Hemodiafiltration with endogenous reinfusion of the ultrafiltrate (HFR) is a dialysis technique characterized by a resin cartridge with adsorptive properties that combines the mechanisms of diffusion, convection, and adsorption in a single therapeutic regimen. After nearly 20 years of clinical experience with HFR, this article reviews the accumulated evidence with this technique, considering whether adsorption reduction, as a third purification mechanism, should be the next step in the treatment of hemodialysis patients. HFR, beyond producing an extensive removal of uremic toxins, has demonstrated to reduce the loss of nutrients and other physiological components during the dialysis session as compared to online hemodiafiltration, ameliorating the inflammatory state and oxidative stress in this population. In addition to its ease of use, the technique is also highly biocompatible and can be used in patients with a compromised vascular access. Based on these observations, HFR appears to be an especially useful therapy for high-comorbidity patients, including those with frailty, malnutrition, or cardiovascular disease. In this review, we, as a consensus panel of nephrologists experienced with HFR, survey existing literature and summarize our views on when to use this technique, which patients may be best suited for HFR, and how to effectively prescribe and monitor this modality of dialysis in daily clinical practice.
La hemodiafiltración con reinfusión endógena del ultrafiltrado (HFR) es una técnica de diálisis caracterizada por un cartucho de resina con poder adsorbente que combina los mecanismos difusión, convección y adsorción en un solo esquema terapéutico. Después de cerca de 20 años de experiencia clínica con HFR, el presente artículo revisa la evidencia acumulada con esta técnica, planteando si la adición de la adsorción, como tercer mecanismo depurativo, debería ser el siguiente paso en el tratamiento de los pacientes en hemodiálisis. La HFR, a pesar de producir una extensa eliminación de toxinas urémicas, ha demostrado reducir la pérdida de nutrientes y componentes fisiológicos durante la sesión de diálisis frente a la hemodiafiltración on-line, mitigando el estado inflamatorio y el estrés oxidativo en esta población. Además de su facilidad de uso, la técnica también es altamente biocompatible y puede utilizarse en situaciones de un acceso vascular comprometido. En base a estas observaciones, la HFR parece ser una técnica especialmente útil para pacientes con elevada comorbilidad, incluyendo aquellos con fragilidad, desnutrición o enfermedad cardiovascular. En esta revisión, como panel de consenso de nefrólogos con experiencia clínica en HFR, examinamos la literatura existente y resumimos nuestros puntos de vista sobre cómo usar esta técnica, qué perfil de paciente puede ser más adecuado para la HFR, y cómo prescribir y monitorizar de manera práctica esta modalidad de diálisis.
The ultimate goal of dialysis is to increase the survival of patients on renal replacement therapy, improving their quality of life and minimizing complications related to the dialysis technique itself.1 To achieve this goal, dialysis prescriptions have been modified in recent decades, improving the biocompatibility of dialysis membranes and increasing the efficiency of removal of small and medium molecular weight uremic solutes by optimizing diffusion and convection. Although on-line hemodiafiltration (OL-HDF) with high convective volume has undoubtedly been a breakthrough in reducing morbidity and mortality in hemodialysis (HD) patients,2,3 the quality of life and survival of the dialysis population remains low.4,5
Have we reached the maximum dialysis efficiency that we can obtain by manipulating pore size alone? Time for adsorptionAlthough the development of high-flux membranes, including those of medium cut-off, has improved the clearance of medium size molecules,6 molecules above 70 kDa are not removable by filtration or diffusion.7,8 Similarly, dialytic clearance of p-cresyl sulfate (pCS), and other protein-bound uremic toxins (PBUTs), is poor with diffusive treatment and limited with OL-HDF,9 and only a few studies have explored how to improve their clearance by other extracorporeal therapies.10–14 This is of particular relevance, given that PBUTs are highly correlated with cardiovascular complications in patients with chronic kidney disease (CKD) on HD,11–13,15,16 being the leading cause of death in this population.17,18 At this point, it seems very difficult to improve the clearance of uremic toxins by simply manipulating pore size without paying the price of increased loss of albumin and other beneficial substances such as amino acids, vitamins, hormones, growth and coagulation factors.7,19 In this context, adsorption, based on mass separation by a solid agent called sorbent,20 could be an interesting strategy to remove PBUTs and molecules larger than the limit imposed by the "albumin size".7,8,15,21
