Patients with chronic kidney disease (CKD) on hemodialysis present high cardiovascular comorbidity. Peripheral arterial disease (PAD) is associated with higher mortality and the interest in its early detection and treatment is increasing. The objective of this study is to determine the frequency and severity of symptomatic PAD, and to establish its relationship with mortality in HD patients that have received treated early and compare them with a cohort of our center already reported.
Material and methodsRetrospective study on a cohort of incident patients since 2014 and followed up until December 2019. Demographic data, cardiovascular risk, the presence of symptomatic PAD at baseline and during follow-up were collected. Trophic lesions were graded using the Rutherford scale.
ResultsInitially, there were 91 patients and 7 cases that were not included in the study were lost to follow-up. Age 64 ± 16 years, men 51.6% (47/91). The percentage of baseline PAD was 10.7% (9/84). During a median follow-up of 35 months (20−57), the diagnosis of PAD increased to 25% (21/84). Half of the patients with PAD 52.38% (11/21) obtained a score greater than 3 in the Rutherford Clinical Classification, which corresponds to severe disease. 13/21 patients required reoperation due to recurrence of symptoms (61.9% of cases with PAD).
The development of PAD was significantly associated with: an elevated index of Charlson (3.9±2.1 vs. 7.7 ± 3.5; P = 0.001),being male (19 vs. 2; P = 0.001), diabetic (no: 7; yes: 15; P = 0.001) and with a history of chronic ischemic heart disease (no: 13; yes: 8; P = 0.001), 38.1% (8/21) had ischemic heart disease in patients who developed PAD, while in the absence of PAD the presence of ischemic heart disease was 9.5% (6/63). Furthermore, more than half (66.7% [14/21]) of those who developed PAD were diabetic.
Univariate analysis showed that age, C reactive protein, albumin, and number of surgical interventions, but not PAD, were associated with mortality. In the multivariate analysis adjusted for other factors, only C reactive protein was related to overall survival Exp β: 2.17; P = 0.011; CI (1.19–3.97). Regarding cardiovascular mortality, in the multivariate Cox analysis, only PAD was related to mortality of cardiovascular origin Exp β: 1.73; P = 0.006; CI (1.17–2.56).
ConclusionsA significant number of patients on hemodialysis develop PAD requiring peripheral vascular surgery. PAD was not associated with overall mortality in our cohort, but it did show an association with cardiovascular mortality. Prospective studies with a larger sample size are necessary. New surgical treatments and Follow-up by vascular surgeons could improve the severity of PAD and the long-term prognosis.
Los pacientes con enfermedad renal crónica (ERC) en hemodiálisis presentan gran comorbilidad cardiovascular. La enfermedad arterial periférica (EAP) se asocia a mayor mortalidad y ha incrementado el interés en su detección precoz y tratamiento. El objetivo del presente trabajo es determinar la frecuencia y gravedad de EAP sintomática, establecer su relación con la mortalidad en pacientes en HD que han sido tratados precozmente y compararlos con una cohorte de nuestro centro ya reportada.
Material y metodosEstudio retrospectivo sobre una cohorte de todos los pacientes incidentes desde 2014 y seguidos hasta diciembre de 2019. Se recogieron datos demográficos, riesgo cardiovascular, la presencia de EAP sintomática basal y durante el seguimiento. Con la escala Rutherford se graduaron los síntomas o lesiones tróficas.
ResultadosInicialmente eran 91 pacientes y se perdió seguimiento de 7 casos que no incluyeron en el estudio. Edad 64 ± 16 años, hombres 51,6% (47/91). El porcentaje de EAP basal fue del 10,7% (9/84). Durante una mediana de seguimiento de 35 meses (20–57), el diagnóstico de EAP aumentó al 25 % (21/84). La mitad de los enfermos con EAP 52,38% (11/21) obtuvo una puntuación mayor de 3 de la Clasificación clínica de Rutherford que corresponde con estadios severos. Requirieron reintervención por reaparición de los síntomas 13/21 pacientes (61.9% de los casos con EAP).
El desarrollo de EAP se asoció de forma significativa con la presencia de un índice de Charlson elevado (3,9 ± 2,1 vs. 7,7 ± 3,5; p: 0,001), con ser varón (19 vs. 2; p = 0,001), diabético (no: 7; sí: 15; p = 0,001) y con el antecedente de cardiopatía isquémica crónica (no: 13; sí:8; p = 0,001), de forma que un 38,1% (8/21) presentó cardiopatía isquémica en los pacientes que desarrollaron EAP mientras que en ausencia de EAP la presencia de cardiopatía isquémica fue de un 9,5% (6/63). Además, más de la mitad (66,7% [14/21]) de los que desarrollaron EAP eran diabéticos.
