:50.1±16.2D:48.0±14.1 | C:21/29D:20/36 | 50 | 56 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Doi 1996 | Case–control | JapanAsian | C:62±12D:61±13 | C:28/36D:50/74 | 64 | 124 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Dudley 1995 | Case–control | BritainEurope | NR | NR | 163 | 267 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Eroglu 2008 | Case–control | TurkeyAsian/Europe | C:58.3±10.5D:52.3±9.5 | C:19/27D:22/34 | 46 | 56 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 7 |
| Freire 1998 | Case–control | IsraelAsian | C:10±6D:11±7 | C:48/29D:39/50 | 77 | 89 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 6 |
| Fu 2002 in Chinese | Case–control | ChinaAsian | NR | NR | 44 | 47 | PB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Gallego 2008 | Case–control | AustraliaAustralia | C:4.0–10.6D:5.9–11.9 | C:16/25D:199/213 | 41 | 412 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 6 |
| Gao 2014 in Chinese | Case–control | ChinaAsian | C:57.6±11.3D:54.6±16.8 | C:19/9D:21/9 | 28 | 30 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Grzeszczak 1998 | Case–control | PolandEurope | C:61.8±9.4D:62.7±8.3 | NR | 462 | 254 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Gu 2010 in Chinese | Case–control | ChinaAsian | NR | NR | 75 | 100 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Guo 2007 in Chinese | Case–control | ChinaAsian | 27–83 | NR | 27 | 33 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Gutiérrez 1997 | Case–control | SpainEurope | C:60.1±10.6D:64.2±9.2 | C:28/32D:47/53 | 60 | 100 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Hadjadj 2003 | Case–control | FranceEurope | C:65.7±8.3D:65.0±7.3 | C:2285/854D:292/313 | 3139 | 605 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 8 |
| Hadjadj 2007 | Case–control | Denmark,Finland,France.Europe | C:42.0±10.2D:44.8±11.0 | C:757/544D:671/744 | 1301 | 1415 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 8 |
| Hibberd 1997 | Case–control | BritainEurope | C:43.0±11.6D:50.9±13.6 | C:34/38D:45/41 | 72 | 86 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 6 |
| Hsieh 2000 | Case–control | TaiwanAsian | C:59.6±9.5D:59.5±10.4 | C:87/92D:68/89 | 179 | 157 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 7 |
| Huang 1998 | Case–control | FinlandEurope | 56.2±7.2 | NR | 13 | 46 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Huang 2004 in Chinese | Case–control | ChinaAsian | C:59.8±7.5D:57.3±6.4 | C:44/49D:46/48 | 93 | 94 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Ilić 2014 | Case–control | SerbiaEurope | C:25.8±6.8D:28.1±5.8 | NR | 46 | 33 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 6 |
| Jayapalan 2010 | Case–control | MalaysiaAsian | C:59.8±10.2D:57.0±10.2 | C:79/96D:31/50 | 175 | 81 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Jeffers 1997 | Case–control | USAAmerica | NR | NR | 50 | 459 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Lee 2002 | Case–control | TaiwanAsian | NR | NR | 294 | 417 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Li 2003 in Chinese | Case–control | ChinaAsian | C:64.2±1.2D:63.5±1.0 | NR | 97 | 105 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Li 2004 in Chinese | Case–control | ChinaAsian | C:63.6±12.6D:64.2±10.3 | C:116/102D:35/45 | 218 | 80 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Li 2005 in Chinese | Case–control | ChinaAsian | NR | NR | 38 | 21 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Liao 2002 in Chinese | Case–control | ChinaAsian | NR | C:20/14D:31/21 | 34 | 52 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Liu 2015 in Chinese | Case–control | ChinaAsian | NR | NR | 100 | 100 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Liu 2018 in Chinese | Case–control | ChinaAsian | C:45.9±9.0D:46.0±9.4 | C:126/110D:98/93 | 236 | 191 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Liu 2019 in Chinese | Case–control | ChinaAsian | C:61.4±10.8D:62.3±11.2 | C:216/84D:215/85 | 300 | 300 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Mansouri 2017 | Case–control | MoroccoAfrica | C:63.7±9.2D:60.1±8.9 | C:50/80D:15/70 | 130 | 85 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Marre 1994 | Case–control | FranceEurope | C:39±14D:43±18 | C:37/25D:30/32 | 62 | 62 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 6 |
| Marre 1997 | Case–control | FranceEurope | C:43±13D:46±13 | C:193/144D:84/73 | 337 | 157 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 7 |
| Miura 1999 | Case–control | JapanAsian | C:34.8±7.3D:33.5±8.6 | C:33/65D:51/47 | 98 | 103 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 6 |
| Möllsten 2008 | Case–control | SwedenEurope | C:47.0±10.7D:43.9±11.3 | C:30/43D:88/109 | 73 | 197 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 6 |