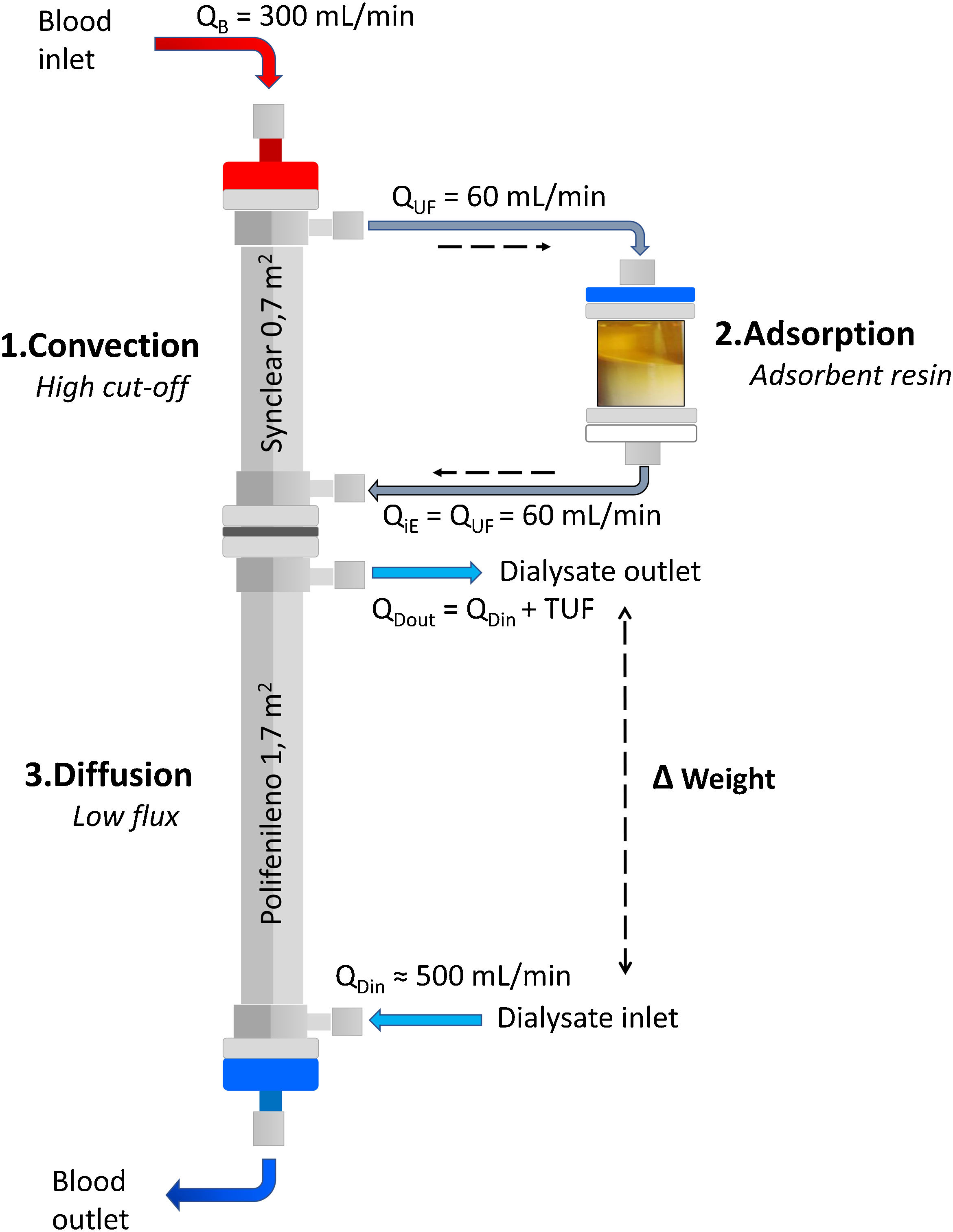
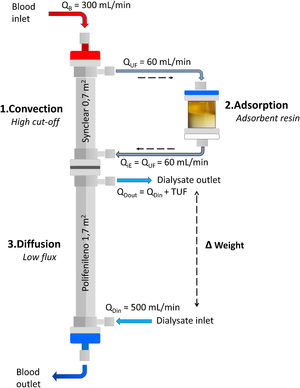
Adsorption: what techniques are available?Beyond the adsorption capacity of some membranes such as polymethylmethacrylate (PMMA) membranes,22–24 the two properly adsorptive techniques available for CKD patients on HD are hemoperfusion (HP) and HDF with on-line ultrafiltrate regeneration (HFR, Hemo-Filtrate-Reinfusion).7,25,26 Although both techniques use a cartridge with an adsorbent material, HP requires the sorbent to be highly biocompatible, as there is direct contact of the adsorbent with blood cells, which historically has limited its use.26–28 In contrast, HFR uses a dual-chamber dialyzer plus an adsorbent cartridge (Fig. 1), where the patient's own ultrafiltrated fluid is regenerated and subsequently re-infused into the patient's blood, avoiding direct contact between the blood cells and the adsorbent material, thus overcoming the tolerability and problems of blood coagulation initially observed with HP.29
The purpose of this review is twofold: firstly, to analyze, synthesize and discuss the evidence accumulated in recent years on the use of HFR, and secondly, as a consensus panel of nephrologists with clinical experience with this dialysis modality, to summarize our points of view on how to use this technique and in which patient profile it is especially indicated, offering the clinician a practical vision when prescribing and monitoring this technique in his or her clinical activity.
What HFR is and how it worksHFR concept: combining diffusion, convection and adsorptionHFR is a renal replacement therapy that uses a double chamber dialyzer plus an adsorptive cartridge, combining in a single therapeutic scheme the mechanisms of convection, adsorption and diffusion (Fig. 1).7 In the first segment, the blood passes through a very high permeability dialyzer where it is ultrafiltrated by means of a pressure gradient (convective phase), generating an endogenous ultrafiltrate (or plasma water) which is then regenerated by passing through the cartridge (adsorptive phase) where an adsorbent (carbon or resin) retains different toxins and cytokines. In this convection/adsorption stage there is no net loss of fluid or albumin, the clean ultrafiltrate is being re-infused back into the blood to undergo conventional HD. The regeneration of large volumes of ultrafiltrate plasma obtained in the first HFR filter allows a large volume of plasma water to be treated, and at the same time, enables the return of many valuable substances such as hormones, small peptides and many vitamins.25 The blood with the clean ultrafiltrate then passes through a low-flux filter (diffusive phase) which allows the removal of small molecules, as well as the ultrafiltration necessary to achieve an adequate water balance in the patient.30
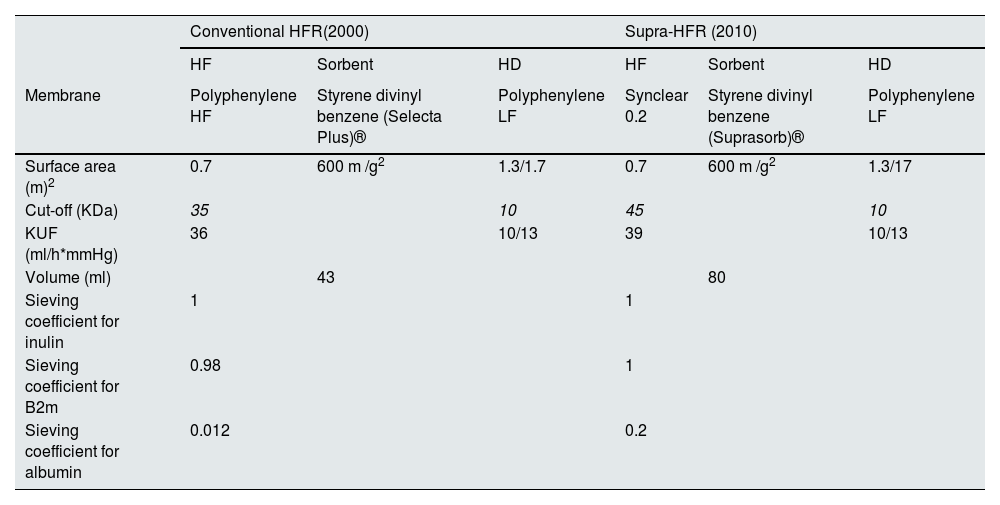
Historical evolution of HFRHFR evolved from HDF with separate diffusion and convection (PFD, Paired Filtration-Dialysis) using a hydraulically separated dual-chamber filter of different permeability,31 to which a carbon adsorbent cartridge was added.32,33 From the beginning, HFR proved to be a well-tolerated and highly biocompatible technique, with a lower inflammatory response and the ability to scavenge medium-sized molecules such as β 2-microglobulin (β 2-m).34,35 The development of HFR was a complex process that underwent numerous evolutionary changes to find a suitable adsorbent that would retain a wide variety of mediators and at the same time be suitable for extracorporeal treatment, with HFR-supra being the most effective and current version (Table 1).36–46
Characteristics and historical evolution of the various HFR modalities.40.