El análisis univariante mostró que la edad, la proteína C reactiva, la albúmina y el número de intervenciones quirúrgicas, pero no la EAP, se asociaban con la mortalidad. En el análisis multivariante ajustado por otros factores solo la proteína C reactiva, se relacionó con la supervivencia global Exp (β): 2,17; p = 0,011; IC (1,19–3,97). Con respecto a la mortalidad cardiovascular, en el análisis multivariante de Cox, solo la EAP se relacionó con la mortalidad de origen cardiovascular Exp (β): 1,73; p = 0,006; IC (1,17–2,56).
ConclusionesUn número significativo de paciente en hemodiálisis desarrollan EAP, requiriendo cirugía vascular periférica. La EAP no se asoció a mortalidad global en nuestra cohorte, pero mostró asociación con la mortalidad cardiovascular. Aunque son necesarios estudios prospectivos con mayor tamaño muestral, los nuevos tratamientos quirúrgicos y el seguimiento por los cirujanos vasculares podrían mejorar la gravedad de la EAP y el pronóstico a largo plazo.
Key concepts
- •
Cardiovascular disease and specifically peripheral arterial disease (PAD) are important causes of mortality in people with chronic kidney disease on hemodialysis.
- •
Being male, diabetic, having inflammation and the presence of ischemic heart disease are related to the occurrence of PAD.
- •
Multidisciplinary follow-up and surgery by vascular surgeons has been modified in recent years, with a decrease in the number of major amputations.
- •
Early follow-up and management could have implications for reducing mortality.
- •
The persistence of inflammatory parameters such as C-reactive protein carries a higher risk of cardiovascular mortality in patients on hemodialysis and PAD.
Chronic kidney disease (CKD) is an independent risk factor for the development of peripheral arterial disease (PAD).1,2 The prevalence of PAD in some series is up to 25%3,4 and has been associated with cardiovascular mortality, morbidity, hospitalization and worse quality of life.5–7
A factor that promotes PAD in hemodialysis (HD) patients is vascular calcification, not only due to calcification of the intima secondary to atherosclerosis, but also to calcification of the medial layer. This is favored by an alteration in the balance between factors that favor calcification, such as osteoblastic transformation of the smooth muscle cell, induced by increased phosphorus and inflammation, and the decrease in factors that inhibit calcification, such as the decrease in fetuin A, a chaperone-binding protein, called calciproteins, that under normal conditions inhibits the crystallization of calcium phosphate particles.8,9
Early diagnosis of PAD is a challenge; the aim is to achieve diagnosis in those who are asymptomatic to offer appropriate treatment. Since 2017, European guidelines for the diagnosis and treatment of PAD suggested screening by determining the ankle brachial index (ABI) in population with CKD.10–12
In terms of treatment, revascularization after critical lower limb ischemia produces worse outcomes in patients with CKD than in those without CKD.13–15 In the Medicare program of 1991 and 1994, two-thirds of amputee patients who had advanced CKD before and after initiation of renal replacement therapy died within 2 years of the procedure. The amputation rate was 4.8–6.2 % in that population, compared to 4.3% amputations in the population without advanced CKD.16,17
In a 2017 meta-analysis, the hazard ratios for the occurrence of PAD were 1.22 and 2.06 for eGFR of 45 and 15 mL/min/1.73 m2 respectively as compared to patients with eGFR of 95 ml/min/1.73 m2. Risk factors associated with mortality were coronary artery disease, cerebrovascular disease, dementia, ASA greater than 4, cognitive impairment, and the presence of renal disease.18
It is important to know the benefit of early treatment of HD patients with PAD; therefore, the specific objective of the present study was to determine the overall and cardiovascular mortality of patients on HD with symptomatic PAD treated early after the development of new surgical techniques in recent years. In addition, we compared the results with data previously collected in our own center. As a secondary objective, we evaluated other variables: demographics, inflammation, and cardiovascular risk.
Material and methodsStudy designRetrospective observational study of a cohort that included all patients older than 18 years in renal replacement therapy on HD in our center from 2014 to December 2019.
Baseline variable collection- -
Cause of renal disease, type of vascular access, presence of cardiovascular events prior to HD entry, and cardiovascular risk factors.
- -
Analytical parameters of inflammation and nutrition were collected including: cholesterol, triglycerides, albumin, prealbumin, C-reactive protein and other parameters related to CKD such as mineral metabolism (calcium, phosphorus, parathormone, magnesium) and anemia (hemoglobin, transferrin saturation index, and prescription of erythropoietin analogues).
