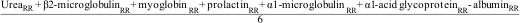New generation helixone dialyzers has recently been developed as part of the ongoing effort to improve dialyzer hemocompatibility and avoid adverse reactions to synthetic dialyzers. This study aimed to assess the performance and albumin loss of this new dialyzer series in hemodiafiltration and compare it with the previous generation helixone series.
Material and methodsA prospective study was conducted in 19 patients. Each patient underwent eight dialysis sessions with the same routine dialysis parameters; only the dialyzer varied: FX60 CorDiax, FX CorAL 60, FX600 CorDiax, FX CorAL 600, FX80 CorDiax, FX CorAL 80, FX800 CorDiax, and FX CorAL 800. The reduction ratios (RR) of urea, creatinine, ß2-microglobulin, myoglobin, kappa-free immunoglobulin light chains (κFLC), prolactin, α1-microglobulin, α1-acid glycoprotein, lambda immunoglobulin light chains (λFLC), and albumin were compared intra-individually. Dialysate albumin loss was also measured.
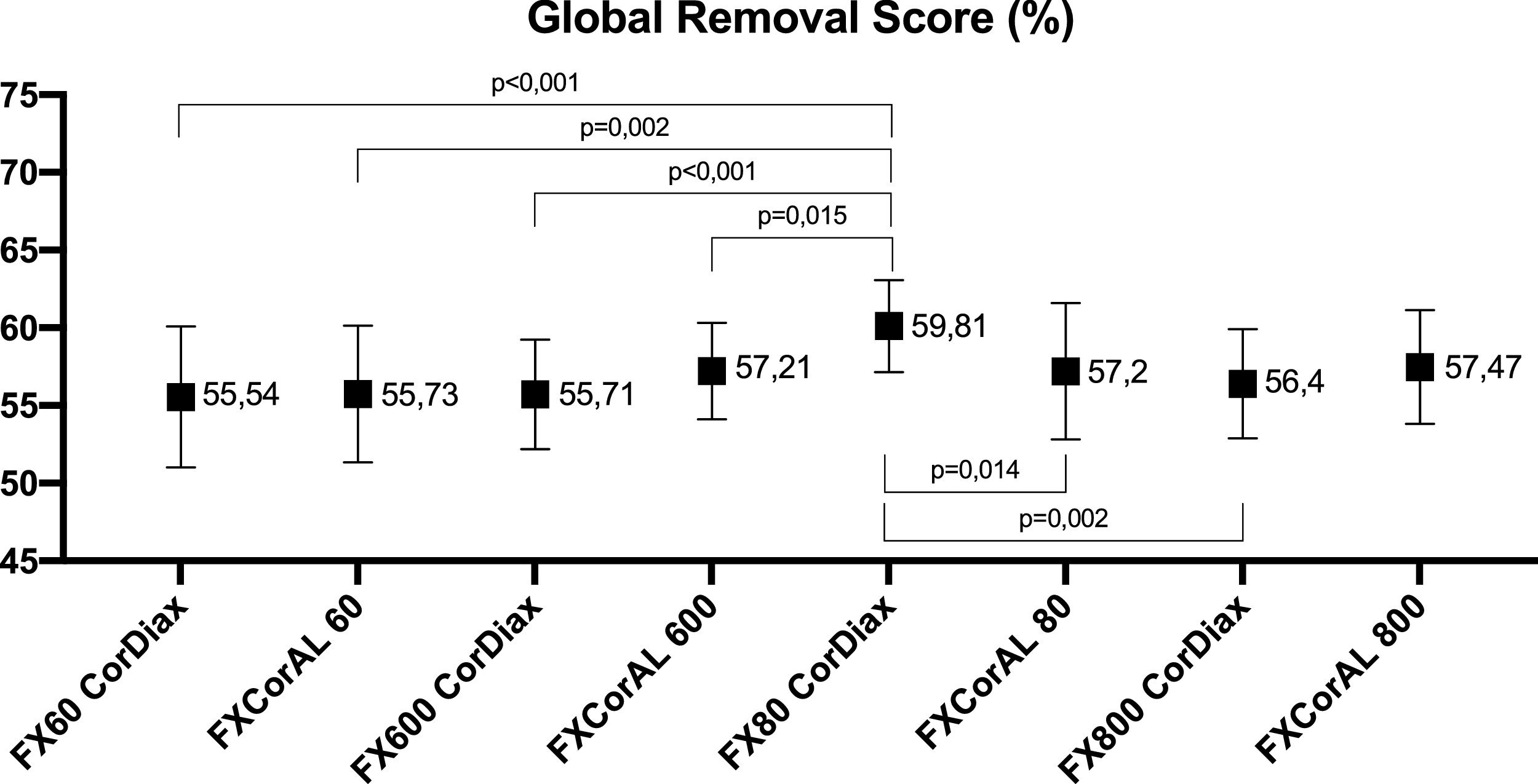
ResultsAll treatments were well tolerated. The mean amount of replacement fluid ranged from 31 to 34 L. Comparison of dialysis treatments showed no differences between small molecules and even up to those the size of β2-microglobulins. Little differences were found between myoglobin, κFLC, prolactin, α1-microglobulin, and λFLC RRs, and only FX80 CorDiax was slightly superior to the others. Mean dialysate albumin losses were similar, with less than 2.5 g lost in each dialyzer. The FX80 CorDiax showed slightly higher global removal scores than the other dialyzers evaluated, except for FX CorAL 800.
ConclusionThe new generation helixone dialyzers series has been updated to minimise the risk of adverse reactions, while maintaining the effectiveness and albumin loss achieved by the previous most advanced helixone generation.