| Conventional HFR(2000) | Supra-HFR (2010) | |||||
|---|---|---|---|---|---|---|
| HF | Sorbent | HD | HF | Sorbent | HD | |
| Membrane | Polyphenylene HF | Styrene divinyl benzene (Selecta Plus)® | Polyphenylene LF | Synclear 0.2 | Styrene divinyl benzene (Suprasorb)® | Polyphenylene LF |
| Surface area (m)2 | 0.7 | 600 m /g2 | 1.3/1.7 | 0.7 | 600 m /g2 | 1.3/17 |
| Cut-off (KDa) | 35 | 10 | 45 | 10 | ||
| KUF (ml/h*mmHg) | 36 | 10/13 | 39 | 10/13 | ||
| Volume (ml) | 43 | 80 | ||||
| Sieving coefficient for inulin | 1 | 1 | ||||
| Sieving coefficient for B2m | 0.98 | 1 | ||||
| Sieving coefficient for albumin | 0.012 | 0.2 | ||||
Conventional HFR emerged in 2000 with the replacement of the carbon-only sorbent used in HDF with separate diffusion and convection (PFD, Paired Filtration-Dialysis), by a cartridge with higher adsorptive capacity (Selecta Plus®, Bellco). That cartridge contained two adsorbent materials: white spherical particles of macroporous, insoluble resin (20 ml) and uncoated activated carbon (240 ml).36 Due to the ultrafiltered nature of the regenerated fluid, HFR originally allowed the use of carbon adsorbents in an "uncoated form", of higher adsorptive capacity, overcoming the bioincompatibility and blood coagulation problems initially observed with HP, which necessitated the use of carbons in "coated" form, more biocompatible, but with a lower adsorption capacity.25 The resin, of a nonionic and hydrophobic nature, allowed the adsorption of medium and high molecular weight solutes, includingβ 2-m, proinflammatory cytokines, and advanced glycation end-products (AGEs); whereas the carbon was able to adsorb creatinine and uric acid and other low molecular weight solutes.36–39 Finally, since mid-2010 we have a new improved HFR technique, called Supra-HFR, in which the high-flux polyphenylene convective membrane (with a cut-off of 35 kDa) has been replaced by a super-high-flux membrane (Synclear 0.2®, Bellco/Medtronic), with a cut-off >55 kDa and an albumin screening coefficient of 0.2 (similar to high cut-off filters),40 and in which a new cartridge (Suprasorb®, Bellco/Medtronic) is used, containing twice as much resin as the Selecta cartridge® used in conventional HFR,41 further improving the convective and adsorptive capacities of the technique.42–46
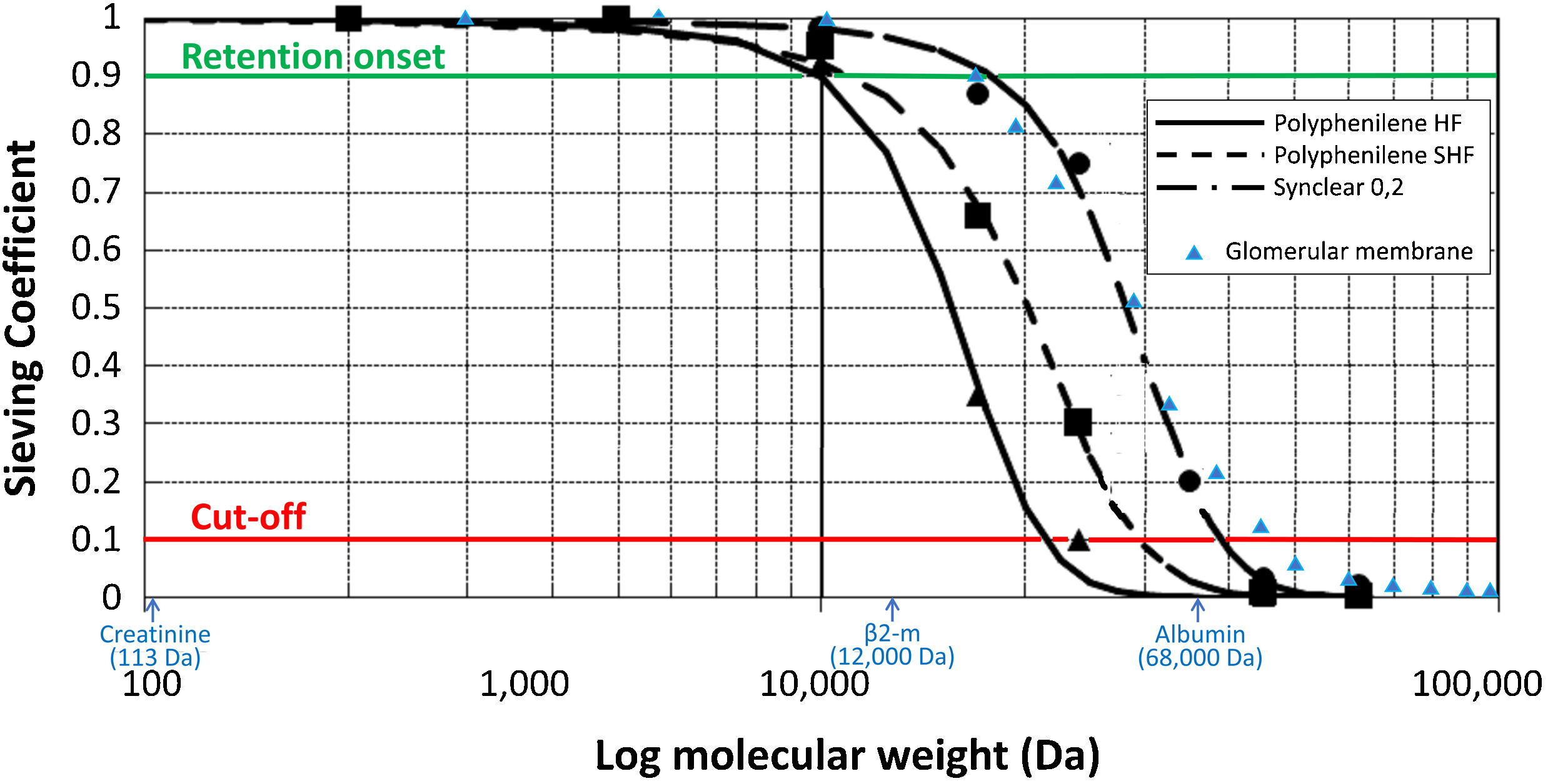
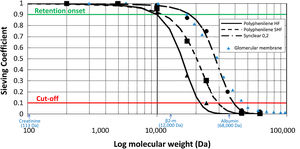
Endogenous reinfusion, a unique feature of HFR, has made it possible to progressively increase the permeability of the membrane used in this first convective phase beyond the limit imposed for albumin (Table 1),22,40,47–49 since the resin does not adsorb the albumin present in the ultrafiltrate, and therefore it will be reinfused into the patient's blood (Fig. 1). Supra-HFR uses in this first convective phase a 0.7 m2 super-high-flux surface membrane (Synclear 0.2®, Bellco/Medtronic), characterized by a high cut-off (>55,000 Da), much higher than the other high-flux membranes commonly used in OL-HDF and with a screening coefficient that much more closely resembles that of the glomerulus (Fig. 2).40 Compared to the high-flux polyphenylene membrane used in traditional HFR, the ultrafiltrate contains significantly higher concentrations of medium-high weight molecules (which may be subsequently adsorbed) such as IL-6 (24,700 Da) orα 1-glycoprotein (43,500 Da) and, obviously, albumin (66,500 Da).22,48,49 The ultrafiltrate is conducted from this hemofilter to an 80 g neutral styrene resin with an adsorption area of 35,000 m2.50
Schematic representation of the sieving curves of the different membranes used in HFR (Polyphenylene HF, Polyphenylene SHF, Synclear 0.2). The points on the curve where the sieving coefficient is 0.1 and 0.9 for a given molecular weight determine the cut-off and retention onset for each membrane, respectively. While the cut-off reflects the molecular weight at which 90% of the solutes will be retained, the retention onset indicates the molecular weight at which more than 10% of the solutes will be retained. As can be seen in the graph, Synclear 0.2 differs greatly from Polyphenylene HF and SHF in both its retention onset and cut-off. This translates into a higher screening coefficient of the Synclair membrane and thus a higher clearance of medium molecular weight substances and protein-bound toxins once they pass through the resin. Adapted from: Grandi et al.,40 Boschetti-de-Fierro et al.98 and from Garcia-Prieto et al.99 Data referring to the glomerular membrane (triangles in blue) have been added for comparison according to data collected by Axelsson et al.100 β2-m: β2-microglobulin.