| Movva 2007 | Case–control | IndiaAsian | C:57.2±10.5D:55.4±10.8 | C:122/52D:133/42 | 174 | 175 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Nakajima 1996 | Case–control | JapanAsian | C:57.0±7.9D:55.0±6.0 | C:65/36D:24/17 | 101 | 41 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Oh 1996 | Case–control | KoreaAsian | C:34.6±12.6D:35.7±9.8 | C:13/18D:16/12 | 31 | 28 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 6 |
| Ohno 1996 | Case–control | JapanAsian | C:60.5±7.2D:60.3±8.6 | C:42/37D:30/23 | 79 | 53 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Okuno 2003 | Case–control | JapanAsian | C:68.6±8.1D:67.6±10.0 | C:6/6D:18/20 | 12 | 38 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Oue 1999 | Case–control | JapanAsian | C:61±12D:51±10 | C:15/12D:20/20 | 21 | 30 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Panagiotopoulos 1995 | Case–control | AustraliaAustralia | C:61.9±1.8D:64.4±0.9 | C:33/17D:49/66 | 50 | 115 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Park 2005 | Case–control | KoreaAsian | C:60.3±10.1D:60.1±11.0 | NR | 103 | 88 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Pong 2001 in chines | Case–control | ChinaAsian | C:74.6±7.7D:73.9±7.5 | NR | 62 | 78 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Powrie 1994 | Case–control | BritainEurope | NR | NR | 19 | 85 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 6 |
| Prasad 2006 | Case–control | IndiaAsian | C:57±12.8D:60.6±11.5 | C:65/131D:76/149 | 196 | 225 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Ringel 1997# | Case–control | GermanyEurope | C:38.9±13.1D:35.7±11.4 | C:76/58D:130/96 | 134 | 226 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 7 |
| Ringel 1997# | Case–control | GermanyEurope | C:61.4±10.6D:58.6±9.6 | C:84/77D:69/71 | 161 | 140 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 7 |
| Schmidt 1995# | Case–control | GermanyEurope | C:45±15.5D:44±15.4 | C:71/43D:75/58 | 114 | 133 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 7 |
| Schmidt 1995# | Case–control | GermanyEurope | C:65±9.3D:63±9.7 | C:119/128D:81/127 | 247 | 208 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 7 |
| Schmidt 1997 | Case–control | GermanyEurope | C:65±9D:63±9 | C:153/158D:158/189 | 311 | 347 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 7 |
| Seruga 2017 | Case–control | SloveniaEurope | C:64.7±9.2D:63.7±8.0 | C:163/143D:196/179 | 276 | 375 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Sun 2006 in Chinese | Case–control | ChinaAsian | C:54.9±7.8D:47.4±6.6 | C:26/14D:19/11 | 40 | 30 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Tarnow 1995 | Case–control | DenmarkEurope | C:40.9±9.6D:42.7±10.2 | C:121/77D:118/72 | 198 | 190 | HB | Type 1 | PCR-RFLP | Type 1 diabetic patients | 6 |
| Tien 2009 | Prospective observational | TaiwanAsian | C:61.0±14.4D:59.5±10.9 | NR | 47 | 202 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Viswanathan 2001 | Case–control | IndiaAsian | C:56.7±8.9D:56.7±9.3 | C:57/29D:15/8 | 86 | 23 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Wang 1999 in Chinese | Case–control | ChinaAsian | C:63.3±8.5D:59.1±9.1 | C:16/33D;26/28 | 49 | 54 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Wang 2007 in Chinese | Case–control | ChinaAsian | 46–69 | 74/70 | 80 | 64 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Wyawahare 2017 | Case–control | IndiaAsian | C:55.4±9.4D:56.2±8.5 | NR | 129 | 50 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Xu 2001 in Chinese | Case–control | ChinaAsian | C:59.5±7.4D:57.5±8.2 | C:55/56D:68/70 | 111 | 138 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Xue 2000 in Chinese | Case–control | ChinaAsian | C:60.1±10D:60.9±11.6 | C:76/64D:48/33 | 140 | 81 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Yan 2008 in Chinese | Case–control | ChinaAsian | C:57.7±8.9D:60.2±8.1 | C:66/59D:56/36 | 125 | 92 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Yang 2003 in Chinese | Case–control | ChinaAsian | C:59.1±10.8D:55.2±11.3 | C:19/42D:31/40 | 61 | 71 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Young 1998 | Case–control | ChinaAsian | C:57.4±11.5D:53.5±9.0 | C:19/37D:20/34 | 56 | 54 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 6 |
| Zhang 2011 in Chinese | Case–control | ChinaAsian | 38–71 | 72/96 | 42 | 126 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Zhao 2001 in Chinese | Case–control | ChinaAsian | NR | NR | 61 | 47 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Zhong 2005 in Chinese | Case–control | ChinaAsian | C:52.7±9.6D:51.6±8.9 | C:52/41D:53/49 | 93 | 102 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
| Zhong 2008 in Chinese | Case–control | ChinaAsian | NR | C:22/31D:30/24 | 53 | 54 | HB | Type 2 | PCR-RFLP | Type 2 diabetic patients | 5 |
C: case subjects; H: diabetic subjects; HB: hospital-based; PB: population-based; NR: not reported; PCR: polymerase chain reaction; RFLP: restriction fragment length polymorphism. * Months.