Recientemente se han desarrollado dializadores de helixona de nueva generación como parte del esfuerzo continuo para mejorar la biocompatibilidad del dializador y evitar reacciones adversas a los dializadores sintéticos. Este estudio tuvo como objetivo evaluar el rendimiento y la pérdida de albúmina de esta nueva serie de dializadores en hemodiafiltración y compararla con dializadores de helixona de la generación anterior.
Material y métodosSe realizó un estudio prospectivo en 19 pacientes. Cada paciente recibió ocho sesiones de diálisis con los mismos parámetros de diálisis de rutina; sólo varió el dializador: FX60 CorDiax, FX CorAL 60, FX600 CorDiax, FX CorAL 600, FX80 CorDiax, FX CorAL 80, FX800 CorDiax y FX CorAL 800. Los índices de reducción (RR) de urea, creatinina, ß2-microglobulina, mioglobina, cadenas ligeras kappa (κFLC), prolactina, α1-microglobulina, α1-glicoproteína ácida, cadenas ligeras lambda (λFLC) y albúmina se compararon intraindividualmente. También se midió la pérdida de albúmina del dializado.
ResultadosTodos los tratamientos fueron bien tolerados. La media de líquido de reposición osciló entre 31 y 34 litros. La comparación de los tratamientos de diálisis no mostró diferencias entre moléculas pequeñas e incluso del tamaño de las β2-microglobulinas. Se encontraron pocas diferencias entre los RR de mioglobina, κFLC, prolactina, α1-microglobulina y λFLC. Las pérdidas de albúmina en el dializado fueron similares, con menos de 2,5 gramos por sesión. El dializador FX80 CorDiax mostró puntuaciones de eliminación global ligeramente más altas que el resto de los evaluados, a excepción del FX CorAL 800.
ConclusiónLa nueva generación de dializadores de helixona, actualizado para minimizar el riesgo de reacciones adversas, ha mantenido la eficacia y la pérdida de albúmina conseguidas por la generación de helixona previa.
Currently, the most widely used synthetic dialyzers are composed of polysulfone (PSU) and polyethersulfone (PES). These materials are mainly used in hemodialysis (HD) and online hemodiafiltration (HDF). The PVP has been shown to impact priming procedure and saline circulation, reducing protein fouling and platelet adsorption,1–4 thus it can be asserted that it reduces the risk of intradialytic coagulation, albeit at the expense of increasing the risk of adverse reactions.5 In fact, a small percentage of patients exhibit intolerance to synthetic dialyzers, manifested as low blood pressure and desaturation. Consequently, cellulose dialyzers, such as cellulose triacetate (CTA),6–8 or polymethylmethacrylate (PMMA) membranes,9 have been reintroduced as an alternative to avoid these clinical complications.
The new generation helixone, FX CorAL™ dialyzer series, has recently been developed as part of the ongoing effort to enhance dialyzer hemocompatibility. This dialyzer contains a membrane that combines a blend of polysulfone and PVP with small amounts of α-tocopherol added to stabilise the blood-side surface of the membrane.10 This modification facilitates the formation of a more robust hydrophilic layer. This hydrophilic layer on the membrane surface augments the hemocompatibility of polysulfone membranes by improving their antifouling ability, resulting in less protein adsorption and lower coagulation activation.11–13 To date, various studies have been published that report no hypersensitivity events to the new dialyzer.14,15
Data from the recently published com-PERFORM study indicate that the reduced secondary membrane formation of this dialyzer may be associated with its increased capacity for removing small and middle-sized molecules and the limited loss of albumin during high-volume HDF treatments.15
This study aimed to assess the performance and albumin loss associated with the new generation helixone dialyzer series during HDF treatments and compare them with the previous generation series. To do this, we assessed the removal of a wide range of molecular weight molecules and evaluated the risk of hypoalbuminemia by quantifying albumin loss in both blood and dialysate.
MethodsThis prospective, single-centre study was carried out in 19 patients (fourteen men, five women) with a mean age of 74.7 ± 9.3 years (range 57–83) who were stable on a thrice-weekly hemodialysis programme for an average of 61 ± 80 months. Prevalent adult patients on a maintenance hemodialysis program in our unit were considered for inclusion. Patients were excluded if they were in unstable clinical condition, with a scheduled living donor transplantation, or did not accept to participate. Underlying renal diseases were chronic glomerulonephritis (two patients), diabetic nephropathy (three patients), interstitial nephritis (three patients), nephroangiosclerosis (five patients), systemic disease (two patients), urological (two patients), and aetiology unknown (two patients). All patients signed an informed consent form. The study was approved by the local ethics committee and was conducted according to the Declaration of Helsinki.
