Increased acetataemia during haemodialysis sessions has been associated with a number of abnormalities, including increased oxidative stress, pro-inflammatory cytokines and nitric oxide synthesis. Citric acid may play an alternative role to acetate as a dialysate stabilizer given that the effect of citrate on complement and leukocyte activation is different to that of acetate. The purpose of this study was to compare the inflammatory effect in immunocompetent blood cells of acetate dialysate and citrate dialysate.
Materials and methodsThe effect of acetate and/or citrate was investigated in the whole blood of uremic patients and in healthy in vitro samples. Four types of dialysate were tested: dialysate 1, acetate-free with 1mmol/L of citrate; dialysate 2, with 0.8mmol/L of citrate and 0.3mmol/L of acetate; dialysate 3, citrate-free with 3mmol/L of acetate; and dialysate 4, citrate-free with 4mmol/L of acetate. The cell types used were: human monocyte culture (THP-1); and peripheral blood mononuclear cells (PBMCs) from healthy subjects and uremic patients on haemodialysis. ICAM-1 was determined and levels of reactive oxygen species and total microvesicles were quantified.
ResultsUnlike the citrate dialysates, the dialysates with acetate (dialysate 3 and dialysate 4) induced increased ICAM-1 expression density in THP-1 cells; an increase in ICAM-1 expression was observed in the immunocompetent cells of healthy subjects with acetate dialysate (dialysate 3 and dialysate 4) but not with citrate dialysate (dialysate 1 and dialysate 2). No significant ICAM-1 differences were found between the different dialysates in the cells of haemodialysed patients. Reactive oxygen species expression and the number of microvesicles increased significantly with acetate dialysate but not with citrate dialysate in the cells of both healthy subjects and haemodialysed patients.
ConclusionAt the concentrations in which it is generally used in clinical practice, acetate-based dialysate increases oxidative stress and the total number of microvesicles and may induce another pro-inflammatory stimuli in uremic patients on haemodialysis. Citrate dialysates do not induce this activation, which could make them a suitable alternative in clinical practice.
El líquido de diálisis con citrato no induce in vitro estrés oxidativo ni inflamación en comparación con el acetato.
El incremento de la acetatemia durante la sesión de hemodiálisis se ha asociado a una serie de alteraciones: aumento del estrés oxidativo, de las citocinas proinflamatorias y de la síntesis de óxido nítrico. El ácido cítrico puede jugar un papel alternativo al acetato como estabilizante del líquido de diálisis (LD). El citrato en comparación con el acetato tiene un patrón diferente en cuanto a la activación leucocitaria y del complemento. El objetivo de este estudio es comparar el acetato con el citrato en el LD respecto a su efecto inflamatorio en las células inmunocompetentes de la sangre.
Material y métodosEl efecto del acetato o citrato fue investigado en sangre completa de pacientes urémicos y controles sanos in vitro, enfrentada a 4tipos de LD: el LD 1, con 1mmol/L de citrato y libre de acetato; LD 2, con 0,8mmol/L de citrato y 0,3mmol/L de acetato; LD 3, con 3mmol/L de acetato sin citrato y LD 4, con 4mmol/L de acetato sin citrato. Los tipos de células utilizados fueron: cultivo de monocitos humanos (THP-1); células mononucleares de sangre periférica (PBMC) de controles sanos y pacientes urémicos en HD. Se determinó ICAM-1, la cuantificación de los niveles de especies reactivas de oxígeno (ROS) y la cuantificación de microvesículas totales.
ResultadosLos LD con acetato (L3 y L4) indujeron un incremento en la densidad de expresión de ICAM-1 en las células THP-1, no así los de citrato; con células inmunocompetentes de sujetos sanos los LD con acetato (L3 y L4) respecto a los con citrato (L1 y L2) observamos un incremento en la expresión de ICAM-1; con células de pacientes en hemodiálisis no existían diferencias significativas entre los diferentes LD. Tanto en las células de sujetos sanos como en las de los dializados, se incrementaron significativamente la expresión de especies reactivas de oxígeno y las microvesículas con los LD con acetato y no con citrato.
ConclusionesEl acetato en el LD, en las concentraciones que se utilizan habitualmente en la práctica clínica, aumenta el estrés oxidativo y las microvesículas totales, y puede actuar como coadyuvante de los otros estímulos proinflamatorios a los que están sometidos los pacientes urémicos en hemodiálisis. Los LD con citrato no producen esta activación, por lo que podrían ser una alternativa en la clínica.