The Supra-HFR cartridge consists of a hydrophobic styrene resin with single particle diameter granules or beads of approximately 100μ m and internal pores measuring between 20 and 50 Å (macropores). The chemical nature of the resin, coupled with its large adsorbent surface area relative to its volume (approximately 700 m2/g resin), gives it a high capacity to adsorb various uremic toxins in the medium- to high-molecular-weight range of molecules, includingβ 2-m, homocysteine, parathyroid hormone, FGF23 and various cytokines, such as IL-6, TNF-alpha and TGF-alpha.20,36,41–44,51–53 Small molecules such as creatinine and uric acid are also retained in the cartridge, while urea, phosphorus and potassium are reinfused unchanged in the regenerated ultrafiltrate and subsequently dialyzed in the diffusive filter. Similarly, calcium, bicarbonate, albumin and various trace elements such as manganese, selenium or zinc are not modified as they pass through the resin.25
Adsorption is a physicochemical phenomenon that involves the interaction of a molecule with the resin surface through hydrophobic, electrostatic or ionic attractions.54 Although no theory for predicting adsorption has been universally accepted, there are several factors that influence the adsorptive capacity of the resin, such as the packing density of the adsorbent, the length and inner diameter of the cartridge, or the distance between the granules, the discussion of this features are beyond the scope of this manuscript, and have recently been reviewed.7,55 However, for the clinician it is of interest to understand that the final kinetics of adsorptive transport depends on the initial solute concentration and, especially, the ultrafiltrate flux (QUF) through the cartridge. While some molecules adsorb well over a wide range of QUF, others may have a significant decrease in their adsorption as QUF through the cartridge increases, as the time of interaction between the resin and plasma water decreases, and thus the adsorptive efficiency of the technique is reduced.55 In order to optimize the adsorptive mechanism, it is recommended a QUF of around 60−70 ml/min in Supra-HFR, which translates into approximately 14−17 l of endogenous reinfusion volume in a typical 4 h50 session. The Flexya dialysis monitor™ (Bellco/Medtronic) that performs HFR has a software program that automatically determines the best QUF based initially on the maximum linear velocity (the flow rate that provides the best adsorption). The monitor also automatically determines the patient's hematocrit and transmembrane pressure to adjust the QUF according to these parameters, so that the QUF is usually higher at the beginning of the dialysis session than at the end.29,50
Endogenous re-infusion and diffusionAfter adsorption, the toxin-free ultrafiltrate is reinfused into the patient's blood which, in turn, passes through the second filter chamber, where conventional HD will take place (Fig. 1). This is a 1.7 m2 low-flow polyphenylene surface area filter (Kuf = 13 ml/h/mmHg) where small molecule removal and correction of metabolic acidosis takes place by diffusive mechanism.47 It is at this level that the net water balance of the technique is also produced by ultrafiltration to achieve the patient's target dry weight.29,47,50
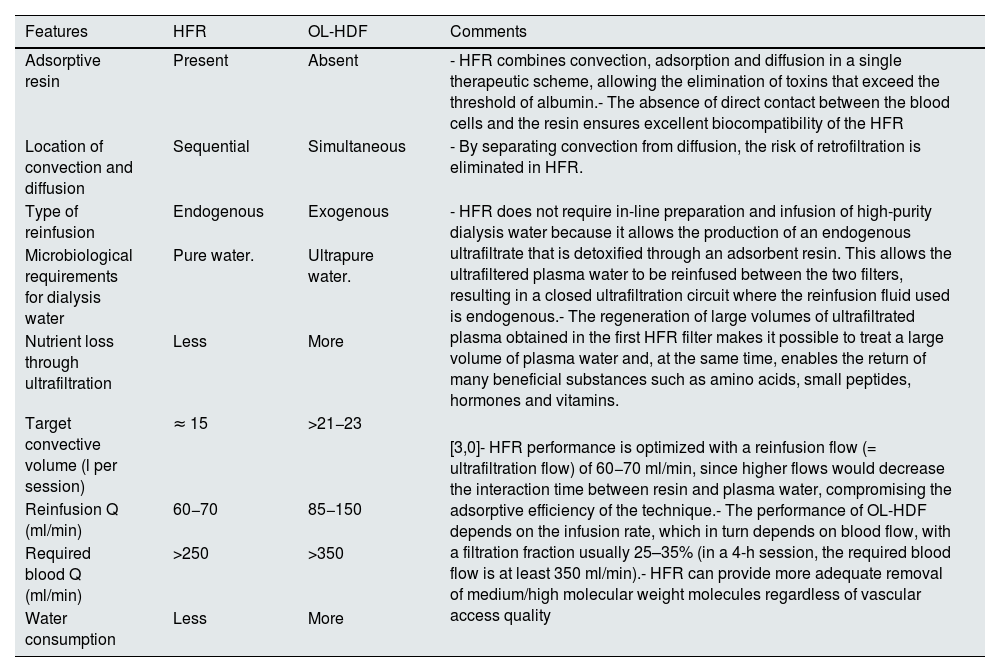