Each patient underwent eight dialysis sessions in post-dilution HDF modality, with routine dialysis parameters: dialysis buffer with bicarbonate, blood flow rate (Qb) 450 ml/min, dialysate flow 400 ml/min, and dialysis time (Td) 289 ± 18 min (range 240−300 min). Fifteen patients were dialyzed through an arteriovenous fistula, one through a prosthetic arteriovenous fistula, and the other three with a tunnelled catheter. The anticoagulation used was low molecular-weight heparin (tinzaparin) in 53% of the patients and heparin sodium in 37%; the remaining 10% were dialyzed without heparin. Net fluid removal was set individually, depending on the patient’s clinical needs. All patients were anuric with <50 ml/day urine volume. Fresenius 5008 CorDiax or 6008 CAREsystem dialysis monitors were used (Fresenius, Bad Homburg, Germany). The only difference among the eight sessions in each patient was the dialyzer:
FX60 CorDiax™, helixone, Fresenius Medical Care
FX CorAL 60™, helixone hydro, Fresenius Medical Care
FX600 CorDiax™, helixone, Fresenius Medical Care
FX CorAL 600™, helixone hydro, Fresenius Medical Care
FX80 CorDiax™, helixone, Fresenius Medical Care
FX CorAL 80™, helixone hydro, Fresenius Medical Care
FX800 CorDiax™, helixone, Fresenius Medical Care
FX CorAL 800™, helixone hydro, Fresenius Medical Care
The order of the different treatment sessions was randomly assigned. The dialyzer characteristics are summarised in Table 1. Blood and dialysis fluid samples for analyses were taken from each patient in the same dialysis session of the week.
In vitro dialyzer performance.
| FX60 CorDiax | FX CorAL 60 | FX600 CorDiax | FX CorAL 600 | FX80 CorDiax | FX CorAL 80 | FX800 CorDiax | FX CorAL 800 | |
|---|---|---|---|---|---|---|---|---|
| Membrane | Helixone (polysulfone) | Helixone hydro (polysulfone) | Helixone (polysulfone) | Helixone hydro (polysulfone) | Helixone (polysulfone) | Helixone hydro (polysulfone) | Helixone (polysulfone) | Helixone hydro (polysulfone) |
| KUF, ml/h/mmHg | 47 | 61 | 46 | 60 | 64 | 75 | 62 | 73 |
| Wall thickness, µm | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 |
| Inner diameter, µm | 185 | 185 | 210 | 210 | 185 | 185 | 210 | 210 |
| SC B2m | 0.90 | 1.00 | 0.90 | 1.00 | 0.90 | 1.00 | 0.90 | 1.00 |
| SC Myoglobin | 0.50 | 0.60 | 0.50 | 0.60 | 0.50 | 0.60 | 0.50 | 0.60 |
| SC Albumin | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Surface, m2 | 1.4 | 1.4 | 1.6 | 1.6 | 1.8 | 1.8 | 2.0 | 2.0 |
| Sterilization | Steam | Steam | Steam | Steam | Steam | Steam | Steam | Steam |
KUF: ultrafiltration coefficient; SC: sieving coefficient.
The dialysis parameters collected in each session were as follows: real duration, dialyzer, blood flow rate (Qb), recirculation index measured by the temperature module, arterial and venous pressures, transmembrane pressure (TMP), initial and final hematocrit automatically measured by BVM® biosensor, initial and final body weights, volume of blood processed, and replacement volume.
Laboratory measurements included concentrations of urea (60 Da), creatinine (113 Da), ß2-microglobulin (11,800 Da), myoglobin (17,200 Da), kappa-free immunoglobulin light chains (κFLC, 22,500 Da), prolactin (23,000 Da), α1-microglobulin (33,000 Da), α1-acid glycoprotein (41,000 Da), lambda-free immunoglobulin light chains (λFLC, 45,000 Da), and albumin (66,000 Da), in serum at the beginning and at the end of each session to calculate the percentage reduction ratio (RR) of these solutes. The final concentration of ß2-microglobulin, myoglobin, κFLC, prolactin, α1-microglobulin, α1-acid glycoprotein, λFLC, and albumin were corrected for the degree of haemoconcentration and the volume of distribution (approximate extracellular volume) according to Bergström and Wehle.16 Throughout the treatment, a proportional part of the dialysis fluid was collected to quantify albumin loss.
We used the global removal score (GRS) to evaluate the efficacy of a global removal dialyzer, including the RR of molecules from 60 Da to 41,000 Da and considering albumin RR as negative values, calculated with the following formula17:
The results are expressed as the arithmetic mean ± standard deviation. For the analysis of the statistical significance of quantitative parameters, we used the Student t-test for paired data, and ANOVA for repeated data followed by Bonferroni’s post-hoc comparisons test for parametric data. P-values <.05 was considered statistically significant. Analyses were performed using SPSS software version 23 (SPSS, Chicago, IL, USA).
ResultsAll dialysis sessions were conducted without notable clinical incidents, using the same dose of heparin and without major coagulation of the extracorporeal circuit. There were no differences in dialysis parameters: Qb, total blood processed, vascular access recirculation, real session duration, initial weight, final weight, weight gain, initial and final hematocrit, arterial pressure, venous pressure, and TMP (Table 2). The replacement fluid in post-dilution HDF was also similar, between 31 and 34 L, with no significant changes with variations in internal diameter and minimal changes with the increase in the surface of the dialyzers (Table 2).
Comparison of dialysis parameters in the eight study sessions.