Characteristics of the studies evaluating the effects of ACE I/D gene polymorphisms on DKD risk.
| Author (year) | Gene sites | Case | Control | HWE(p) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ACE I/D | DD | ID | II | Total | DD | ID | II | Total | ||
| Ahluwalia 2009 | 132 | 64 | 44 | 240 | 89 | 117 | 49 | 255 | 0.3445 | |
| An 2015 in Chinese | 23 | 37 | 26 | 86 | 15 | 73 | 57 | 145 | 0.2327 | |
| Araz 2001 | 34 | 64 | 18 | 116 | 43 | 57 | 23 | 123 | 0.5945 | |
| Arzu 2004 | 9 | 11 | 5 | 25 | 24 | 21 | 5 | 50 | 0.8974 | |
| Azar 2001 | 23 | 27 | 2 | 52 | 1 | 7 | 2 | 10 | 0.1903 | |
| Bai 2012 in Chinese | 23 | 28 | 18 | 69 | 14 | 34 | 27 | 75 | 0.5720 | |
| Barnas 1997 | 14 | 27 | 9 | 50 | 4 | 21 | 15 | 40 | 0.3901 | |
| Bu 2008 in Chinese | 26 | 25 | 14 | 65 | 21 | 42 | 29 | 92 | 0.4429 | |
| Chen 2010 in Chinese | 62 | 43 | 15 | 120 | 17 | 34 | 23 | 74 | 0.5188 | |
| Cheng 2005 in Chinese | 28 | 9 | 0 | 37 | 40 | 31 | 1 | 72 | 0.0635 | |
| Chowdhury 1996 | 78 | 124 | 40 | 242 | 55 | 79 | 32 | 166 | 0.7033 | |
| De Cosmo 1999 | 73 | 79 | 23 | 175 | 65 | 53 | 18 | 136 | 0.1803 | |
| Ding 2012 in Chinese | 12 | 18 | 20 | 50 | 15 | 20 | 21 | 56 | 0.0379 | |
| Doi 1996 | 14 | 30 | 20 | 64 | 12 | 56 | 56 | 124 | 0.7105 | |
| Dudley 1995 | 47 | 85 | 31 | 163 | 70 | 148 | 49 | 267 | 0.0591 | |
| Eroglu 2008 | 16 | 17 | 13 | 46 | 19 | 24 | 13 | 56 | 0.3200 | |
| Freire 1998 | 33 | 32 | 12 | 77 | 34 | 45 | 10 | 89 | 0.3930 | |
| Fu 2002 in Chinese | 17 | 22 | 5 | 44 | 8 | 16 | 23 | 47 | 0.0972 | |
| Gallego 2008 | 15 | 17 | 9 | 41 | 102 | 204 | 103 | 409 | 0.9607 | |
| Gao 2014 in Chinese | 17 | 6 | 5 | 28 | 9 | 13 | 8 | 30 | 0.4684 | |
| Grzeszczak 1998 | 129 | 230 | 103 | 462 | 73 | 118 | 63 | 254 | 0.2685 | |
| Gu 2010 in Chinese | 25 | 34 | 16 | 75 | 30 | 48 | 22 | 100 | 0.7352 | |
| Guo 2007 in Chinese | 7 | 14 | 6 | 27 | 6 | 13 | 14 | 33 | 0.3493 | |
| Hadjadj 2003 | 1119 | 1468 | 552 | 3139 | 208 | 282 | 115 | 605 | 0.2662 | |
| Hibberd 1997 | 21 | 42 | 9 | 72 | 36 | 43 | 7 | 86 | 0.2341 | |
| Hsieh 2000 | 40 | 59 | 80 | 179 | 21 | 50 | 86 | 157 | 0.0038 | |
| Huang 1998 | 4 | 9 | 0 | 13 | 19 | 20 | 7 | 46 | 0.6498 | |
| Huang 2004 in Chinese | 32 | 37 | 24 | 93 | 18 | 40 | 36 | 94 | 0.2585 | |
| Ilić 2014 | 10 | 23 | 13 | 46 | 10 | 12 | 11 | 33 | 0.1181 | |
| Jayapalan 2010 | 21 | 77 | 77 | 175 | 19 | 31 | 31 | 81 | 0.0504 | |
| Lee 2002 | 40 | 137 | 117 | 294 | 39 | 170 | 208 | 417 | 0.6181 | |
| Li 2003 in Chinese | 19 | 43 | 35 | 97 | 10 | 42 | 53 | 105 | 0.6910 | |
| Li 2004 in Chinese | 50 | 93 | 75 | 218 | 22 | 35 | 23 | 80 | 0.2641 | |
| Li 2005 in Chinese | 38 | 47 | 16 | 101 | 21 | 42 | 38 | 101 | 0.1477 | |
| Liao 2002 in Chinese | 16 | 14 | 4 | 34 | 13 | 22 | 17 | 52 | 0.2832 | |
| Liu 2015 in Chinese | 17 | 58 | 25 | 100 | 16 | 54 | 30 | 100 | 0.3097 | |
| Liu 2019 in Chinese | 45 | 129 | 126 | 300 | 22 | 124 | 154 | 300 | 0.6633 | |
| Mansouri 2017 | 76 | 42 | 12 | 130 | 47 | 32 | 6 | 85 | 0.8627 | |