Differential characteristics of HFR with respect to OL-HDFAlthough both techniques combine diffusion and convection in order to improve the purification of medium and large molecules, there are notable differences between the two techniques beyond the adsorptive capacity associated with HFR (Table 2). First, the double chamber of the HFR filter allows complete separation of convection and diffusion,7 so that the same volume of ultrafiltrate obtained in the first chamber is returned as replacement fluid to the blood circuit just before the second chamber of the filter, allowing lower dialysis water consumption and zero risk of back-filtration.25 Secondly, the regeneration of ultrafiltrate after its passage through the adsorber cartridge minimizes the risk of loss of albumin and of other nutrients that has been described with OL-HDF.56,57 Third, in HFR the reinfusion volume delivered is about 15 L per session,50 whereas in OL-HDF the convective target is at least 21−23 L per session in postdilution,58 the latter being an unfeasible target in patients with suboptimal vascular access.58–60 Finally, while the performance of OL-HDF benefits from an increase in infusion flow (QI) by increasing QB,61 in the case of HFR the excessive increase in endogenous QUF facilitated by an increase in QB may be counterproductive by decreasing the adsorptive efficacy of the technique.44,55,62,63 In conclusion, HFR allows more adequate removal of medium molecular weight molecules than OL-HDF in comorbid patients with limited vascular access who do not reach a high convective volume.50,59
Differential characteristics between HFR and OL-HDF.
| Features | HFR | OL-HDF | Comments |
|---|---|---|---|
| Adsorptive resin | Present | Absent | - HFR combines convection, adsorption and diffusion in a single therapeutic scheme, allowing the elimination of toxins that exceed the threshold of albumin.- The absence of direct contact between the blood cells and the resin ensures excellent biocompatibility of the HFR |
| Location of convection and diffusion | Sequential | Simultaneous | - By separating convection from diffusion, the risk of retrofiltration is eliminated in HFR. |
| Type of reinfusion | Endogenous | Exogenous | - HFR does not require in-line preparation and infusion of high-purity dialysis water because it allows the production of an endogenous ultrafiltrate that is detoxified through an adsorbent resin. This allows the ultrafiltered plasma water to be reinfused between the two filters, resulting in a closed ultrafiltration circuit where the reinfusion fluid used is endogenous.- The regeneration of large volumes of ultrafiltrated plasma obtained in the first HFR filter makes it possible to treat a large volume of plasma water and, at the same time, enables the return of many beneficial substances such as amino acids, small peptides, hormones and vitamins. |
| Microbiological requirements for dialysis water | Pure water. | Ultrapure water. | |
| Nutrient loss through ultrafiltration | Less | More | |
| Target convective volume (l per session) | ≈ 15 | >21−23 | [3,0]- HFR performance is optimized with a reinfusion flow (= ultrafiltration flow) of 60−70 ml/min, since higher flows would decrease the interaction time between resin and plasma water, compromising the adsorptive efficiency of the technique.- The performance of OL-HDF depends on the infusion rate, which in turn depends on blood flow, with a filtration fraction usually 25–35% (in a 4-h session, the required blood flow is at least 350 ml/min).- HFR can provide more adequate removal of medium/high molecular weight molecules regardless of vascular access quality |
| Reinfusion Q (ml/min) | 60−70 | 85−150 | |
| Required blood Q (ml/min) | >250 | >350 | |
| Water consumption | Less | More |
Different studies have demonstrated the ability of HFR to remove a broad spectrum of uremic toxins, including PBUTs (Table 3). Esquivias-Motta et al.45 performed a randomized, crossover trial comparing OL-HDF and HFR in 17 HD patients without inflammation. In a sequential 8-week OL-HDF and HFR scheme, uremic toxins and 13 other molecules markers of inflammation, endothelial dysfunction, and oxidative stress were measured, with each patient being its self-control. HFR showed a greater reduction in indoxyl sulfate (IS) than OL-HDF. Riccio et al.44 in 12 patients with inflammation showing a CRP level greater than 3 mg/l, revealed that the adsorbent cartridge retained pCS by 53% compared to 35% with HD. In another study conducted in China with 37 patients,63 one session of HFR in patients on OL-HDF resulted in a mean reduction of pCS and IS by 40.9% and 43.6%, respectively. Other studies obtain variable results depending on the design and population included.50
Clinical studies with Supra-HFR.