- -
In addition, we recorded the presence of anticoagulant or antiplatelet therapy. Comorbidity was reflected by the Charlson index, considering 0–1 points mild comorbidity; 2 low, and high ≥3 high.
We recorded the presence of symptomatic PAD before starting HD and the degree of damage. At the end of the study, we recorded the development of symptomatic PAD and the degree of involvement during follow-up.
Symptomatic PAD was defined as the presence of symptoms (gait claudication reported by the patient) or the presence of serious ischemia of the lower limbs (due to the presence of pain at rest, trophic lesions, tissue loss, or gangrene). We used the Rutherford classification to assess the degree of involvement. Categories 1–3 correspond to mild to severe claudication, and 4–6 correspond to the categories in which intervention is recommended due to the presence of pain at rest or tissue loss. Thus: category 1 corresponds to mild claudication; category 2 is moderate claudication and category 3 severe claudication. Category 4 corresponds to the presence of pain at rest, category 5 to minor tissue loss and category 6 major tissue loss defined by severe ischemic ulcers or gangrene.
Variables collected during follow-upDuring follow-up, we collected mortality and evolution variables including: the development of symptomatic PAD, the treatment and number of interventions received, in those who required reintervention, and how many continued follow-up for peripheral vascular surgery (PVS). In addition to the patient’s current situation (ongoing dialysis, if they received transplantation, loss to follow-up or death) and in cases of mortality whether it had a vascular cause. We also documented the Rutherford score at the end of follow-up.
Statistical analysisIn the descriptive analysis, we present quantitative variables as mean ± standard deviation, and categorical variables as frequencies and percentages.
Chi-square was used for the contrast of hypotheses in the cross-tabulations, according to the qualitative variable, and Student's t test was used for the qualitative-quantitative cross-tabulations. For multiple comparisons, we used Kaplan-Meier survival analysis and Cox regression analysis.
A p < 0.05, and 95% CI was considered a statistically significant finding. For data analysis and processing we used the SPSS V17.0 statistical software (Chicago, Illinois®, USA).
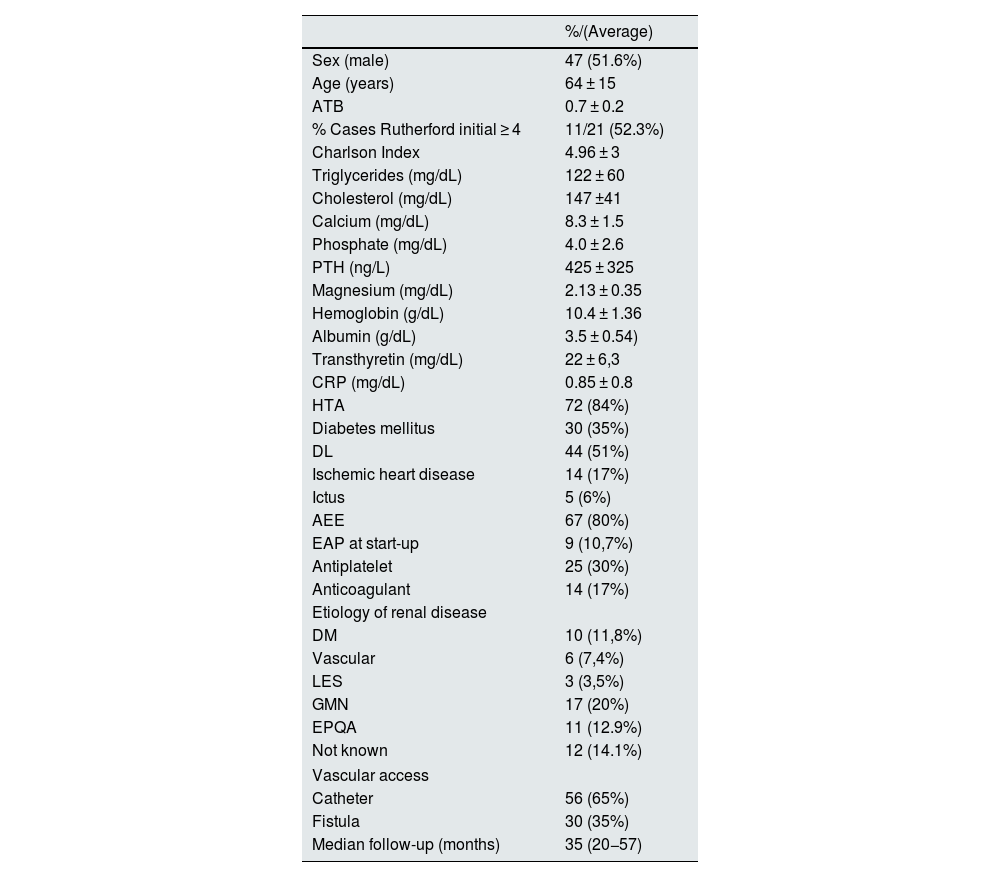
ResultsBaseline characteristicsThe population consisted of a total of 91 patients, 7 patients were lost to follow-up and 84 remained for the study. 51.6% were male with a median age of 64 ± 15 years. The median follow-up was 35 (20−57) months.