| Marre 1994 | 23 | 35 | 4 | 62 | 19 | 28 | 15 | 62 | 0.4640 | |
| Marre 1997 | 119 | 168 | 50 | 337 | 48 | 69 | 40 | 157 | 0.1368 | |
| Miura 1999 | 13 | 49 | 36 | 98 | 10 | 58 | 35 | 103 | 0.0459 | |
| Möllsten 2008 | 16 | 45 | 12 | 73 | 48 | 113 | 36 | 197 | 0.0335 | |
| Movva 2007 | 39 | 88 | 47 | 174 | 27 | 74 | 74 | 175 | 0.2415 | |
| Nakajima 1996 | 14 | 50 | 37 | 101 | 4 | 19 | 18 | 41 | 0.7529 | |
| Oh 1996 | 10 | 9 | 12 | 31 | 7 | 10 | 11 | 28 | 0.1518 | |
| Ohno 1996 | 15 | 38 | 26 | 79 | 5 | 15 | 33 | 53 | 0.1178 | |
| Okuno 2003 | 3 | 8 | 1 | 12 | 5 | 12 | 21 | 38 | 0.1521 | |
| Oue 1999 | 5 | 8 | 8 | 21 | 0 | 15 | 15 | 30 | 0.0679 | |
| Panagiotopoulos 1995 | 15 | 25 | 10 | 50 | 43 | 44 | 28 | 115 | 0.0175 | |
| Park 2005 | 27 | 49 | 27 | 103 | 7 | 51 | 30 | 88 | 0.0220 | |
| Pong 2001 in chines | 14 | 23 | 25 | 62 | 7 | 33 | 38 | 78 | 0.9656 | |
| Powrie 1994 | 7 | 8 | 4 | 19 | 24 | 37 | 24 | 85 | 0.2328 | |
| Ringel 1997# | 35 | 68 | 31 | 134 | 57 | 130 | 39 | 226 | 0.0177 | |
| Ringel 1997# | 44 | 84 | 33 | 161 | 35 | 69 | 36 | 140 | 0.8662 | |
| Schmidt 1995# | 52 | 38 | 24 | 114 | 55 | 55 | 12 | 122 | 0.7442 | |
| Schmidt 1995# | 101 | 105 | 41 | 247 | 83 | 91 | 34 | 208 | 0.2886 | |
| Schmidt 1997 | 121 | 129 | 61 | 311 | 131 | 154 | 62 | 347 | 0.1577 | |
| Seruga 2017 | 90 | 143 | 43 | 276 | 115 | 169 | 91 | 375 | 0.0659 | |
| Sun 2006 in Chinese | 15 | 17 | 8 | 40 | 6 | 10 | 14 | 30 | 0.1221 | |
| Tarnow 1995 | 63 | 95 | 40 | 198 | 67 | 77 | 46 | 190 | 0.0134 | |
| Viswanathan 2001 | 24 | 45 | 17 | 86 | 5 | 8 | 10 | 23 | 0.1956 | |
| Wang 1999 in Chinese | 15 | 20 | 14 | 49 | 9 | 27 | 18 | 54 | 0.8337 | |
| Wang 2007 in Chinese | 19 | 27 | 34 | 80 | 7 | 35 | 22 | 64 | 0.2082 | |
| Wyawahare 2017 | 21 | 56 | 52 | 129 | 6 | 26 | 18 | 50 | 0.4640 | |
| Xu 2001 in Chinese | 42 | 48 | 21 | 111 | 30 | 72 | 36 | 138 | 0.5934 | |
| Xue 2000 in Chinese | 42 | 45 | 53 | 140 | 19 | 35 | 27 | 81 | 0.2520 | |
| Yan 2008 in Chinese | 40 | 64 | 21 | 125 | 12 | 22 | 58 | 92 | 0.0005 | |
| Yang 2003 in Chinese | 19 | 24 | 18 | 61 | 14 | 27 | 30 | 71 | 0.0940 | |
| Young 1998 | 3 | 30 | 24 | 57 | 8 | 20 | 26 | 54 | 0.2207 | |
| Zhang 2011 in Chinese | 12 | 22 | 8 | 42 | 24 | 42 | 60 | 126 | 0.0021 | |
| Zhao 2001 in Chinese | 15 | 23 | 23 | 61 | 5 | 17 | 25 | 47 | 0.4239 | |
| Zhong 2005 in Chinese | 16 | 54 | 23 | 93 | 15 | 56 | 31 | 102 | 0.2041 | |
| Zhong 2008 in Chinese | 10 | 31 | 12 | 53 | 8 | 30 | 16 | 54 | 0.3174 | |
The forest plot concerned the impact of ACE I/D gene polymorphism on the risk of DKD in 63 trials. The pooled analysis indicated that ACE I/D gene polymorphism was correlated with the risk of DKD in the overall populations (D allele vs I allele: OR=1.316, 95% CI: 1.213–1.42, P=0.000; DD genotype vs ID+II genotype: OR=1.414, 95% CI: 1.253–1.595, P=0.000; II genotype vs DD+ID genotype: OR=0.750, 95% CI: 0.647–0.869, P=0.000) (shown in Table 3).