| FX60 CorDiax | FX CorAL 60 | FX600 CorDiax | FX CorAL 600 | FX80 CorDiax | FX CorAL 80 | FX800 CorDiax | FX CorAL 800 | |
|---|---|---|---|---|---|---|---|---|
| Blood processed, L | 126.5 ± 8.2 | 127.2 ± 8.0 | 126.6 ± 7.9 | 127.5 ± 8.1 | 124.5 ± 10.0 | 126.9 ± 8.1 | 126.5 ± 7.7 | 126.7 ± 7.8 |
| Recirculation, % | 16.0 ± 4.8 | 15.4 ± 4.0 | 16.9 ± 4.1 | 16.6 ± 4.4 | 16.5 ± 4.7 | 17,2 ± 5,6 | 15.8 ± 3.8 | 16.0 ± 4.2 |
| Real dialysis time, min | 283.7 ± 18 | 283.8 ± 18 | 282.7 ± 18 | 283.7 ± 18 | 282.7 ± 18 | 283.2 ± 18 | 283.1 ± 18 | 283.1 ± 18 |
| Initial weight, kg | 69.75 ± 12.0 | 69.95 ± 12.4 | 69.94 ± 12.6 | 69.98 ± 12.6 | 69.87 ± 12.7 | 69.47 ± 12.7 | 70.15 ± 12.4 | 70.02 ± 12.6 |
| Final weight, kg | 67.64 ± 12.5 | 67.70 ± 12.5 | 67.79 ± 12.6 | 67.89 ± 12.7 | 67.65 ± 12.7 | 67.58 ± 12.7 | 67.83 ± 12.5 | 67.88 ± 12.6 |
| Weight gain, kg | 2.11 ± 1.12 | 2.25 ± 0.73 | 2.15 ± 0.82 | 2.10 ± 0.57 | 2.23 ± 0.90 | 1.89 ± 0.94 | 2.32 ± 0.88 | 2.14 ± 0.69 |
| Initial hematocrit, % | 27.4 ± 5.8 | 28.4 ± 5.0 | 28.8 ± 4.5 | 28.3 ± 4.5 | 27.9 ± 4.7 | 27.5 ± 4.7 | 28.0 ± 4.5 | 28.8 ± 5.0 |
| Final hematocrit, % | 31.9 ± 7.4 | 33.2 ± 5.9 | 33.7 ± 5.7 | 32.9 ± 5.0 | 32.8 ± 5.8 | 31.5 ± 6.3 | 33.8 ± 5.8 | 33.8 ± 5.9 |
| Arterial pressure, mmHg | −219 ± 27 | −212 ± 26 | −213 ± 31 | −210 ± 30 | −217 ± 21 | −216 ± 26 | −218 ± 34 | −218 ± 27 |
| Venous pressure, mmHg | 211 ± 31 | 206 ± 28 | 208 ± 32 | 206 ± 26 | 200 ± 30 | 205 ± 23 | 207 ± 28 | 210 ± 25 |
| TMP, mmHg | 188 ± 39 | 178 ± 48 | 189 ± 40 | 187 ± 24 | 174 ± 32 | 172 ± 34 | 183 ± 27 | 186 ± 30 |
| Substitution volume, L | 32.7 ± 5.4 | 30.9 ± 4.8 | 31.2 ± 5.2 | 32.8 ± 5.2 b | 33.5 ± 5.6 | 34.3 ± 6.6 a,b | 34.1 ± 5.7 c | 33.9 ± 5.1 b |
| Kt, L | 72.8 ± 7.1 | 71.0 ± 6.1 | 70.5 ± 7.2 | 72.8 ± 6.8 b | 73.3 ± 8.0 | 74.2 ± 9.1 | 75.1 ± 7.9 b | 74.4 ± 8.0 b |
TMP: transmembrane pressure.
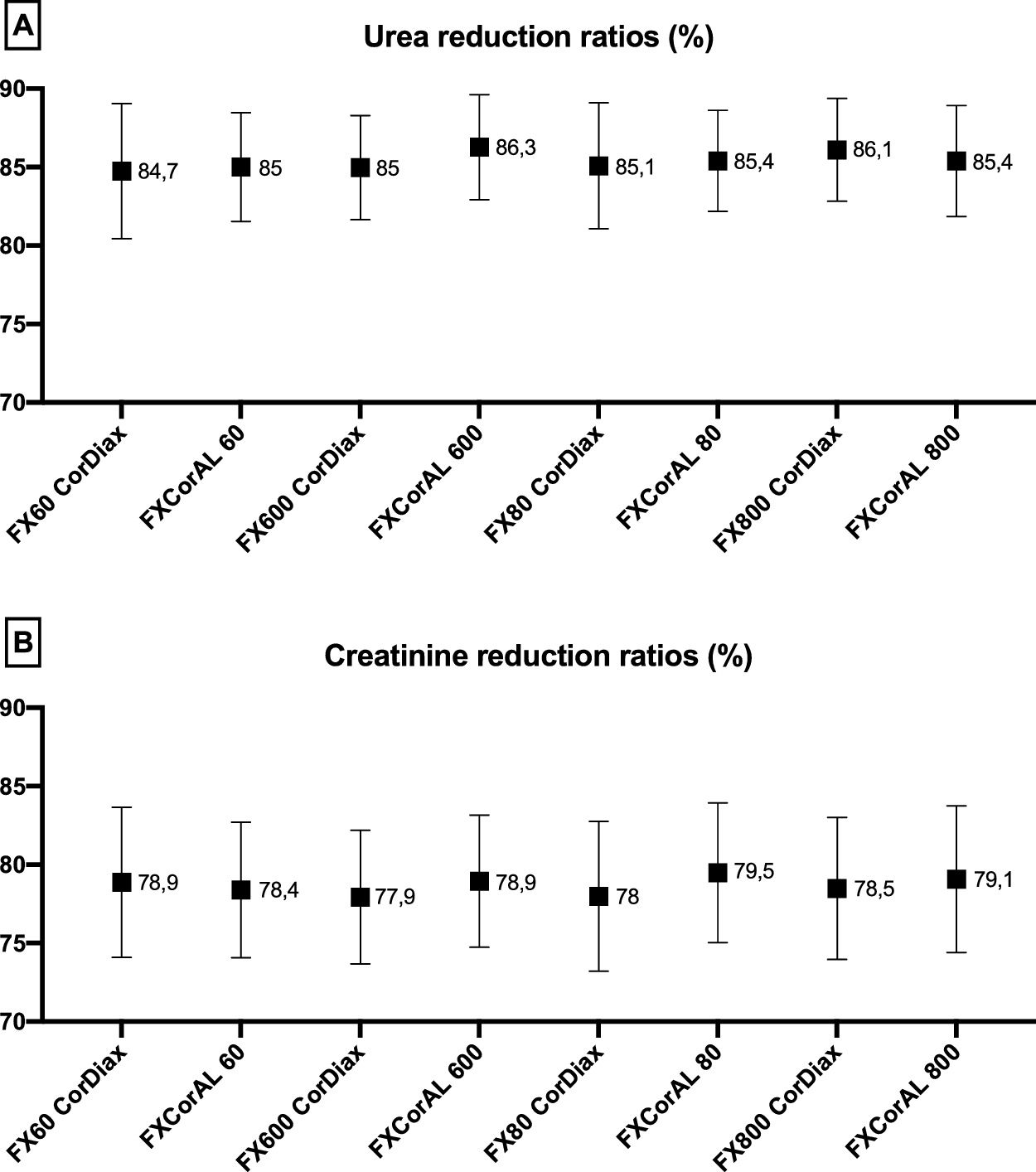
The dialysis dose, measured with ionic dialysance, showed that Kt was similar in all study situations, between 70 and 74 L, with very few statistically significant differences (Table 2). Similar urea and creatinine RRs were observed in all eight dialyzers evaluated (shown in Fig. 1).