Hemodialysis (HD) machines are able to produce dialysis fluid (DF) containing calcium and magnesium salts and, at the same time, bicarbonate. To maintain the solubility of these salts it is necessary to add an acid that maintains the pH, between 7.1 and 7.6.1 The acid that has been added to the DF is acetic acid, generally in concentrations of 3 or 4mmol/L. The transition from acetic acid to acetyl-coenzyme-A and its metabolization in the citric acid cycle produces equimolar amount of bicarbonate. In HD with bicarbonate and 3–4mmol/L of acetate, up to 25% of the alkalization produced comes from the acetate. In most patients during the HD session there is an increase in the blood levels of acetate, since the transfer of acetate from DF to the blood is greater than the capacity to be metabolized in the body. This increase in acetate blood level is superior with high-efficiency techniques, like on-line hemodiafiltration (HDF-OL),2 and it is also more evident in patients with a low capacity to metabolize acetate, i.e. individuals with reduced muscle mass, or liver insufficiency.3
The increase in acetate levels during the HD session has been associated to a number of abnormal effects: increased oxidative stress, synthesis of proinflammatory cytokines and generation of nitric oxide.4–8 Up to 50% of HD patients have chronic low-grade inflammation. An elevation of acetate level would worsens inflammation that is produced by exposure to pyrogenic substances or bioincompatible materials. Also high acetate levels in blood has been associated with hemodynamic intolerance to HD.9
To avoid these negative effects of acetate, an effort has been made to replace the acetate of the DF by another acid, or just remove it from the DF. The latter was achieved by the technique of hemodiafiltration called “acetate free biofiltration” (AFB), in which the DF does not contain acetate or bicarbonate, instead this is infused intravenously. The removal of acetate in the AFB resulted in no activation of polymorphonuclear cells and monocytes10 and the increased synthesis of nitric oxide did not occur.6 Alternatively, DF acetate can been successfully replaced by hydrochloric or citric acid.8,11,12
Citric acid may be an alternative to acetate as a stabilizer for the DF. As compared to acetate, Citrate behave differently in relation to the activation leukocytes and complement.13 Citrate used as a regional anticoagulant (RAC) in HD, has shown favorable effects on inflammation14–16; although it is true that the concentrations of citrate as RAC are substantially higher than that of the DF containing citrate. In a HD with 1mmol/L of citrate in DF, the blood returning to the patient has a citrate concentration of 0.58μmol/L, while the concentration in normal blood is 0.1μmol/L. After 4h of HD, the systemic concentration of citrate would be 0.25–0.3mmol/L.17 With 0.8mmol/L of citrate in the DF, the blood citrate level at the end of the HD would be within 0.2–0.25mmol/L; in HDF-OL citrate would reach a concentration of 0.3mmol/L. At these concentrations, the activation of complement and granulocytes would be reduced13 as compared with acetate. HDF-OL with citrate would achieve lower levels of CRP and β2-microglobulin than with acetate.17
In the above-mentioned studies, it is difficult to separate the beneficial effects on inflammation produced by acetate removal from DF from those resulting from the addition of citrate.
Presently we have 2 types of DF containing citrate. The first contains 1mmol/L of citrate and does not have acetate; the second has 0.8mmol/L citrate and 0.3μmol/L acetate. Our standard DF for HD contains 3–4mmol/L acetate.
In this work we have evaluated in vitro the oxidative stress, adhesion molecules and production of microparticles in cells from uremic patients exposed to DF with citrate alone, with citrate plus acetate and with acetate only, in concentrations of 3 or 4mmol/L. The aim of this study is to compare the effects of the DF acetate and citrate on the inflammatory response of immunocompetent blood cells.
Materials and methodsThe effect of both acetate and citrate was investigated in whole blood from uremic patients and healthy controls in vitro. Blood was obtained from patients that did not received anti-inflammatory medication during the previous days.
Types of blood samplesWe obtained 2 pools of blood stored in the biobank of the Renal Research Network (REDinREN, Madrid). The first pool corresponds to blood from 12 patients with chronic kidney disease (CKD) on regular HD: there were 3 experiments performed and the results of each experiment are the average of the 3 wells. The other pool came from 4 healthy controls, with similar characteristics of age (average of 60 years), and gender (50% men). Two of the HD patients were diabetic. Blood samples were obtained pre-HD. With these samples, 3 experiments were done and the data are the average of 3 wells.
Hemodialysis fluidsFour types of HD fluids (DF) were prepared
- -
DF 1, contained citrate (1mmol/L) without acetate: SelectBag Citrate® produced in the HD AK200-ultra-S machine.
- -
DF 2, with citrate (0.8μmol/L) and acetate (0.3μmol/L): Citrasate® 323°C produced in Therapeutic Systems 5008 FMC®.
- -
LD 3, with acetate (3mmol/L) without citrate: SoftPac® produced on AK200-ultra-S HD machines.
- -
LD 4, with acetate (4mmol/L) without citrate: produced in Therapeutic Systems 5008 FMC®.
The composition of the DF is shown in Table 1. They are ranked from lowest to highest concentration of acetate in DF. The LD meets the characteristics of ultrapure.18 Samples were taken from the hansen to the dialysate input, once the machine had been stabilized (for conductivity and temperature). The samples were subsequently sealed.
Composition of dialysis fluids used.
| Formula | Reference | Na | Ca | Mg | K | Cl | Citrate | Acetate | Bicarbonate | Glucose | Osmolaridad |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mM/L | mM/L | mM/L | mM/L | mM/L | mM/L | mM/L | mM/L | mOsm/L | |||
| DF1 Citrate Gambro® | CX 265 G | 140 | 1.65 | 0.5 | 2 | 109.3 | 1 | 0 | 34 | 8.33 | |
| DF 2 Citrasate® | 303-C (A+B) | 139.3 | 1.665 | 0.475 | 1.9 | 104.37 | 0.8 | 0.3 | 35 | 8.33 | 287.22 |
| DF 3 Acetato Gambro® | G295 | 140 | 1.5 | 0.5 | 2 | 109 | 0 | 3 | 35 | 8.33 | 294 |
| DF 4 Acetate FMC® | ACF 3A5 | 140 | 1.5 | 0.5 | 2 | 107 | 0 | 4 | 35 | 8.33 | 296 |
Human monocytes (THP-1 cell line, Sigma, Cat # 88081201) were cultured in RPMI 1640 medium with 10% penicillin-streptomycin, both supplied by Lonza (Basel, Switzerland) with10% fetal bovine serum FBS from Sigma (St. Louis, USA).