| Country | Reference | Year | N° patients/n° centers | Comparator | Main results |
|---|---|---|---|---|---|
| Uremic Toxins | |||||
| Italy | Murgia et al.70 | 2022 | 13/1 | Supra-HFR vs. standard HD | Improved prognosis of MM and acute renal failure. |
| China | Chen et al.63 | 2020 | 37/1 | Supra-HFR vs. standard HD | Decrease in B2 microglobulin, p-cresol and indoxyl sulfate. IL-6 and CRP were not modified |
| Spain | Pendón-Ruiz de Mier et al.43 | 2020 | 9/1 | Supra-HFR vs. standard HD in patients with MM | Serum kappa and lambda levels were reduced by 57% and 33%, respectively; 33% of patients recovered renal function, and 44% required maintenance dialysis. |
| Italy | Riccio et al.44 | 2014 | 12/11 | Supra-HFR vs. OL-HDF in inflamed patients | Increased adsorption of IL-6 and p-cresol on the Supra-HFR resin cartridge. |
| Inflammation and oxidative stress | |||||
| Italy | Donati et al.50 | 2022 | 9/1 | Supra-HFR vs. OL-HDF | Significant reduction of FGF23 with both techniques. IL-6 is not modified. Significant decrease of TNF-α and TGF-α with HFR-supra, IL-8 is not modified. |
| Spain | Esquivias-Motta et al.45 | 2017 | 17/1 | Supra-HFR vs. OL-HDF in non-inflamed patients | Decrease in inflammatory markers and oxidative stress. |
| Italy | Palleschi et al.76 | 2016 | 41/19 | Supra-HFR vs. OL-HDF | Decrease in oxidative stress markers |
| Spain | González-Diez et al.39 | 2014 | 40/multicenter | Supra-HFR vs. standard HD in patients with ferritin <600 μg/l | Decrease in oxidative stress markers |
| Italy | Calò et al.84 | 2010 | 14/6 | Supra-HFR vs. standard HD in non-inflamed patients | Decrease in oxidative stress markers |
| Anemia | |||||
| Italy | Tessitore et al.62 | 2018 | 28/1 | Supra-HFR vs. low-flux and high-flux HD | Significant hepcidin decrease |
| Italy | Bolasco et al.74 | 2011 | 30/14 | Supra-HFR vs. standard HD in non-inflamed patients | Increased hemoglobin and decreased ESA requirements |
| Nutrition and albumin losses | |||||
| Italy | Borrelli et al.72 | 2014 | 24/8 | Supra-HFR in inflamed patients | Decrease in inflammatory markers, with increased albumin levels and no loss of amino acids. |
| Italy | Borrelli et al.82 | 2010 | 48/10 | Supra-HFR vs. standard HD | No loss of amino acids |
| Quality of life | |||||
| Italy | Scaparrotta et al.78 | 2021 | 1/1 | Supra-HFR vs. standard HD | Improvement of pruritus and quality of life in a liver transplant patient. |
| Italy | Solano et al.90 | 2015 | 1/1 | Supra-HFR vs. standard HD | Improvement of quality of life in a patient with lupus nephritis. |
| Italy | Borrelli et al.77 | 2016 | 114/18 | Supra-HFR vs. standard HD | Improvement of the physical component of quality of life |
One of the most explored applications of HFR is its ability to clear light chains, whose precipitation at the tubular level causes acute kidney damage in multiple myeloma. While current chemotherapy treatments decrease the production of light chains, whose half-life ranges from hours to several days, the elimination of these chains from the circulation constitutes a coadjuvant treatment in these patients. Techniques such as plasmapheresis or the use of high cut-off dialyzers (45–60 Kd) have been used over the last few years, without showing in different randomized trials to decrease renal damage in myeloma patients.64–68 Both techniques require albumin supplementation, and plasmapheresis must also be associated with HD, which implies a high cost. In 2010, Testa et al.69 demonstrated thatsupra-HFR removed light chains. In a recent Spanish study,43 in 9 patients with acute renal failure secondary to multiple myeloma and treated with HFR, serum kappa and lambda levels were reduced by 57% and 33%, respectively, while serum albumin levels remained stable; 33% of patients recovered renal function, 22% died during the first year and 44% required maintenance dialysis. In another Italian study involving 13 patients with myeloma and renal failure,70 the mean rate of reduction at the end of the cycle with HFR-supra was 85% for kappa chains and 40% for lambdas; 46% of patients showed partial recovery of renal function within the third month, with 38% remaining on dialysis. However, the effect of HFR on renal histology, the main prognostic parameter in renal involvement in myeloma, is unknown.71
Biocompatibility: the effect on inflammation and oxidative stressThe HFR is a highly biocompatible dialysis technique that, while producing extensive removal of uremic toxins, minimizes nutrient loss and inflammatory response during treatment. The synthetic resin cartridge is capable of absorbing cytokines and other uremic toxins, while allowing nutrients and antioxidants, such as amino acids and vitamins, to pass through, thereby decreasing inflammation and oxidative stress. These characteristics suggest that HFR should be used in HD patients affected by overt and idiopathic chronic inflammation. Borrelli et al.72 confirmed in a multicenter study in 24 inflamed patients with CRP >5 mg/dl and albumin <4 mg/dl, who after 8 months of HFR treatment showed a significant decrease in CRP of 35%, a 14% increase in albumin levels, and a decrease in proinflammatory cytokines such as IL-6, IL-1β and TNFα, with no changes in branched amino acid levels. The ability of the HFR cartridge to adsorb IL-6 has been proven by Riccio et al.,44 proposing this technique as a new strategy to decrease systemic inflammation in dialyzed patients. Although most of the studies performed have compared low-flux or high-flux HD with HFR, there are some which compare HFR with OL-HDF. In a randomized crossover trial, Esquivias-Motta et al.45 demonstrated a greater decrease in IL-6 levels with HFR versus OL-HDF. In a study conducted in China in 37 patients on OL-HDF, a decrease in TNF-α of 28.1% was observed after a single session of HFR, while IL-6 and CRP concentrations remained unchanged.63 In relation to the inflammatory status of HD patients, the response to treatment of anemia with erythropoietic agents (EAAE) and ferrotherapy has also been explored with HFR, where its effect on hepcidin seems to play a crucial role.73 Although hepcidin has a low PM of 2,8 kD and can be easily removed by conventional HD, some small studies show a greater decrease in hepcidin with HFR as compared with HD, with a decrease in the AAEE requirements.62,74
Beyond its favorable effect on the inflammatory status in HD patients, different studies support the antioxidant capacity of HFR by showing a slight decrease in oxidative stress parameters such as LDL oxidase, superoxide bismutase, catalase, glutathione peroxidase, and hemoperoxidase levels. González-Diez et al.39 performed a multicenter study to assess oxidative stress parameters in 40 patients with ferritin less than 600 μg/l and residual diuresis <300 ml/day, after 12 months on HFR it was found a significant decrease in the levels of total antioxidant capacity and peroxide bismutase. The superiority of HFR with respect to OL-HDF has also been proven in some studies, where a significant decrease in most of the inflammatory and oxidative stress parameters is objectified.45 In conclusion, in most of the published studies (Table 3) the switch from HD to HFR led to an improvement in inflammation with a significant decrease in serum levels of CRP, IL-6, IL-1 and TNF and a significant increase in albumin and prealbumin. Whether these favorable effects can modify the clinical outcomes of these high-risk patients should be confirmed by well-designed ad-hoc studies, differentiating inflamed from non- inflamed patients and comparing HFR with OL-HDF.
Nutritional statusThe HFR, despite increasing the elimination of uremic toxins and inflammatory markers, minimizes the loss of nutrients such as amino acids and vitamins with respect to high-flux HD and OL-HDF, which are associated with a loss amino acid l of 4−5 g/per session.75 In small studies It has been proven that the elimination of both essential and branched amino acids is lower with HFR than with acetate-free HDF, probably due to the absence of amino acid adsorption by the cartridge and the endogenous reinfusion of plasma.67 Another small randomized study showed less vitamin C losses in 41 patients using the HFR technique,76 while several studies have shown less albumin loss associated with HFR compared to OL-HDF.44,45,63 The reduced loss of amino acids, vitamins and albumin postulates HFR as a technique of great use in malnourished patients with nutritional supplementation requirements.