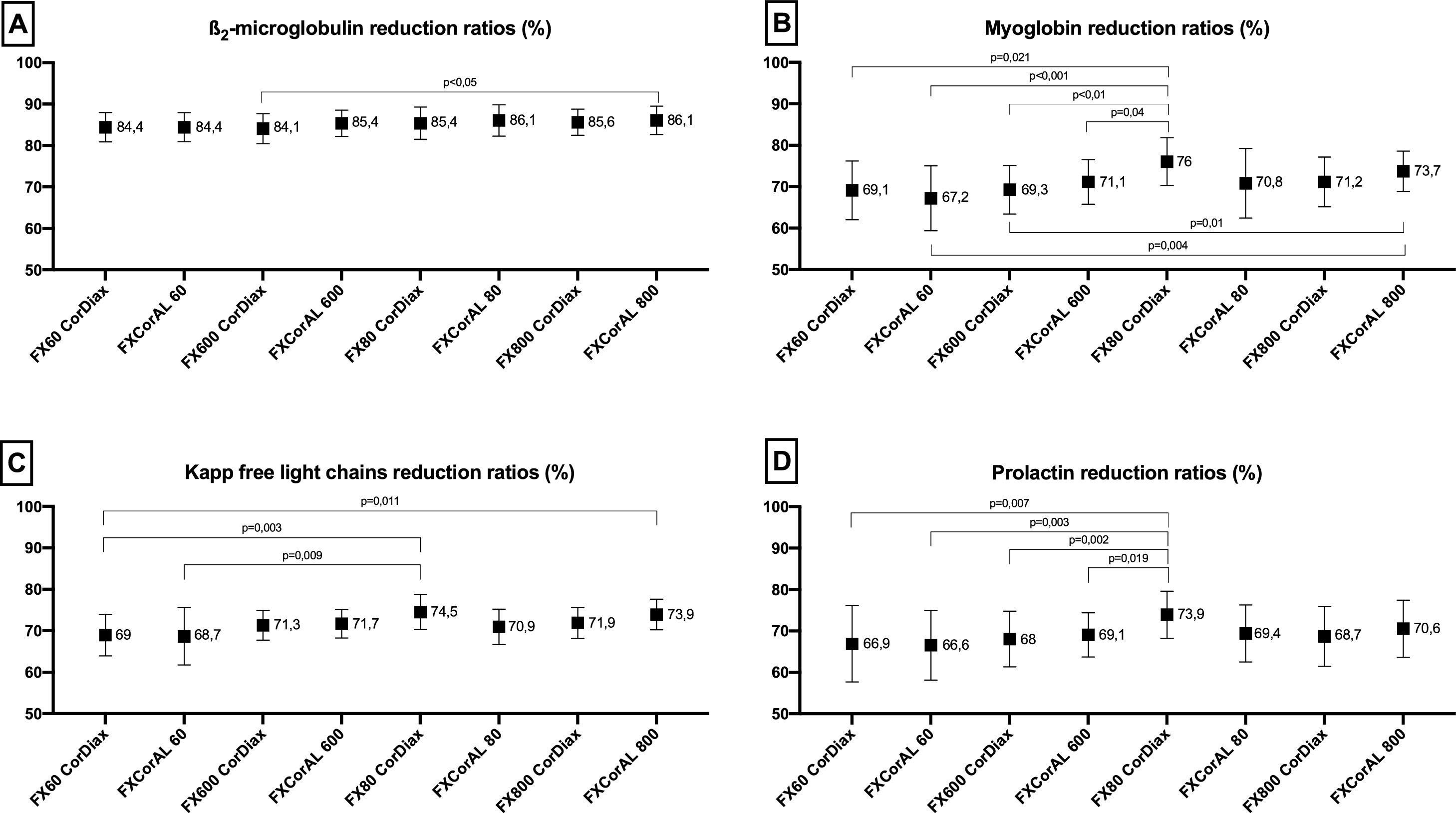
Medium-sized moleculesThe average values of β2-microglobulin RRs were between 84% and 86% for all treatments. The trend was slightly superior for the CorAL series compared with the FX CorDiax series but statistically significant difference were only observed between FX CorAL 80 and FX CorAL 800 versus FX600 CorDiax (shown in Fig. 2).