The baseline characteristics and biochemical data of the study population are shown in Table 1. At the beginning of follow-up, 10.7% (9/84) had PAD. Up to one third of the patients (29.7% [25/84]) were receiving antiplatelet therapy and 14/84 (17%) were anticoagulated. The mean Charlson index score was 4.98 ± 3.0, and in 77% of patients the score was ≥3.
Baseline characteristics of the study population.
| %/(Average) | |
|---|---|
| Sex (male) | 47 (51.6%) |
| Age (years) | 64 ± 15 |
| ATB | 0.7 ± 0.2 |
| % Cases Rutherford initial ≥ 4 | 11/21 (52.3%) |
| Charlson Index | 4.96 ± 3 |
| Triglycerides (mg/dL) | 122 ± 60 |
| Cholesterol (mg/dL) | 147 ±41 |
| Calcium (mg/dL) | 8.3 ± 1.5 |
| Phosphate (mg/dL) | 4.0 ± 2.6 |
| PTH (ng/L) | 425 ± 325 |
| Magnesium (mg/dL) | 2.13 ± 0.35 |
| Hemoglobin (g/dL) | 10.4 ± 1.36 |
| Albumin (g/dL) | 3.5 ± 0.54) |
| Transthyretin (mg/dL) | 22 ± 6,3 |
| CRP (mg/dL) | 0.85 ± 0.8 |
| HTA | 72 (84%) |
| Diabetes mellitus | 30 (35%) |
| DL | 44 (51%) |
| Ischemic heart disease | 14 (17%) |
| Ictus | 5 (6%) |
| AEE | 67 (80%) |
| EAP at start-up | 9 (10,7%) |
| Antiplatelet | 25 (30%) |
| Anticoagulant | 14 (17%) |
| Etiology of renal disease | |
| DM | 10 (11,8%) |
| Vascular | 6 (7,4%) |
| LES | 3 (3,5%) |
| GMN | 17 (20%) |
| EPQA | 11 (12.9%) |
| Not known | 12 (14.1%) |
| Vascular access | |
| Catheter | 56 (65%) |
| Fistula | 30 (35%) |
| Median follow-up (months) | 35 (20−57) |
ESA, erythropoiesis-stimulating agents; DL, dyslipidemia; DM, diabetes mellitus; PAD, peripheral arterial disease; PKD, adult polycystic kidney disease; GMN, glomerulonephritis; HTN, hypertension; ABI, ankle brachial index; SLE, systemic lupus erythematosus; CRP, C-reactive protein; PTH, parathormone.
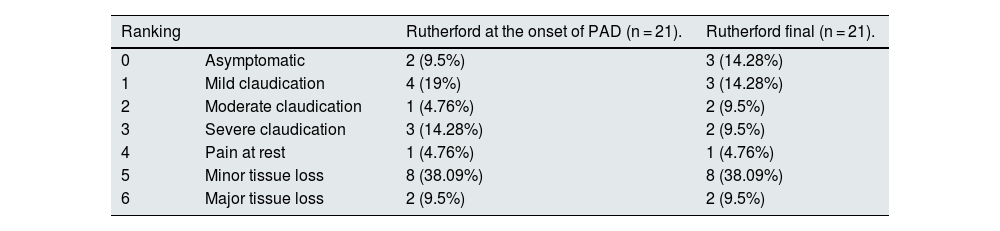
10.7% (9 patients) had PAD. This percentage increased to 25% (21/84 patients) at the end of the study. Most of them (18 patients) remained in follow-up in the PVD clinic. The progression of the vascular involvement was manifested by the Rutherford classification, collected at diagnosis and after follow-up (Table 2). At the time of onset of symptomatic PAD, 11/21 (52.3%) had a score>3 which corresponds to severe stages and usually involves intervention. At follow-up, the percentage of patients classified with Rutherford>3 did not change, and only 3/21 patients with PAD showed progression in their Rutherford score at the end of follow-up with respect to the onset of symptomatic PAD (Table 2).