Peripheral blood mononuclear cellsPeripheral blood mononuclear cells (PBMCs) were obtained from 10ml of blood collected in EDTA tubes by density gradient using Lymphocyte Isolation Solution (Rafer, Zaragoza, Spain).
Quantification of adhesion moleculesCell adhesion molecule 1, CD54/ICAM-1 were determined in both THP-1 and PBMC cells following the same protocol.
Cells were plated at a density of 106cells/ml in 6-well plates (Nunclon™ Delta, NUNC® Thermo Fisher Scientific, Waltham, Massachusetts, United States). They were incubated overnight with the various DF and using a phosphate buffer (PBS, Sigma, Sant Louis, Missouri, USA) as a control. The determination of adhesion molecules was performed by immunofluorescence techniques using the FITC-conjugated monoclonal antibody MEM-111 against the CD54 molecule, from Thermo Fisher Scientific (Waltham, Massachusetts, USA). We used the FACSCalibur flow cytometer from Becton Dickinson (San Jose, California, USA) and the Cyflogic software to analyze the mean fluorescence channel.
Quantification of reactive oxygen species levelsReactive oxygen species (ROS) activity was measured using the method described by Wang and Josep (Wang and Joseph, 1999) which is based on the fluorescence emitted by 2′,7′-dichlorodihydrofluorescein when oxidized to 2′,7′-dichlorofluorescein.
PBMCs were plated in 6-well plates at a density of 106 cells/ml. Cells were incubated overnight with the corresponding DF. Thereafter cells were washed with PBS phosphate buffer (Sigma, Sant Louis, Missouri, USA) and incubated with H2DCFDA at a concentration of 5μM (Thermo Fisher Scientific, Waltham, Mass., USA) in fresh medium for 30min. The analysis was performed by flow cytometry FACSCalibur (Becton Dickinson) and the mean flow channel was analyzed with Cyflogic software.
Quantification of microvesiclesMicrovesicles were quantified in the supernatant of cultured PBMCs treated overnight with the different DF. An aliquot was labeled with annexin V conjugated with FITC supplied by Becton Dickinson. The analysis was performed in FACSCalibur (Becton Dickinson) flow cytometry. First the population of microvesicles was selected according to size (FSC/forward scatter) and complexity (SSC/side scatter) using the Flow Cytometry Sub-Micron Size Reference Kit, Green Fluorescent (Life Technologies, Paisley, USA) and then the Annexin V positive microvesicles were selected. A known number of latex flow count microspheres (Beckman Coulter, Brea, California, USA) was used to quantify the concentration of microvesicles.
Measurements units and statisticsBaseline values of expression of adhesion molecules or ROS activity levels in the PBMC cells were very variable. Thus the results are presented by assigning the arbitrary value of one to the control sample, and expressing an increase or decrease with respect to this value after cells were cultured in the presence of the different DF that were the objective of the study. The values obtained are expressed as fold change vs control.
The results are expressed as mean±SD of the experiments done in triplicate. One-step analysis of variance was used to compare the results of the five groups in each of the tests. This was after performing Levene test. If necessary, a non-parametric test was used: Kruskal–Wallis. Paired Student's t-test or the Mann–Whitney test, were used as appropriate, to compare two groups; p<0.05 was considered significant. Statistical analysis was performed using SPSS 15.0 Inc. (Chicago, IL, USA).