The average values of myoglobin RRs ranged between 67% and 76% for all treatments; the myoglobin RRs obtained with FX80 CorDiax were significantly higher than those with FX60 CorDiax, FX CorAL 60, FX600 CorDiax, and FX CorAL 600 (shown in Fig. 2). Additionally, the FX CorAL 800 dialyzer was slightly superior to the FX CorAL 60.
The average values of κFLC RRs varied between 68% and 75% for all treatments; only the κFLC RRs obtained with the FX80 CorDiax dialyzer were significantly higher than those obtained with the FX60 CorDiax and FX CorAL 60 dialyzers (shown in Fig. 2).
The average values of prolactin RRs ranged from 66% to 74% for all treatments; only those obtained with the FX80 CorDiax dialyzer were significantly higher than those obtained with the FX60 CorDiax, FX CorAL 60, FX600 CorDiax, and FX CorAL 80 dialyzers (shown in Fig. 2).
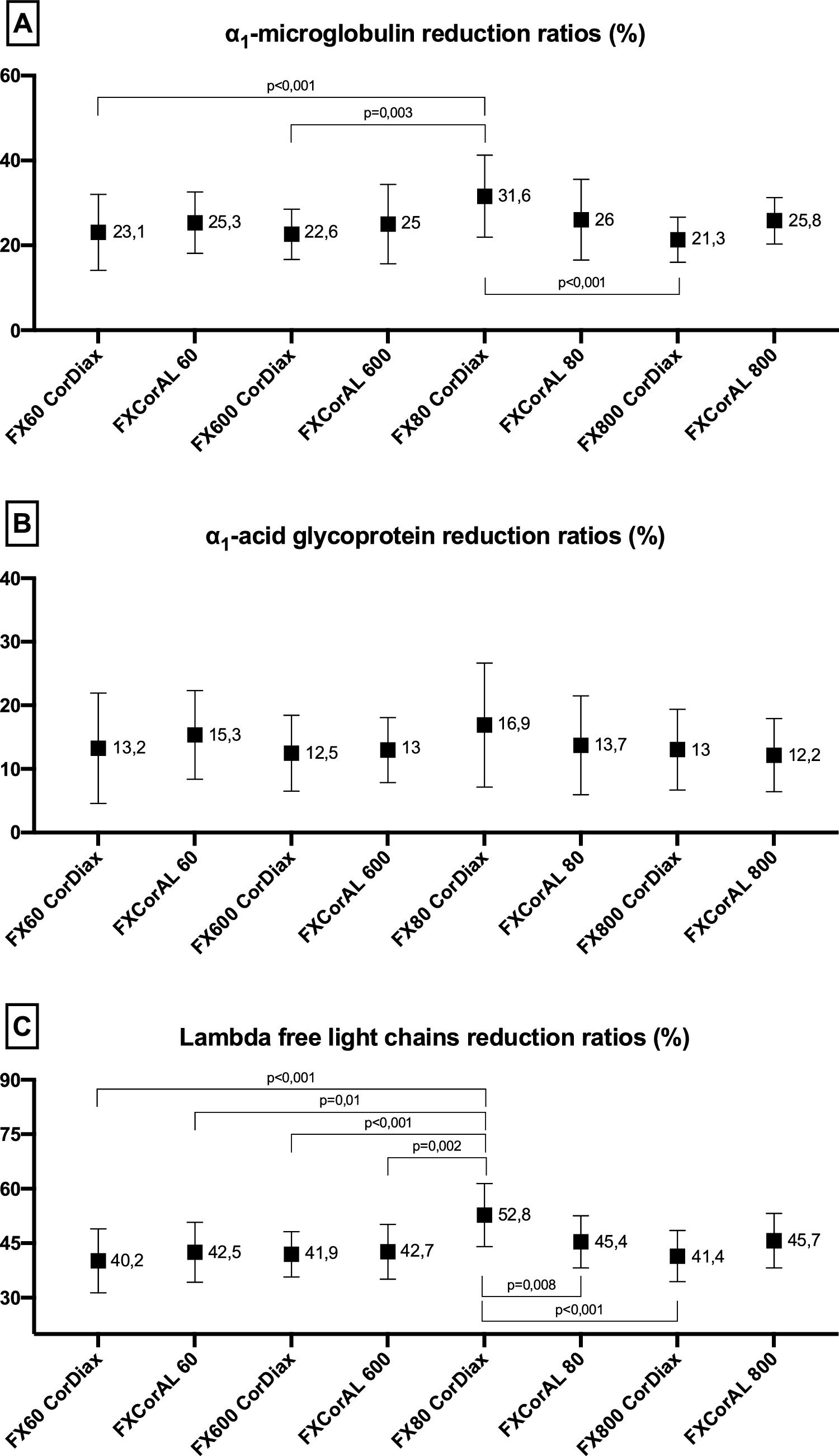
The average values of α1-microglobulin RRs were between 22% and 32% in the treatments, and only the FX80 CorDiax dialyzer was significantly superior to the FX60 CorDiax and FX600 CorDiax dialyzers (shown in Fig. 3).
The average values of α1-acid glycoprotein RRs varied between 12% and 17% for all treatments, with no statistically significant differences (shown in Fig. 3).
The average values of λFLC RRs ranged between 40% and 53% for all treatments; again, only the FX80 CorDiax dialyzer was significantly superior to the other evaluated dialyzers (shown in Fig. 3).