Meta analysis of the association of ACE I/D gene polymorphisms on DKD risk.
| Genetic contrasts | Group and subgroups | Studies number | Q test P value | Model selected | OR (95% CI) | P value | Begg's test |
|---|---|---|---|---|---|---|---|
| D versus I | Overall | 63 | 0.000 | Random | 1.316 (1.213–1.427) | 0.000 | 0.006 |
| Asian | 41 | 0.000 | Random | 1.513 (1.363–1.679) | 0.000 | – | |
| Caucasian | 20 | 0.167 | Random | 1.058 (0.975–1.149) | 0.176 | – | |
| Chinese | 27 | 0.002 | Random | 1.552 (1.368–1.760) | 0.002 | – | |
| Non-Chinese | 36 | 0.000 | Random | 1.169 (1.066–1.281) | 0.000 | – | |
| Type 1 diabetic | 13 | 0.022 | Random | 1.139 (0.952–1.364) | 0.155 | – | |
| Type 2 diabetic | 50 | 0.000 | Random | 1.361 (1.243–1.490) | 0.000 | – | |
| DD versus ID+II | Overall | 63 | 0.000 | Random | 1.414 (1.253–1.595) | 0.000 | 0.016 |
| Asian | 41 | 0.016 | Random | 1.819 (1.559–2.122) | 0.016 | – | |
| Caucasian | 20 | 0.755 | Random | 1.023 (0.92–1.127) | 0.755 | – | |
| Chinese | 27 | 0.112 | Fixed | 1.929 (1.666–2.234) | 0.000 | – | |
| Non-Chinese | 36 | 0.008 | Random | 1.137 (1.045–1.237) | 0.003 | – | |
| Type 1 diabetic | 13 | 0.153 | Fixed | 1.103 (0.884–1.377) | 0.153 | – | |
| Type 2 diabetic | 50 | 0.000 | Random | 1.503 (1.310–1.726) | 0.000 | – | |
| II versus DD+ID | Overall | 63 | 0.000 | Random | 0.750 (0.647–0.869) | 0.000 | 0.107 |
| Asian | 41 | 0.000 | Random | 0.678 (0.547–0.840) | 0.000 | – | |
| Caucasian | 20 | 0.021 | Random | 0.858 (0.719–1.025) | 0.092 | – | |
| Chinese | 27 | 0.040 | Random | 0.650 (0.548–0.771) | 0.000 | – | |
| Non-Chinese | 36 | 0.000 | Random | 0.845 (0.683–1.046) | 0.123 | – | |
| Type 1 diabetic | 13 | 0.011 | Random | 0.803 (0.568–1.134) | 0.212 | – | |
| Type 2 diabetic | 50 | 0.000 | Random | 0.738 (0.626–0.870) | 0.000 | – | |
| ID versus DD+II | Overall | 63 | 0.005 | Random | 0.999 (0.914–1.091) | 0.981 | 0.822 |
| Asian | 41 | 0.002 | Random | 0.949 (0.829–1.085) | 0.443 | – | |
| Caucasian | 20 | 0.516 | Fixed | 1.075 (0.981–1.178) | 0.121 | – | |
| Chinese | 27 | 0.276 | Fixed | 0.915 (0.803–1.043) | 0.186 | – | |
| Non-Chinese | 36 | 0.003 | Random | 1.055 (0.939–1.185) | 0.369 | – | |
| Type 1 diabetic | 13 | 0.299 | Fixed | 1.048 (0.870–1.263) | 0.622 | – | |
| Type 2 diabetic | 50 | 0.003 | Random | 0.990 (0.896–1.095) | 0.845 | – | |
41 included studies analyzed the correlation between ACE I/D gene polymorphism and DKD risk. A significant correlation was observed between ACE D allele/DD genotype and DKD risk in the Asian diabetic patients (D allele vs I allele: OR=1.513, 95% CI: 1.363–1.679, P=0.000; DD genotype vs ID+II genotype: OR=1.819, 95% CI: 1.559–2.122, P=0.016, Table 3). On the contrary, our pooled analysis indicated that the II genotype might be a protective factor against the DKD risk (II genotype vs DD+ID genotype: OR=0.678, 95% CI: 0.547–0.840, P=0.000, Table 3).
Correlation between ACE I/D gene polymorphism and DKD in Caucasian diabetic patientsThere were 20 trials evaluating the impact of ACE I/D gene polymorphism on DKD susceptibility in Caucasian diabetic patients. The pooled-analysis indicated no significant correlation between ACE I/D gene polymorphism and DKD (D allele vs I allele: OR=1.058, 95% CI: 0.975–1.149, P=0.176; DD genotype vs ID+II genotype: OR=1.023, 95% CI: 0.920–1.127, P=0.755; II genotype vs DD+ID genotype: OR=0.858, 95% CI: 0.719–1.025, P=0.092; ID genotype vs DD+II genotype: OR=1.075, 95% CI: 0.981–1.178, P=0.121, Shown in Table 3).
Correlation between ACE I/D gene polymorphism and DKD risk in Chinese diabetic patients27 studies analyzed the correlation between ACE I/D gene polymorphism and DKD risk in Chinese subjects. It showed a significant correlation between the ACE D allele/DD genotype and DKD in the Chinese population (D allele vs I allele: OR=1.552, 95% CI: 1.368–1.760, P=0.002; DD genotype vs ID+II genotype: OR=1.929, 95% CI: 1.666–2.234, P=0.000, Table 3). On the contrary, our pooled analysis showed that the II genotype might have or induce a protective role against DKD in Chinese diabetic patients (II genotype vs DD+ID genotype: OR=0.650, 95% CI: 0.548–0.771, P=0.000, shown in Table 3).