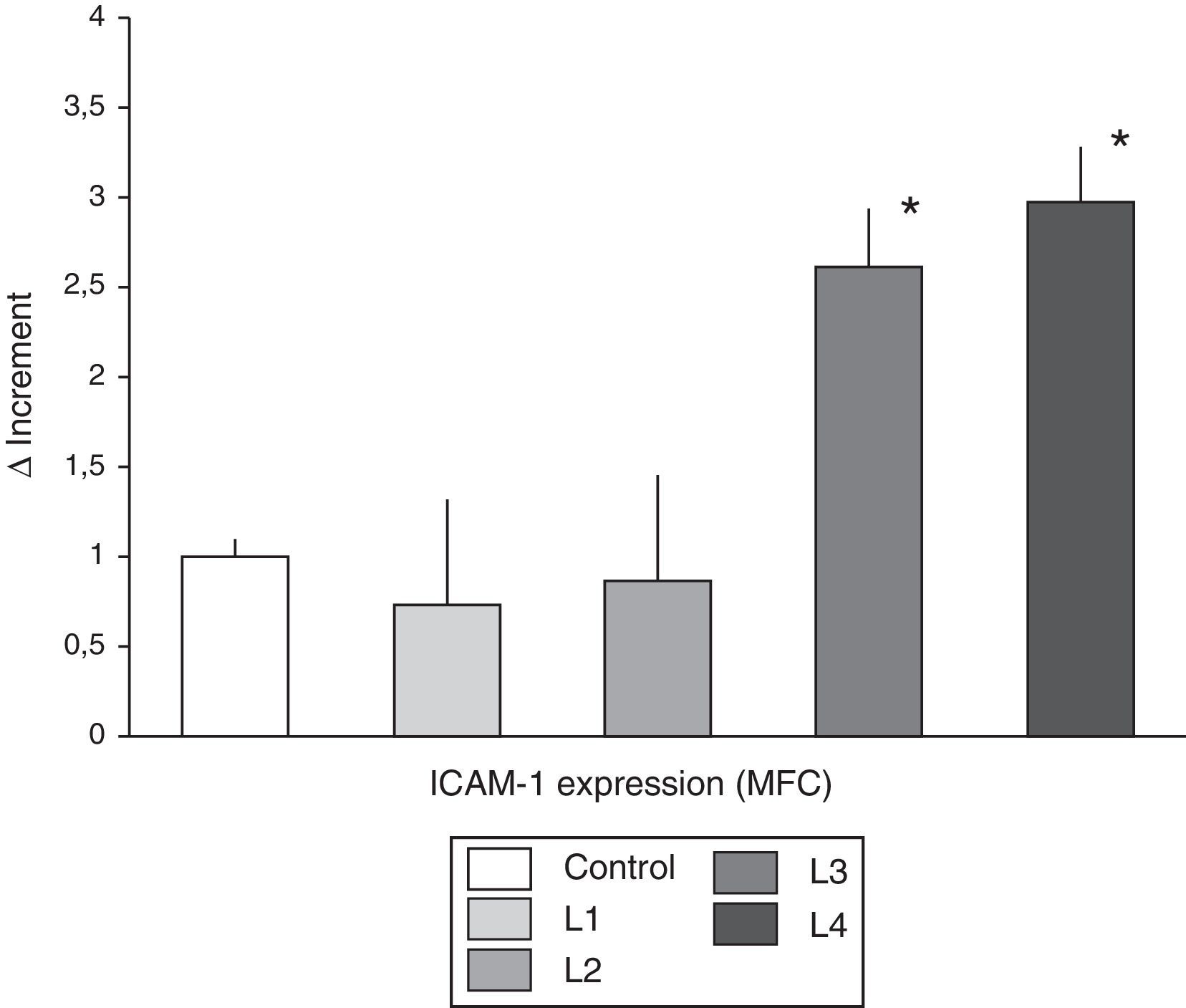
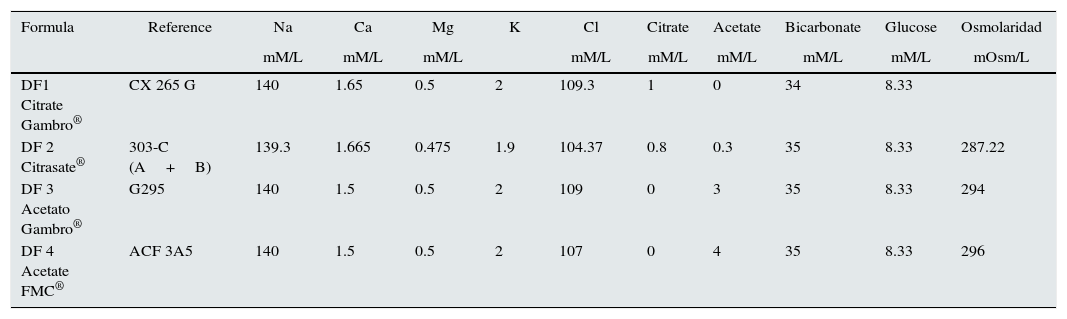
ResultsCharacterization of THP-1 monocyte activation induced by different dialysis fluidsThe THP-1 monocyte cell line, exposed to DF containing acetate (L3 and L4) induced an increase in ICAM-1 expression density (Fig. 1). The mean fluorescence channel for the molecule showed a fold increase of 2.61±0.79 and 2.97±0.81 times, for L3 and L4 respectively, as compared to the mean density observed in control cells (1±0.11). By contrast, citrate-containing fluids (L1 and L2) did not modify the expression of ICAM-1 in THP-1 cells (mean fluorescence channel of 0.73±0.27 and 0.86±0.23, for L1 and L2 respectively).
THP-1, ICAM shows the differences in the expression (mean±SD) of ICAM-1 in the THP1 monocyte cell line cultured in control isotonic solution or in the various hemodialysis fluids. THP-1 cells cultured with acetate-containing fluids showed a significant increase in the mean fluorescence channel as compared with control and cells treated with citrate fluids. MFC: mean fluorescence channel. * p<0.05.