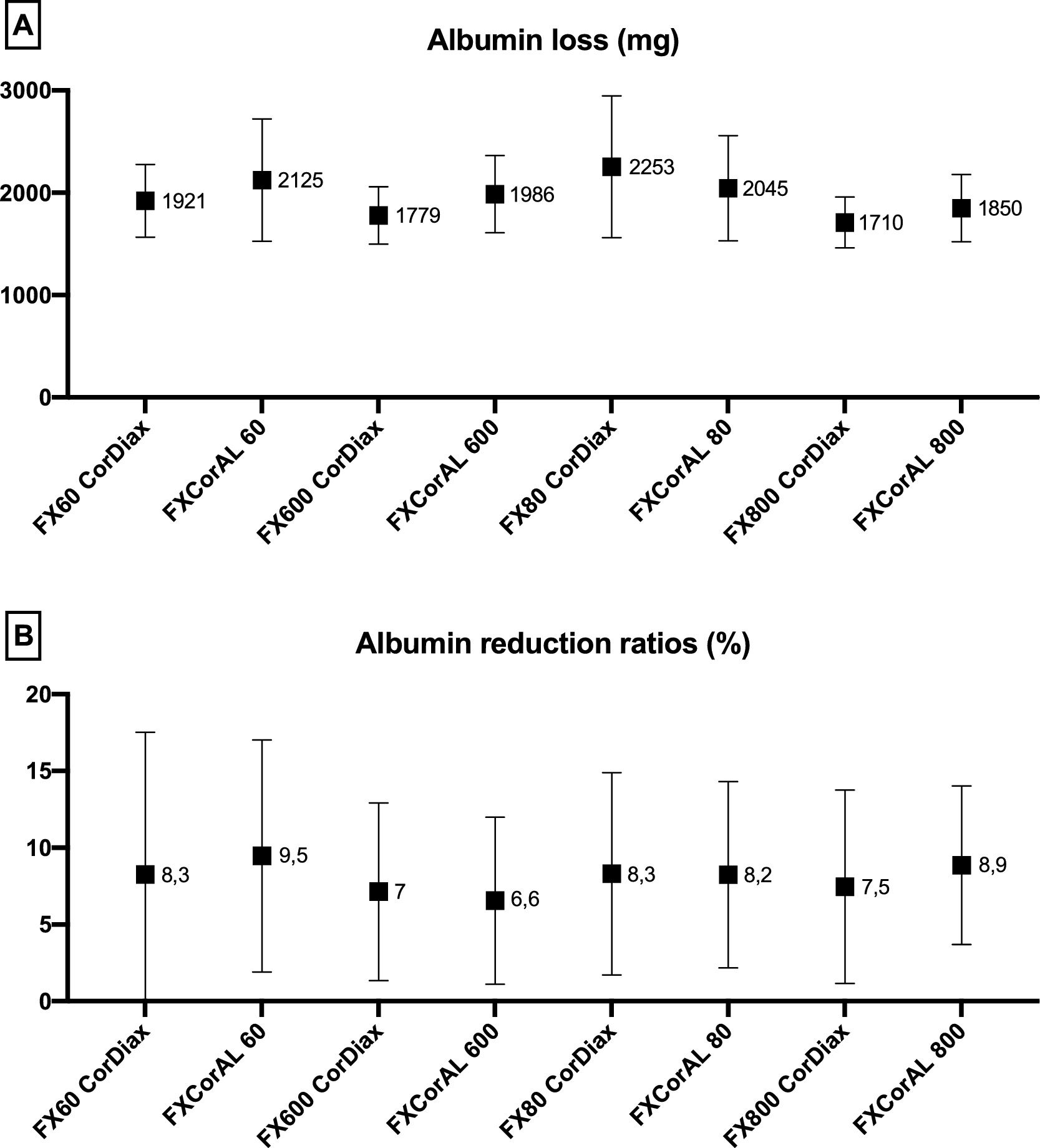
Albumin loss in blood and dialysateThe mean albumin RR was less than 10% in all study situations, with no statistically significant differences (shown in Fig. 4).
The mean amount of dialysate albumin loss was less than 2.5 g in all study situations, ranging between 1.7 and 2.3 g, with a trend to lower albumin loss in dialyzers with a higher internal diameter (shown in Fig. 4).
Global removal scoreThe average GRS ranged between 55% and 60%. Only the GRS obtained with the FX80 CorDiax dialyzer was significantly higher than those obtained with FX60 CorDiax, FX CorAL 60, FX600 CorDiax, FX CorAL 600, and FX CorAL 80 dialyzers (shown in Fig. 5).
DiscussionThe present study is, to our knowledge, the first to analyse in vivo data, with a wide range of molecular weight molecules, on the performance of the new generation helixone dialyzer series versus the previous earlier helixone generation in post-dilution HDF treatments. This study demonstrates that this new dialyzer series achieved similar efficacy and albumin losses to the earlier helixone series, without showing major changes to the variations in membrane surface or capillary fibres’ internal diameter but with the potential advantage of avoiding intolerance reactions to synthetic dialyzers.