Correlation between ACE I/D gene polymorphism and DKD susceptibility in type 1 diabetic patientsThere were 13 studies exploring the impact of ACE I/D gene polymorphism on DKD susceptibility in type 1 diabetic subjects, our pooled analysis showed that there was no association between ACE I/D gene polymorphism and DKD susceptibility in type 1 diabetic patients (D allele vs I allele: OR=1.139, 95% CI: 0.952–1.364, P=0.155; DD genotype vs ID+II genotype: OR=1.103, 95% CI: 0.884–1.377, P=0.153; II genotype vs DD+ID genotype: OR=0.803, 95% CI: 0.568–1.134, P=0.212; ID genotype vs DD+II genotype: OR=1.048, 95% CI: 0.870–1.263, P=0.622, Table 3).
Correlation between ACE I/D gene polymorphism and DKD susceptibility in type 2 diabetic patientsThere were 50 studies exploring the correlation between ACE I/D gene polymorphism and DKD susceptibility in type 2 diabetic subjects, our pooled analysis indicated that the ACE D allele/DD genotype might increase the risk of DKD in type 2 diabetic subjects (D allele vs I allele: OR=1.361, 95% CI: 1.243–1.490, P=0.000; DD genotype vs ID+II genotype: OR=1.503, 95% CI: 1.310–1.726, P=0.000, Table 3). On the contrary, this pooled analysis showed that the ACE II genotype might be a protective factor for DKD in type 2 diabetic patients (II genotype vs DD+ID genotype: OR=0.738, 95% CI: 0.626–0.870, P=0.000, Table 3).
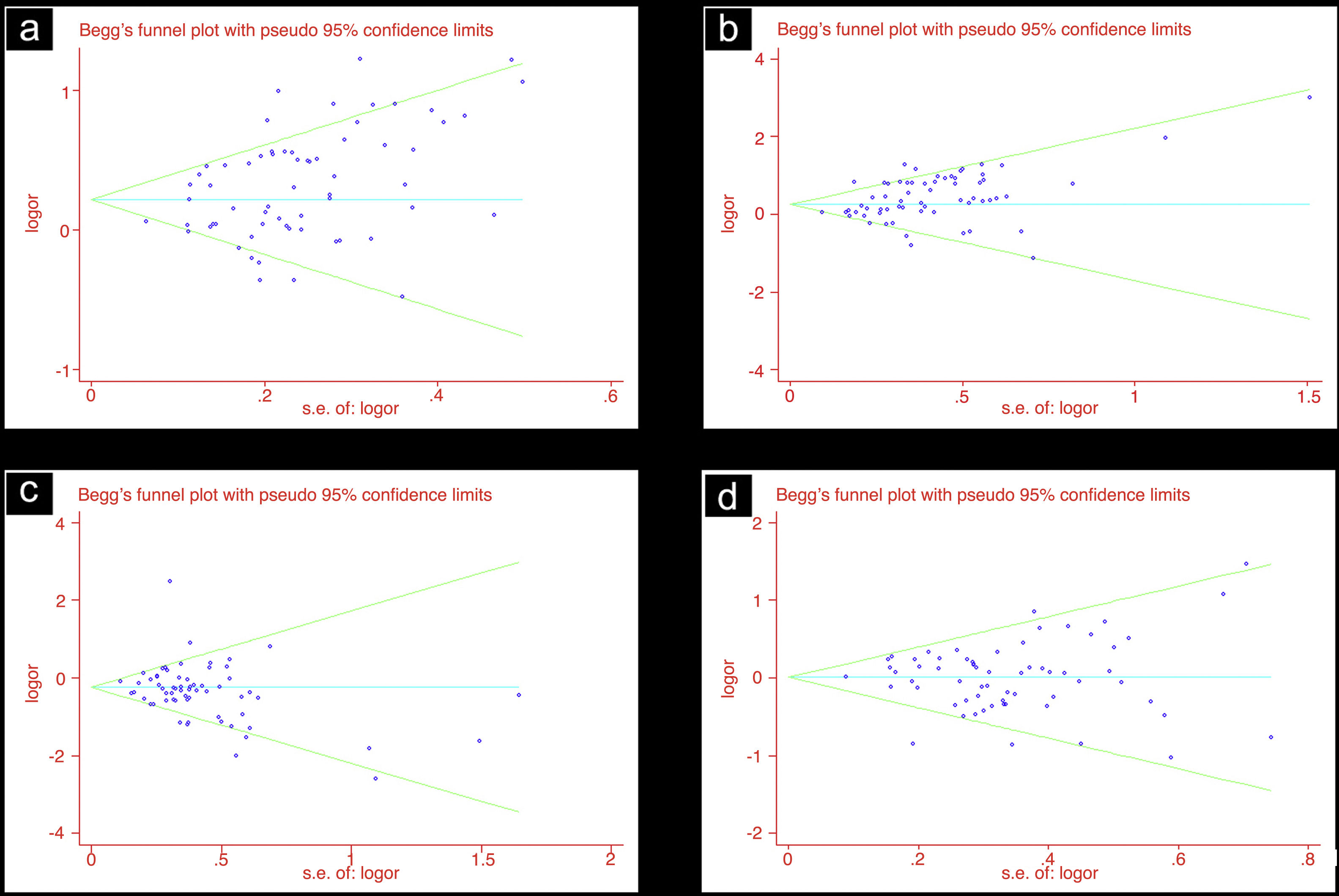
Publication biasIn this study, we used funnel plots and Begg's test to evaluate the publication bias. In the analysis for the association of ACE D allele/DD genotype with DKD susceptibility in overall diabetic patients, there was potential publication bias noted by Begg's test (D vs. I: Begg’ s test P=0.006; DD vs. ID+II: Begg's test P=0.016). In line with this, the funnel plots were asymmetrical (Table 3, Fig. 1).