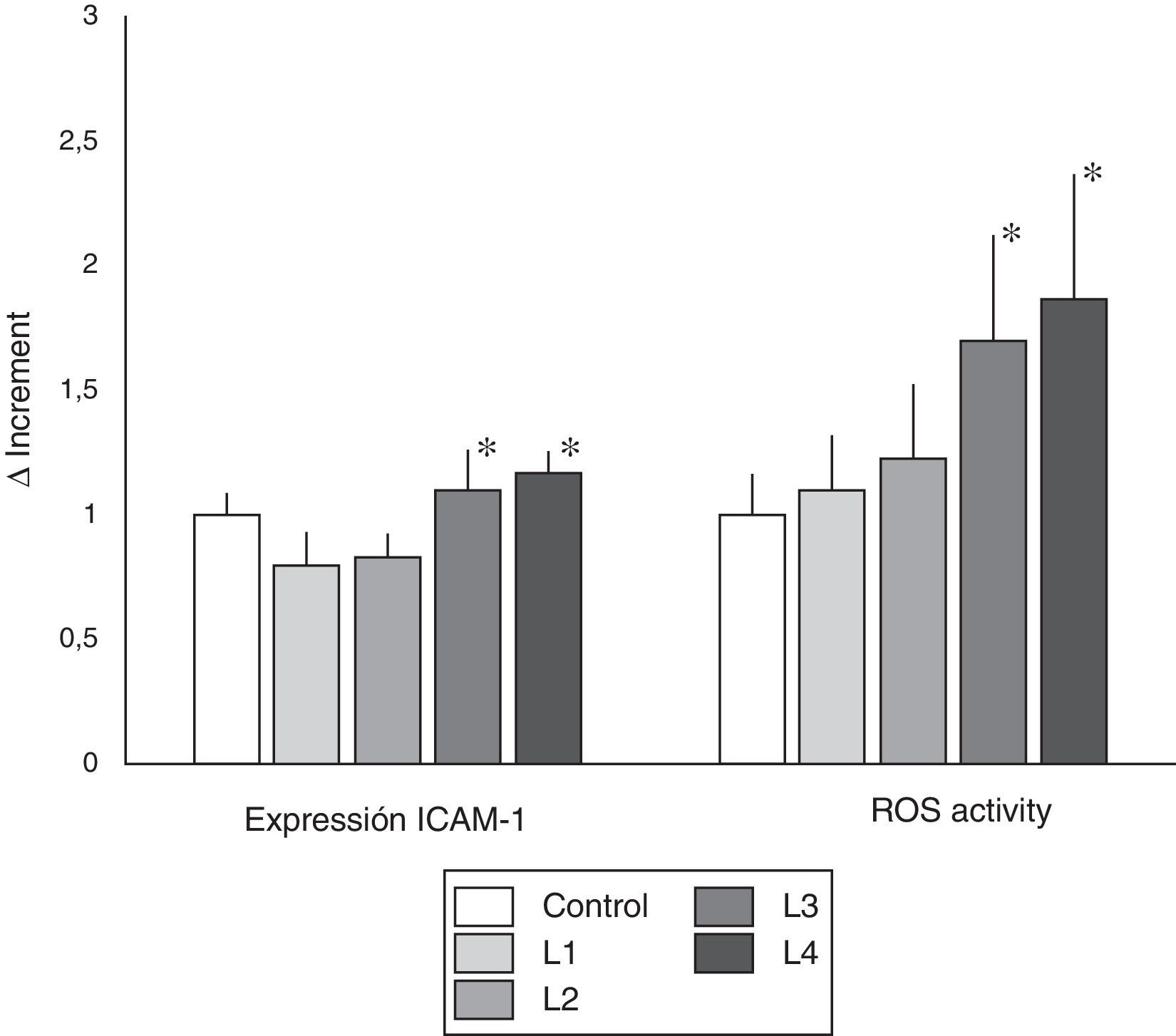
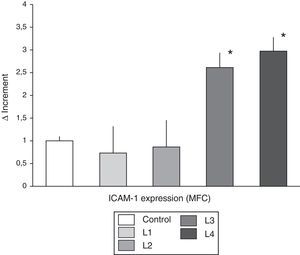
Mean expression of ICAM-1 from immunocompetent cell cultured with the four DF, was not significantly different than control (Fig. 2). However, direct comparison of ICAM-1 expression in cells cultured with citrate containing fluids (L1 and L2) versus cells cultured in acetate (L3 and L4), revealed that cells culture with acetate have an significant higher expression of ICAM-1 than cells cultured in citrate containing fluids.
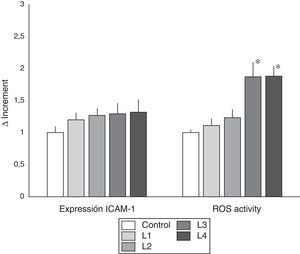
ICAM-1 expression and ROS activity in cells from healthy subjects. Values of ICAM-1 expression and ROS activity (mean±SD) in peripheral blood immunocompetent cells from subjects without known renal disease. The cells were cultured in isotonic control solution or in different hemodialysis fluids. The expression ICAM-1 was not different in the different dialysis fluids as compared with control. However, as compared with citrate fluids, acetate containing fluid induced a greater expression of ICAM-1 (* p<0.05). Regarding ROS activity, cells cultured with fluids containing acetate presented a significant increase in ROS activity as compared with control and with cells treated with citrate fluids (* p<0.05).