Quality of life and survivalAlthough there is positive evidence on the beneficial effect of HFR on quality of life, it is only observational. In an Italian cross-sectional study involving 114 patients from 18 hospitals, the quality of life of 57 patients on conventional dialysis and 57 on HFR were compared, with no baseline differences in terms of age, length of stay on dialysis, comorbidity and functional capacity estimated by the Barthel index.77 Patients treated by HFR showed better scores in the physical component of the SF-36 and particularly in the physical functioning and daily activities components, while there were no differences in the mental component score. Another small study suggests that HFR could alleviate the pruritus associated with CKD,78 a symptom that has a negative impact on quality of life.79 There is a lack studies that specifically compared the mortality associated with HFR with respect to other HD modalities,80 so it is still unknown whether the previously mentioned benefits of the technique on the inflammatory and nutritional status of patients are translated into increased survival.
Practical considerations for HFRWho to prescribe HFR: indications and clinical objectivesAlthough we can indicate HFR in any type of patient, as it is considered a superior technique in terms of biocompatibility, uremic toxin clearance and preservation of nutritional status, there are specific circumstances and patients with specific needs in which this technique would be especially indicated and which must be taken into account in order to prescribe the most individualized dialysis therapy possible, centered on the patient (Table 4).
- none-
Dialysis patients with chronic inflammation. HFR allows mitigation of the chronic inflammatory state and therefore its consequences, being especially useful in patients with central hemodialysis catheters, carriers of non-functioning renal grafts, with chronic low-grade infectious processes (dental or other), neoplasms, etc.45,81
- none-
Patients on chronic dialysis with protein-energy wasting (PED). In addition to the indirect benefit in nutritional status due to improvement of the inflammatory environment, a lower loss of physiological components such as vitamins, Aas, albumin, etc., of HFR compared to other dialysis techniques,29,76,82 guarantees better preservation of the nutritional status of patients compared to other dialysis techniques. In this scenario, HFR would be considered the optimal dialysis technique for patients at risk of malnutrition or malnourished, especially if they are receiving parenteral intradialytic nutrition (PIDN) due to the lower loss of the nutrients administered (endogenous reinfusion of ultrafiltrate), as occurs in other connective dialysis techniques.83
- none-
Patients with high comorbidity. Candidates for this technique are frail patients, with advanced cardiovascular disease, etc., who need to improve functional capacity and reduce the accelerated progression of atherosclerosis, heart disease or sarcopenia,29,84,85 all with the dual objective of improving survival with the best possible quality of life.
- none-
Patients on HDF without ultrapure water availability or with suboptimal vascular access. As we have previously described, HFR is the only HDF technique available in situations of lower microbiological quality of dialysis water, in patients whose vascular access only allows achieving a Qb of 250−300 ml/min (e.g., patients with central venous catheter).50,59
- none-
Young patients with no expectation of transplantation. This would include patients with a high rate of sensitization due to previous transplants, a history of neoplasia, ischemic heart disease, etc., which make their short-term inclusion in the transplant program unlikely.
- none-
Patients with myeloma kidney and acute renal failure. HFR allows highly efficient removal of free light chains without the aforementioned losses of albumin observed with traditional techniques, at lower cost and with good results.41,43,86,87
- none-
Occasional use in other clinical processes. Cases have been reported in the literature in which HFR could be useful in different situations, including myoglobin clearance in acute renal failure secondary to rhabdomyolysis,30 inflammatory bowel disease,88 porphyria cutanea tarda,89 clinical-analytical issue in patient with lupus,90 or nevirapine intoxication in HIV treatment.91
Circumstances especially indicated for HFR.
| Indication | Examples |
|---|---|
| Dialysis patients with chronic inflammation | - Use of central catheter for vascular access- Non-functioning renal graft carrier- Chronic low-grade infections (e.g., periodontitis, bronchiectasis, etc.)- Autoimmune diseases, connective tissue diseases- Neoplasms |
| Patients with energy-protein wasting | - Mean albumin <3.5 g/dl or prealbumin <20 mg/dl levels, during the last 3 months.- Serum creatinine <8 mg/dl during the last 3 months.- Weight loss greater than 10−20% of usual or ideal weight, respectively.- Body mass index <18.5 kg/m2- Subjective global assessment with moderate-severe malnutrition (category B or C).- Decreased caloric intake (<30 kcal/kg/d).- Decreased protein intake (<1.0 g/kg/d).- Documented diagnosis of gastrointestinal disorder (e.g., gastroparesis, malabsorption syndrome) |
| Patients with high comorbidity | - Advanced cardiovascular disease- Long stay on renal replacement therapy- Fragility- Sarcopenia |
| Hemodiafiltration in the absence of ultrapure water. | - Situations of lower microbiological quality of osmosed water- Use of portable osmotizers (e.g., dialysis in critical patient units; dialysis of isolated patients: COVID, C. difficile colonization, vancomycin-resistant S. aureus; etc.) |
| HDF patients with suboptimal vascular access. | - Use of central catheter for vascular access-Arteriovenous fistulas with atherosclerosis of nutritional artery |
| Young patients with no expectation of transplantation | - Hypersensitized due to previous transfusions or transplants.- Comorbidities that contraindicate transplantation (history of neoplasia, ischemic heart disease, etc.). |
| Myeloma kidney patients | - Acute renal failure secondary to light chain deposition |
| Other indications | - Acute renal failure secondary to rhabdomyolysis- Inflammatory bowel disease- Porphyria cutanea tarda- Systemic lupus erythematosus- Nevirapine intoxication in the treatment of HIV. |
After selecting the HFR mode to be used (Supra-HFR being the HFR of choice because of its higher permeability of the convective filter and higher quantity of adsorbent resin), the usual monitor parameters should be stablished:
- •
Qb: 300−350 ml/min, for a maximum ultrafiltrate flow rate of 70 ml/min, allowing sufficient contact time of ultrafiltrate with the sorbent to match the adsorptive capacity of the technique.
- •
Qd: 500 ml/min.
- •
Dialysis bath and heparinization according to patient characteristics.
- •
Recommended time duration: minimum of 4 h in order to optimize treatment.