DiscussionThis pooled-analysis showed that the ACE I/D polymorphism was statistically associated with DKD susceptibility, it indicated that ACE D allele/DD genotype might be a risk factor for DKD. On the contrary, ACE II genotype might be a protective factor for DKD.
Genome-wide association studies (GWAS) research was frequently carried out to explore the relationship between various gene single nucleotide polymorphisms (SNPs) and an array of diseases. In such studies, HWE testing for each SNPs was often the first and quality control step. Those SNPs that did not pass the HWE tests were eliminated before moving on to the next step.89 On the other hand, in case-control genetic association studies, departures from HWE in controls have been associated with problems in the design, genotyping error or selection bias.90 In pooled analysis, checking HWE among controls was a good idea for included trials. Trikalinos et al. has demonstrated that exclusion of trials with departures from HWE may sometimes change the estimate pooled analysis result, they advocated that studies with departures from HWE should be excluded in pooled analysis.91 For this reason, in this pooled analysis, we carefully checked all selected trials and excluded these studies failing to meet the HWE test. In addition, the quality of included studies were generally at the medium level according to the Newcastle-Ottawa Scale (NOS), it indicated that the included studies met the criteria accepted for valid SNP-association studies.
Some previous pooled analysis concerning about the impact of ACE I/D gene polymorphism on DKD risk has been completed. In 2012, a meta analysis included 14,108 DKD cases and 12,472 controls from 63 published studies has been performed, it indicated that ACE I/D polymorphism was associated with DKD development in the Asian type 2 diabetes subjects.92 However, the genotype distributions of the control groups do not conform to HWE in some trials. And because of that, in order to gain a more credible pooled analysis result, we re-examined the related studies and included some other more high quality trials. In line with Wang et al., our study also found that the ACE I/D genotype was correlated with DKD risk in type 2 diabetes patients. Due to the fact that we included a plethora of studies in our analysis compared to the aforementioned analyses, we feel that our pooled results are more convincing.
ACE is a pivotal factor of the renin–angiotensin–aldosterone system (RAAS), it contains 26 exons and 25 introns located on 17q23. In 1990, ACE gene polymorphism was firstly described based on the insertion or deletion (I/D) of a 287bp Alu in the 16th intron.93 Whereafter, a series of ACE polymorphic genetic markers have been found (e.g. A240T, T93C, T594lC). Among these polymorphic marker, I/D polymorphism (rs4340) was the most investigated. On account of this I/D polymorphic marker, we could divide ACE gene polymorphism into DD homozygote, II homozygote and ID heterozygote. It has been demonstrated that ACE I/D polymorphism could affect ACE activity level both in plasma and various tissues.94 Additionally, a great number of previous studies have been carried out and verified the impact of ACE I/D gene polymorphism on various diabetes-related diseases.
DKD is a severe complication both in type 1 and type 2 diabetic patients, it damages about 40% of all diabetic subjects and is a crucial cause of chronic renal failure both in the Eastern and Western world. The pathogenesis of DKD is very complicated, it has been verified that various signaling pathways and molecular factors are activated during DKD, such molecular events include activation of systemic and local RAAS, generation of pro-inflammatory cytokines and excessive reactive oxygen species.3 In addition, recent GWAS studies demonstrated that DKD patients always suffer from genetic damage, and genetic factors are involved in the development of DKD, more critically, some specific gene SNPs might be associated with DKD susceptibility, thus it could provide remarkable clinical significance for preventing and early diagnosing of DKD through detailed illuminating the genetic mechanisms involved in DKD.