The study of ROS activity, indicator of oxidative stress, revealed that fluids with acetate induced greater ROS activity than citrate-containing fluids. As seen in Fig. 2, in PBMC cultured with fluids L3 and L4 induced a significantly higher ROS activity than control (p<0.05), and citrate fluids (L1 and L2) (p<0.05).
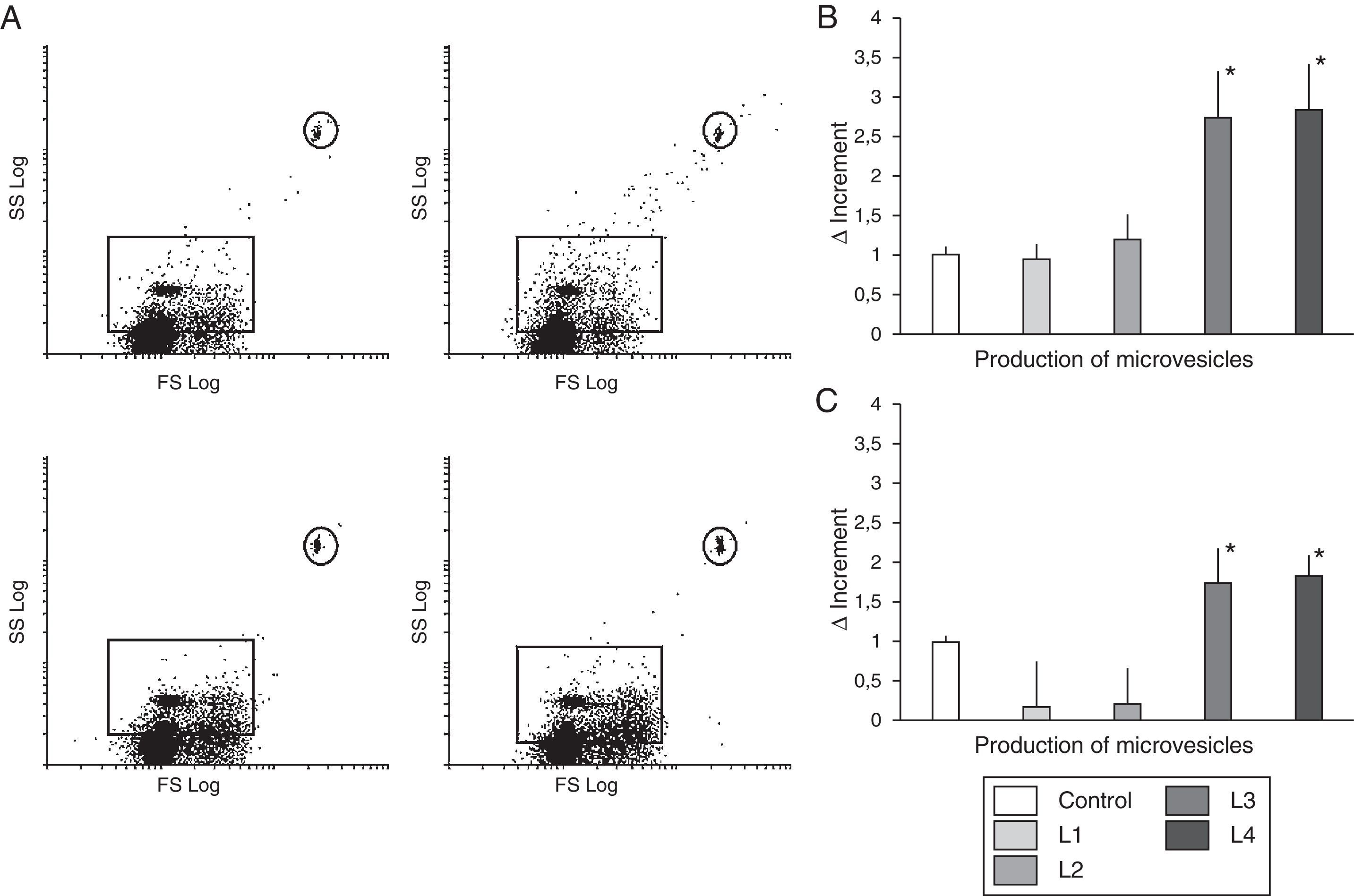
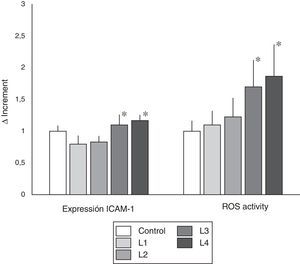
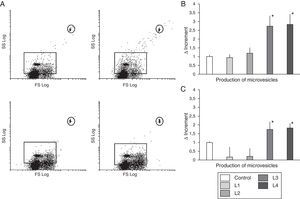
Like the observed with ROS activity, the L3 and L4 liquids that contain acetate induced an increase in microvesicle production (Δ=2.73±0.62 and 2.84±0.59, respectively, with respect to the control, p<0.01) (Fig. 3). However, no differences were observed in cells cultured with L1 and L2 as compared with control (Δ=0.94±0.233 and 1.19±0.31, respectively).