HFR does not require any special monitoring outside normal clinical practice. Clinical success with HFR will be determined by the improvement and/or normalization of the clinical-analytical parameters, the benefits in the tolerance of the procedure, in the quality of life and in the morbidity and mortality of the patient previously mentioned.77,78
Drug dose adjustment in Supra-HFRAlthough there are no specific data in the literature, it is very likely that HFR requires dose adjustment of many drugs, as is the case with other convective and adsorptive techniques.92,93 In critically ill patients, it has bee described high adsorption of piperacillin and vancomycin by coupled plasma filtration adsorption, a type of continuous renal replacement therapy using the same sorbent as HFR with a volume of 140 ml.92 Those drugs within the medium molecular weight range, with low protein binding and neutral or positive charge are those with especially higher clearance during convective techniques compared to conventional HD.93 While waiting for studies analyzing the effect of HFR on antibiotics and other drugs, a practical approach is to administer such drugs after dialysis treatment and perform periodic plasma monitoring.94
Potential barriers to HFR. Economic considerationsThe use of HFR, by using a resin with adsorptive capacity, is associated with a higher cost, this is the main barrier that this technique has had historically to generalize its use. However, even considering the higher costs of HFR vs. OL-HDF (about 50 Є/session vs. 25 Є/session, respectively),50 it should be stressed that HFR allows offering both economic (lower water consumption, absence of need -although advisable- of ultrapure water) and clinical advantages. As previously mentioned, several small-scale clinical trials have shown that HFR can remove more effectively medium and large molecules, as well as PBUTs, compared to current dialysis techniques, including OL-HDF, with greater biocompatibility and without the need for optimal vascular access. We also have observational evidence that, compared to standard HD, RFH could reduce the incidence of cardiovascular events, improve survival and quality of life. All these effects could lead to a potential increase in quality-adjusted life years for patients receiving HFR compared to other dialysis techniques, which would be sufficient to justify the additional cost, as has been described with other adsorptive techniques.95
Guidance for future researchDespite the potential advantages of HFR over high-flux HD and OL-HDF with high convective transport, this technique could benefit from improvements in the design of currently available prototypes. One of the limitations of HFR is the poor synergistic effect between the adsorptive and diffusive phases for the removal of some toxins. For example, the clearance of medium and large molecules such asβ 2-m (11,800 Da), myoglobin (17,200 Da) or kappa (22,500 Da) and lambda (45,000 Da) light chains occurs exclusively by adsorption, since the low permeability of the membrane used during the diffusive phase prevents their elimination at this level.63 To increase the scavenging capacity of HFR, the first step has been to replace this low diffusive membrane with a high permeability one, giving rise to the so-called high flux HFR (HFR-H).46 The available preliminary results obtained with this change, pending final publication, are very interesting as they allow increasing the elimination of medium and high molecular weight uremic toxins, with an improvement in other biological parameters related to inflammation, oxidative stress and senescence. Another possibility would be to replace the diffusive filter with a cut-off medium membrane or even to make modifications to the dialysis monitor that would allow to perform OL-HDF, although this variant could be technically more complex. Finally, resin optimization could be a very interesting alternative to further improve the results obtained with HFR. In addition to the adsorptive effect of activated carbon for PBUTs scavenging, which has recently been detailed,96 nanotechnology-based work demonstrates how metal-organic frameworks (MOFs) can potentially be applied in adsorptive scavenging as well. MOFs are polymers consisting of polydentate organic ligands attached to metal ions that form highly porous networks. In a recent work, it has been described how one of these MOFs, nanoZIF-8, achieves high pCS and IS removal.97 These promising results open the door to the development of new adsorptive methods for the HFR system in the near future. Finally, prospective, controlled, randomized studies with an adequate number of patients followed over the long term are needed to establish the potential advantage of HFR-supra over other dialysis modalities in terms of mortality, hospitalization rate, comorbidity and quality of life.
ConclusionsIn recent years, parallel to the increase in clinical and survival benefits that we have obtained with the widespread use of OL-HDF over conventional HD, we have also witnessed a change in the profile of dialysis patients who are increasingly older and with more comorbidities, and continue to have problems associated with inflammation, oxidative stress, and excess cardiovascular morbidity and mortality. Given this situation, renal replacement therapies should aim to offer treatment that is not only aimed at prolonging survival, but rather at improving quality of life. In this context, HFR represents a biotechnological response that offers a compromise between optimizing the elimination of toxins and the eventual loss of beneficial physiological substances. In summary, HFR is a highly biocompatible procedure, with good clinical tolerance and cardiovascular stability, in which convection is separated from diffusion, eliminating the potential risk of retrofiltration. The regeneration of high volumes of plasma ultrafiltrate using an adsorbing resin allows effective removal of medium molecular size uremic toxins and PBUTs, without loss of albumin and other beneficial substances, avoiding the risks associated with exogenous fluid infusion and the need for optimal vascular access. In addition to its ease of use, HFR is associated with decreased inflammation and oxidative stress, with potential advantages for frail patients and those with high levels of comorbidity. Despite these encouraging data, further long-term studies are needed to confirm these findings and to adequately position the role of HFR within the renal replacement techniques currently available for dialysis patients.
Key points
- •
HFR is a dialysis technique characterized by a resin cartridge with adsorptive power that combines the mechanisms of diffusion, convection and adsorption in a single therapeutic scheme.
- •
Regeneration of high volumes of plasma ultrafiltrate using an adsorbing resin allows effective removal of medium molecular size uremic toxins and PBUTs, without loss of albumin and other beneficial substances, avoiding the risks associated with exogenous fluid infusion and the need for optimal vascular access.
- •
The use of HFR is associated with a decrease in inflammation and oxidative stress relative to conventional HD and online HDF.
- •
HFR is a highly biocompatible procedure, with good clinical and hemodynamic tolerance, being a technique especially indicated for patients with high cardiovascular comorbidity, fragile or malnourished.
;1;
Conflict of interestPablo Molina: lecture fees from Abbott, Baxter, Fresenius-Kabi, Palex and Vifor Pharma; consultancies from Baxter, Fresenius-Kabi, Palex and Vifor Pharma, as well as travel grants from Palex and Vifor Pharma.
Marian Goicoechea: consulting or speaking fees for Medtronic, Astra-Zeneca, Vifor-CSL and Astellas.
Emma Huarte: lecture fees from Abbott, Baxter, Astra-Zeneca.
Francisco Maduell: consulting or speaking fees from Amgen, Baxter, Fresenius Medical Care, Medtronic, Nipro and Vifor.
Alejandro Valero: no conflicts of interest.
Alejandro Martín Malo: has received consulting or speaking fees from Astellas, Astra-Zeneca, Diaverum, Medtronic and Vifor-Pharma.
The authors would like to thank the nursing staff of all our dialysis units for their invaluable dedication to patients undergoing renal replacement therapy. The authors would also like to thank Mr. Federico García Lorente and Mr. Riccardo Barini for their help in writing the article.