Post-dilution HDF has progressively evolved and is now considered a safe, fully established treatment offering multiple clinical advantages. Currently, it is widely regarded as the most effective option for dialysis treatment in terms of efficacy, safety, and survival rates.18–22 This treatment modality mostly uses synthetic dialyzers, although the small percentage of patients with reactions to synthetic dialyzers has prompted the nephrology community to explore alternatives for these patients, such as CTA dialyzers.6–8 One of the most exciting developments in the field has been the introduction of the new generation of helixone dialyzers, which have modified the design of the dialysis membrane to prevent adverse side effects.10–13 In our study, we observed no reactions with this series of dialyzers; however, it is important to note that we only included patients without previous reactions to helixone. Given the scarcity of published studies reporting this type of reaction,14,15 studies with longer observational periods and a larger number of patients are needed to confirm that the modifications introduced in the CorAL series effectively prevent this complication.
In vitro studies have revealed substantial differences in the PVP content and elution, as well as in secondary membrane formation among different dialyzer membranes.10–23 These alterations support the formation of a more robust hydrophilic layer and enhance the hemocompatibility of polysulfone membranes by improving their antifouling ability, resulting in less protein adsorption and lower coagulation activation.10–13 There are other membranes similar to the hydro helixone in terms of hydrophilicity, such as the NV dialyzer (Toray), which introduces a new hydrophilic polymer onto the inner surface of the hollow fiber membrane composed of polysulfone and PVP, inhibiting platelet adhesion, improving blood pressure drop, and reducing inflammation.24 Another notable feature of the FX CorAL dialyzer, manufactured using helixone, is its bisphenol-containing material. However, bisphenols A and S were not detected in the extractable and leachable of this filter, unlike other polysulfone and polyethersulfone membranes.25 This issue, therefore, renders it comparable to CTA dialyzers, which are bisphenols A- and S-free due to their cellulose-based membranes and bisphenol-free polypropylene housing.25
The previous nanotechnology-manufactured helixone generation (FX CorDiax series) was specifically designed for high-volume convective therapies and has demonstrated its superiority over the previous FX series.26 The efficiency observed in this study replicates the results obtained in previous studies with FX 80 CorDiax under similar conditions,27,28 ensuring a comparison with the four dialyzers of the new CorAL series evaluated with minor variations in surface and internal diameter.
There are few published in vivo efficacy studies of the new generation helixone dialyzer series. In 2021, a randomised clinical trial by Ehlerding et al.,14 using FX CorAL 600 with a Qb of 307 ml/min and a duration of 272 min, obtained a replacement volume of 17.4 L and reached a 70% RR of β2-microglobulin. The PERFORM study,15 using FX CorAL 600 with a Qb of 330 ml/min and a duration of 273 min, obtained a replacement volume of 25.4 L and an RR of 85% for β2-microglobulin and 61% for myoglobin. The present study, also using FX CorAL 600 with a Qb of 450 ml/min and a duration of 284 min, obtained a replacement volume of 32.8 L, reaching RRs of 85% for β2-microglobulin and 71% for myoglobin, reaffirming the importance of convective volume to enhance efficacy. Furthermore, our study expanded the removal capacity by evaluating molecules ranging between 22 and 45 kDa.
As in a previous study,29 variations in the internal diameter (FX60 CorDiax vs. FX600 CorDiax and FX80 CorDiax vs. FX800 CorDiax) did not significantly affect the convective volume or removal efficiency. Similar results were also obtained with the new CorAL series, once again raising doubts about the importance of this manufacturing change in achieving a greater convective volume. In addition, as we observed in a previous study,30 the variations in the membrane surface did not lead to significant changes in the convective volume or purification efficiency. When the ultrafiltration coefficient (UFC) exceeds 45 ml/h/mmHg, differences in convective volume and clearance capacity become insignificant. Therefore, it seems reasonable to question the advantage of dialyzers with a larger or smaller surface area when UFCs exceed 45 ml/h/mmHg, since they may obtain an ultrafiltration flow greater than 8.1 L/h or 135 ml/min, which is higher than the amount usually used.
In this study, all eight dialyzers used in post-dilution HDF reached an adequate convective volume and showed a dialysate albumin loss of less than 3 g. These results strongly support their suitability for post-dilution HDF. However, the new CorAL dialyzers series does not surpass the previous FX CorDiax generation in terms of efficiency or albumin loss. Out of the eight dialyzers evaluated, only the FX80 CorDiax dialyzer was slightly superior to the others and, consequently, demonstrated the highest performance. Therefore, if the introduction of the new CorAL series in post-dilution HDF mode shows similar efficacy results to the previous generation, it could serve as a viable alternative to avoid the low incidence of adverse reactions.
However, a limitation of the present study is that it was not specifically designed to determine whether the use of synthetic dialyzers reduces adverse reactions. This is because it was conducted with patients who were already known not to have adverse reactions to polysulfone. Future studies including a large number of incident patients and a reasonable follow-up period are needed to confirm the absence of these adverse reactions.
In conclusion, the results of this study highlight the potential of the new generation helixone dialyzers, the CorAL series. These dialyzers incorporate a modification to the polysulfone membrane by stabilising the polyvinylpyrrolidone on the blood-side surface of the membrane. This modification is promising as it may help prevent adverse reactions while maintaining the effectiveness and albumin loss achieved with the previous generation. Given the multitude of dialysers available on the market, including variations from the same manufacturer, studies such as the present work are needed to allow assessment and comparisons between models, including factors such as the influence of variations in surface size or internal diameter, which can aid their selection and prescription by nephrologists.
Conflict of interest statementThe authors declare no financial support for the project. F.M. has received consultancy fees and lecture fees from Baxter, Fresenius Medical Care, Medtronic, Nipro and Toray. The other authors have no conflicts of interest to declare.
We would like to express our gratitude to all participating patients, as well as to all the staff of the Dialysis Section of our Hospital for their collaboration in this study.