Circulating microvesicles. (A) Scatter plots representative of the distribution of microvesicles produced by immunocompetent cells from healthy subjects with fluids containing citrate (upper panel), or acetate (lower panel). (B) Quantification of the change in microvesicle production (mean±SD) by immunocompetent peripheral blood cells from healthy subjects cultured in control fluid and in fluids containing acetate or citrate. Fluids with acetate significantly increased the production of microvesicles as compared with control and with citrate (* p<0.01). (C) Quantification of the change in microvesicle production (mean±SD) by immunocompetent peripheral blood cells from hemodialysis patients in response to different hemodialysis fluids. Acetate fluids produced a significant increase in microvesicles as compared to control and citrate fluids (p<0.05).
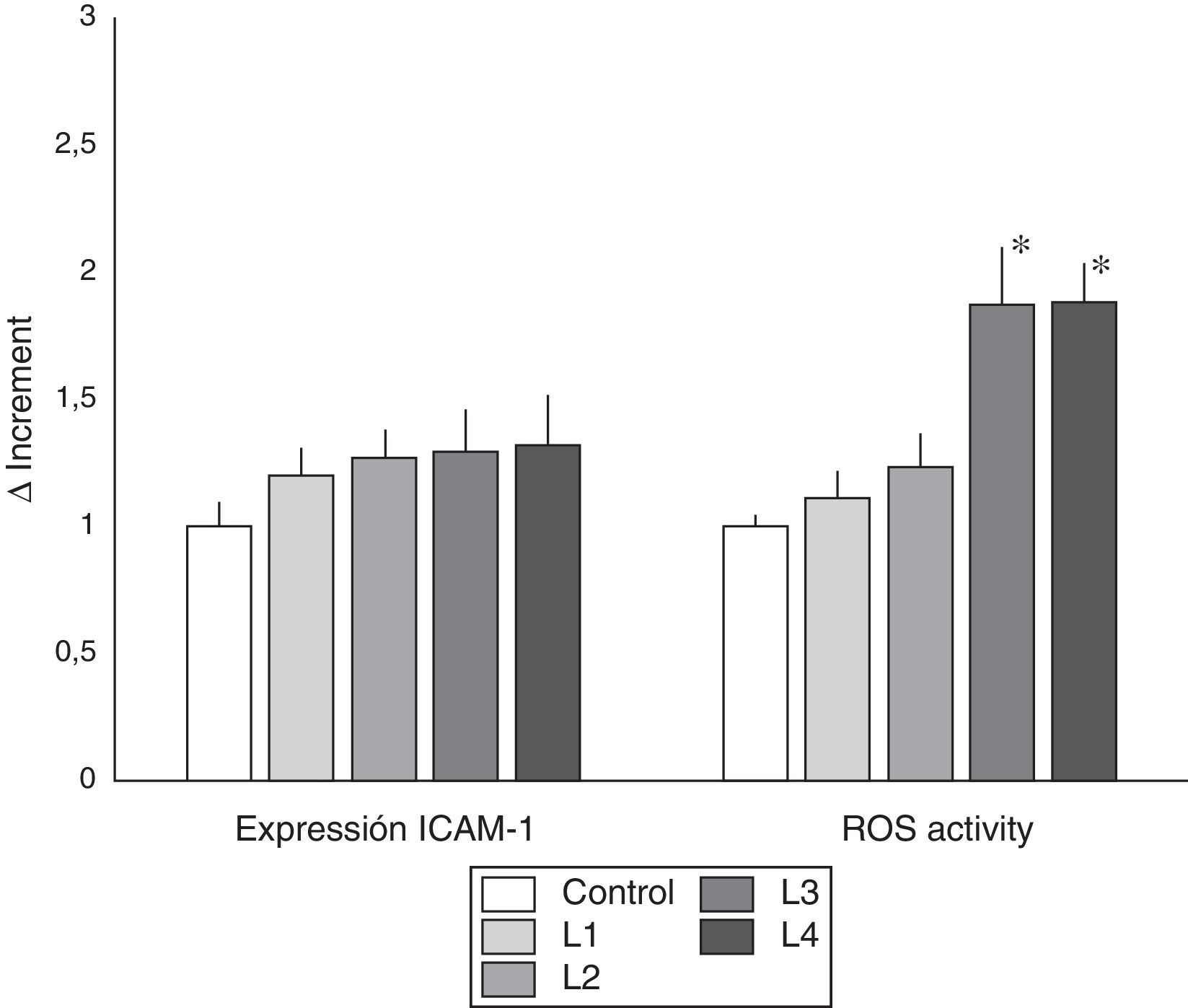
The changes observed in ROS expression and microvesicle production in immunocompetent cells from chronic renal failure patients were parallel to those observed in healthy subjects.
As shown in Fig. 4, acetate containing fluids induced higher ROS activity (p<0.005), as compared with control, and L1 and L2 treated cells. As far as the production of microvesicles (Fig. 3) the increase in the number of microvesicles with L1 and L2 was only 0.16±0.61 and 0.21±0.48, respectively, relative to the control (ns), whereas with L3 and L4 there was an increase of 1.73±0.46 and 1.82±0.29 (p<0.05).
ICAM-1 expression and ROS activity in immunocompetent peripheral blood cells from hemodialysis patients. Incubation in the different dialysis fluids did not produce significant differences in the expression of ICAM-1. However ROS activity was increased in cells cultured with acetate containing dialysis fluids as compared with control and citrate fluids. (* p<0.05).