RAAS activation play a vital role in the occurrence and development of DKD, the RAAS is a pivotal regulator of renal arterial blood pressure by angiotensin II. However, conversion of low activity angiotensin I to high activity angiotensin II was relying on ACE. It has been showed that the ACE level is strongly correlated with ACE I/D polymorphism. Although the ACE I/D gene polymorphism is taken place in the non-coding gene region, the base insertion or deletion itself might alter the splicing process of the ACE precursor mRNA, then influence the stability of ACE mRNA, and ultimately affect the expression or stabilization of ACE. In situ hybridization for ACE mRNA on renal biopsy studies have found that the expression of ACE mRNA was increased in those subjects with the ACE DD genotype.95 Additionally, the serum ACE levels was also higher in the those individuals with D genotype than those with ID genotype or II genotype.93 And because of that, it was reasonable to consider that ACE I/D genetic variation was associated with the development of DKD. ACE D allele carriers had more higher ACE levels both in serum and kidney tissue, which lead to a more efficient activation of angiotensin II, and consequently resulted in the deterioration of DKD. In line with these, our pooled study further demonstrated that DKD risk was higher in those subjects with D allele than I allele carriers. We observed that the presence of II genotype offered a significant protective effect for DKD, whereas the presence of DD genotype conferred remarkable risk for DKD. The detailed mechanistic aspects that underlie the relationship between ACE I/D gene polymorphism and DKD was not completely clear. As mentioned earlier, the impact of ACE I/D gene polymorphism on DKD could be partially attributed to the effect of the ACE I/D polymorphic variant on the expression of the ACE gene. On the other hand, a recent study performed by Mahwish et al. found that ACE I/D genotypes was associated with dyslipidemia in diabetic patients, the DD genotype subgroup subjects were characterized by a significant higher levels of plasma triglycerides and total cholesteroln.96 In addition, the association of ACE I/D genotypes with atherosclerotic risk factors such as hypertension, dyslipidemia, and obesity in type 2 diabetic patients has been reported.97 Taken together, ACE DD genotype might result in the formation of diabetic renal lesions through elevating angiotensin II levels and a key contributor to dyslipidemia in a hyperglycemic environment further culminating in renal complications.
In this study, we found that the impact of ACE I/D gene polymorphism on DKD susceptibility was inconsonant in different types of diabetes and races. The pooled analysis showed that ACE I/D gene polymorphism was correlated with DKD susceptibility in Asian individuals, but there was no obvious correlation in Caucasian subjects. For another, we found that there was no correlation between them among 13 studies concerning type 1 diabetic patients, while ACE I/D gene polymorphism was correlated with the onset of DKD risk in type 2 diabetic patients. It indicates that ACE I/D genetic factors contribute more in patients with type 2 diabetes mellitus. Likewise, this inconsistency was also found in previous pooled analysis, Ng et al. found that ACE gene polymorphism was associated with DKD among type 2 diabetic Asians, while there was a reduced risk of DKD associated with the ACE I/D gene polymorphism among Caucasians with either type 1 or type 2 diabetes.98 Similarly, a pooled analysis performed by Wang et al. further found that the Asian group with T2DM showed a significant association. However, it failed to find any significant effects for different genetic models in T1DM and Caucasian subjects.92 Conversely, another pooled analysis performed by Xu et al. included 17 case-control studies in 2016 showed that ACE I/D polymorphism was correlated with DKD in the Asian groups with type 1 diabetes.9 While Fujisawa et al. found that the association was significant both in Asian populations and in Caucasian populations.10 Some reasons may account for the different results between Asians and Caucasians. Firstly, different lifestyle, environmental exposure, and different socioeconomic status may modify individual DKD susceptibility in different ethnic groups. Secondly, different genetic backgrounds in different racial subjects may influence genetic phenotypes.9 On the other hand, there are some other explanations for the predisposition to DKD in patients with type 2 DM. As mentioned above, the D allele of the ACE gene has been connected with higher ACE activity and increased level of angiotensin II. It has been found that increased angiotensin II could worsen insulin resistance and lipid metabolism disorders.99 In addition, both muscle capillary density and endogenous hepatic glucose production also could be affected by ACE I/D gene polymorphism.100
This study has several potential limitations. Firstly, most included trials were limited number and size. Second, there were evidences of public bias in this pooled study, in addition, the included trials were from various countries and races, which might decrease the reliability of this pooled analysis. Finally, our research was focused on ACE I/D genetic alteration, but previous studies have indicated that gene polymorphism in many other genes including Interleukin-6 -174G/C and angiotensinogen T174M gene polymorphism were correlated with DKD susceptibility,101,102 thus it can be argue that further pooled analysis concerning these genes SNPs are needed.
ConclusionACE I/D gene polymorphism is correlated with DKD risk in Asian, Chinese populations and type 2 diabetic individuals. ACE D allele and DD genotype is a risk factor for DKD. Conversely, ACE II genotype seems to be a protective factor of DKD. However, no correlation between ACE I/D gene polymorphism and the susceptibility of DKD was found in Caucasian or type 1 diabetic patients.
Authors’ contributionShi-kun Yang, Wen-li Zeng, Fen-fen Chu analyzed the data for the manuscript and wrote the manuscript. Shi-kun Yang, Na Song, Wen-li Zeng performed the literature search. Fen-fen Chu, Shi-kun Yang edited the manuscript.
Data availability and ethics committeeThe data used to support the findings of this study are available from the first author and corresponding author upon request. This is a meta analysis using previous relevant published studies. There is no Human participants and/or Animals informed consent. None of the authors is in any condition that may represent a potential conflict of interest. The experiments were carried out according to the Ethics Review Committee of The Third Xiangya Hospital, Central South University.
FundingThis study was supported by the Hunan Provincial Health Commission Project (202103050178). Hunan Provincial Clinical medical technology innovation guide project (2020SK53601). The science and technology planning project of Hengyang City (2019jh011012).
Conflict of interestThe authors declare that they have no conflict of interest.