Autosomal dominant polycystic dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disease. Estimates of prevalence vary depending on the sample, ranging from 1 in 500 to 1 in 2000 persons. It is the fourth leading cause of end-stage renal disease (ESRD), accounting for 6–10 % of the population on renal replacement therapy (RRT).1
It is characterized by the gradual appearance of renal cysts and progression to ESRD, as well as by the association of systemic manifestations such as arterial hypertension, intracranial aneurysms and polycystic liver disease. Cyst growth and renal size are related, in addition to progression to ESRD, with symptoms associated with the abdominal compartment syndrome and compression of other structures, generating satiety, pain and intestinal pseudo-obstruction.2
Knowledge of this disease has been progressing in recent decades. Since the main ADPKD-related genes, PKD1 and PKD2, were identified in the 1990s,3,4 a close genotype-phenotype correlation has been established, as well as the identification of those genes that confer a higher risk of progression. In addition, new genes related with cyst generation (GANAB, DNAJB11 and ALG9)5–7 have been discovered, as well as some of the metabolic pathways involved in cystogenesis, such as the overexpression of cyclic adenosine monophosphate (cAMP). This has led to the development of drugs such as tolvaptan, a vasopressin V2 receptor antagonist, which reduces cAMP levels in the collecting tubule and distal nephron, thus reducing fluid secretion into the cyst and cell proliferation, leading to a slowing of cystic development and disease progression.8
Currently, the indication for tolvaptan is limited to the objective of slowing the progression of renal disease and it is restricted to adult patients with CKD stages 1–4 and evidence of rapid progression.1,8,9 However, there are other potential benefits, such as the case we report below.
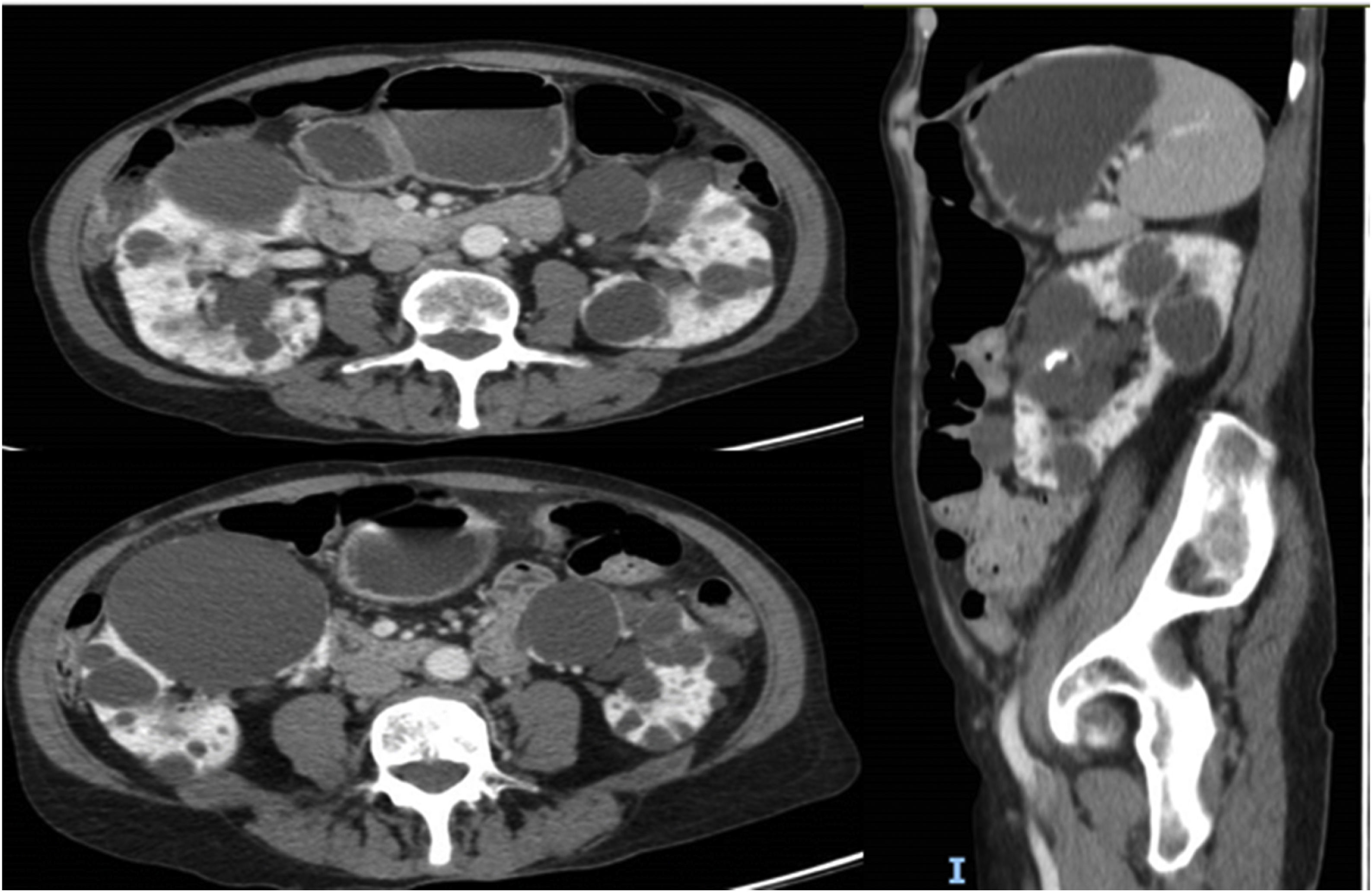
This is a 69-year-old woman with a history of hypertension, axonal sensory polyneuropathy due to Cerebellar Ataxia with Neuropathy and Vestibular Areflexia Syndrome (CANVAS) and ADPKD diagnosed at 40 years of age, with subsequent confirmation of PKD2 mutation. Throughout the course of the disease the following incidences stand out: evacuating puncture of the cysts compressing the renal artery approximately 30 years ago and several episodes of intestinal pseudo-obstruction justified by an acquired megacolon secondary to intestinal compression by the renal cysts (Fig. 1) that required the puncture and sclerosis of a large dominant cyst in the last year. Despite this, the patient continued to have difficulty swallowing and abdominal pain. At that time renal function remained normal and she did not present criteria for rapid progression, precluding the use of tolvaptan according to current indications. However, in September 2022, in agreement with the patient, we decided to trial compassionate treatment with tolvaptan, with the aim of decreasing abdominal volume, as well as better pain control. She was prescribed 45 mg of tolvaptan in the morning and 15 mg eight hours later.
During follow-up in November and December 2022 the patient reported feeling better, with a decrease in the feeling of abdominal pressure, with no new episodes of intestinal pseudo-obstruction and decreased satiety and abdominal pain, which has allowed her to return to sports, travel and gain 1−2 kg of weight. The patient reports good tolerance to the aquaretic effects and no biochemical alterations in the analytical controls.
The effect of "emptying" the renal cysts was rapid, as usually occurs in the first 3–4 weeks after starting treatment with tolvaptan,10 a circumstance that could explain the rapid improvement of the patient.
In conclusion, ADPKD is an entity characterized by the appearance of renal cysts, progression to ESRD, associated systemic manifestations and symptomatology secondary to compression of structures by renal cysts. At present, the indications for the use of tolvaptan are restricted to slowing renal progression; however, the benefits may also include improvement of digestive symptoms, as in our case.