In this in vitro study, acetate in DF, at concentrations of 3 and 4mmol/L, induces an increase in ICAM-1expression, ROS activity and production of circulating microvesicles by immunocompetent blood cells. Citrate does not induce this inflammatory response.
Between 50% and 60% of HD patients show a low-grade chronic inflammation, which has been associated to malnutrition, amyloidosis, atherosclerosis, susceptibility to infections and the high morbidity and mortality of these patients.19,20 This chronic inflammatory state is multifactorial; in part it is due to uremia and partly to specific factors of the dialysis technique.21–23 Among the proinflammatory factors characteristic of HD is the presence of acetate in the DF in a concentration of 20–40 times higher than that of plasma. Acetate transferred to the patient during HD has been associated with increased oxidative stress, generation of proinflammatory cytokines, and nitric oxide synthesis.4–8 During the HD session there are other proinflammatory factors, such as the dialyser membrane, lines, endotoxins and other pyrogenic substances, which make difficult to assess the role of one of them individually, as is the case of acetate. For this reason, an in vitro experiment has been helpful to elucidate the proinflammatory effect of acetate.
The generation of inflammation by DF acetate depends not only on the molecule, but on its concentration. Acetate is a biological molecule however a concentration 30–40 times higher than normal induces an inflammatory response. By contrast, citrate, even in very high concentrations, does not cause such a negative effect. The concentrations of both molecules are neither arbitrary nor easily modifiable. They are required for stabilization of the DF and to maintain an adequate acid-base balance during HD. In this study we have used the usual concentrations of these molecules in DF.
To investigate a possible differential activity of DF on immunocompetent cells, we first determined the expression of ICAM-1 in the THP-1 monocytic cell line. This cell line has been commonly used to investigate vascular damage associated to inflammatory activity and, more specifically, the changes in ICAM-1 expression have been considered to reflect the changes suffered by monocyte cells to favor its interaction with the vascular endothelium.24 The DF with acetate increased the expression of ICAM-1 with respect to the controls, whereas the DF with citrate did not.
Since the DF containing acetate induced activation of THP-1 cells, we wanted to analyze if this effect was due to some specific characteristic of transformed cells. Thus, we decided to culture PBMC from healthy subjects with different DF, this study evaluated other biomarkers associated with cellular activation that could be of clinical use to identify DF-induced cellular activation. In the blood cells from healthy subjects, we did not find differences regarding the expression of ICAM-1 with the DF with acetate as compared with to the control, however there were differences as compared with the DF containing citrate. As far as ROS activity it was observed that the DF with acetate induced greater activity than both control and DF containing citrate. In addition, we decided to evaluate the production of microvesicles produced by PBMC. During recent years it has became apparent the role of microvesicles in the pathogenesis of different diseases, including cardiovascular diseases associated to abnormal inflammatory response.25 As it was the case with ROS determinations, DF with acetate induced an increase in the production of microvesicles.
The chronic inflammatory process of CKD patients is usually associated with activation of immunocompetent cells in peripheral blood. This was confirmed in the present study; as compared to cells from control subjects, cells from CKD patients had higher density of ICAM-1 expression and increased basal levels of ROS (data not provided).
Unlike the observed in control subjects’ cells, the expression of ICAM-1 was not modified with any of the DF. This difference is not easily explained; it is possible that in activated cells the expression of this molecule is so high that a new stimulus fail to produce further increase in expression.
It has been described that citrate produces a direct inhibitory effect of on granulocyte activation, counteracting leukotriene-B4-induced adhesion and interfering with its activation.26,27 In addition, complement activation is reduced, even in concentrations of 0.25mmol/L of citrate.13 These anti-inflammatory effects of citrate would be conditioned by the calcium concentration, in this case it would be the calcium concentration in the DF. Some of our results support this beneficial role of citrate, which is added to the elimination of the negative effect of acetate; certainly the latter would be the critical.
The study has limitations such as the small number of healthy controls and the limited information on the status of the cells from HD patients. Comparison of the effect of the 5 groups using the same cells, works against that limitation. The different composition of the DF with respect to some electrolytes is very small, but an effect even with this small differenced cannot be totally excluded.
We may conclude that acetate in DF, at concentrations commonly used in clinical practice, increases oxidative stress and the number of total microvesicles and may act as an adjunct to the other proinflammatory stimuli to which HD patients are exposed. DF with citrate does not produce such a cell activation, so it could be use as an alternative. The goal should be to eliminate as much as possible the bioincompatible components of HD, one of these components is acetate at high concentrations. This would be of particular benefit in HD patients older than 70 years, that metabolize acetate slowly. In these patients, the elimination of acetate from the DF may contribute to a better survival regardless of their comorbidities.28
Conflict of interestsThe authors have no conflicts of interest to declare.
Please cite this article as: Pérez-García R, Ramírez Chamond R, de Sequera Ortiz P, Albalate M, Puerta Carretero M, Ortega M, et al. El líquido de diálisis con citrato no induce in vitro estrés oxidativo ni inflamación en comparación con el acetato. Nefrologia. 2017;37:630–637.