Rutherford Scale.
| Ranking | Rutherford at the onset of PAD (n = 21). | Rutherford final (n = 21). | |
|---|---|---|---|
| 0 | Asymptomatic | 2 (9.5%) | 3 (14.28%) |
| 1 | Mild claudication | 4 (19%) | 3 (14.28%) |
| 2 | Moderate claudication | 1 (4.76%) | 2 (9.5%) |
| 3 | Severe claudication | 3 (14.28%) | 2 (9.5%) |
| 4 | Pain at rest | 1 (4.76%) | 1 (4.76%) |
| 5 | Minor tissue loss | 8 (38.09%) | 8 (38.09%) |
| 6 | Major tissue loss | 2 (9.5%) | 2 (9.5%) |
PAD, peripheral arterial disease.
Ankle brachial index (ABI) was only available in 7 patients, with a mean value at diagnosis of PAD of 0.86 ± 0.18 and the mean ABI score after follow-up was 0.74 ± 0.32, so analysis of asymptomatic PAD was not possible.
The development of PAD was significantly associated with the presence of an elevated Charlson index (no: 2; yes: 19; p = 0.001), being male (no: 2, yes: 19, p = 0.001), diabetic (no: 7; yes: 14; p = 0.001) and with a history of chronic ischemic heart disease (no: 13; yes: 8. p = 0.001), such that 38.1% (8/21) had ischemic heart disease in the group that developed PAD; however, in the absence of PAD the presence of ischemic heart disease was observed in 9.5% (6/63). More than half (66.7% [14/21]) of those who developed PAD were diabetic.
Interventional treatmentAfter the diagnosis of PAD, we recorded 9 surgical interventions: 5 minor amputations, 1 femorofemoral angioplasty, 1 femoropopliteal bypass, 1 femorofemoral bypass, and 1 major amputation. At follow-up, 13 patients (62%) presented PAD symptoms again and 3 patients required reintervention on several occasions, bringing the total to 18 interventions. Minor amputation continued to be the most frequent intervention. Only one case of major amputation was recorded, the rest received medical treatment.
EvolutionThe median follow-up of all patients from the start of HD in 2014 was 35 months (20−57 months). During this time mortality in the dialysis unit was 30/84 patients (35.7%), 40.5% (34/84) received transplantation, 20.2% (17/84) remained on dialysis, and in 3.6% (3/84) were lost to follow-up.
Mortality of cardiovascular origin occurred in 13 patients, which accounted for 43% of total mortality. None of the 3 patients who required reinterventions died during follow-up.
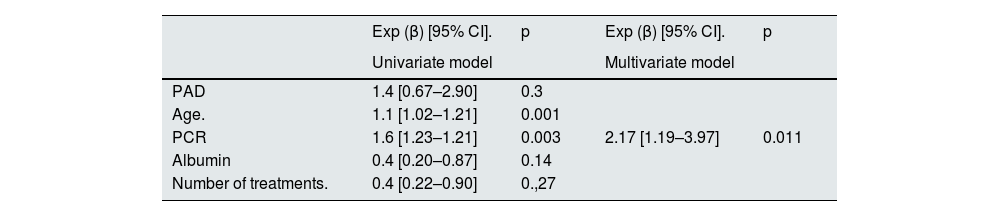
In the univariate analysis, age, C-reactive protein (CRP), albumin, and the number of treatments received, but not PAD, were associated with total mortality. In multivariate analysis after adjustment for other factors, only CRP was related to mortality. Exp (β): 2.17; p = 0.011; CI: 1.19–3.97 (Table 3).
Cox model for overall survival analysis (adjusted for factors PAD, age, CRP, albumin, number of treatments).
| Exp (β) [95% CI]. | p | Exp (β) [95% CI]. | p | |
|---|---|---|---|---|
| Univariate model | Multivariate model | |||
| PAD | 1.4 [0.67–2.90] | 0.3 | ||
| Age. | 1.1 [1.02–1.21] | 0.001 | ||
| PCR | 1.6 [1.23–1.21] | 0.003 | 2.17 [1.19–3.97] | 0.011 |
| Albumin | 0.4 [0.20–0.87] | 0.14 | ||
| Number of treatments. | 0.4 [0.22–0.90] | 0.,27 |
CRP, C-reactive protein; CI, confidence interval; PAD, peripheral arterial disease.
CRP with association with cardiovascular mortality 2.17; CI: 1.19–3.97; p = 0.011.
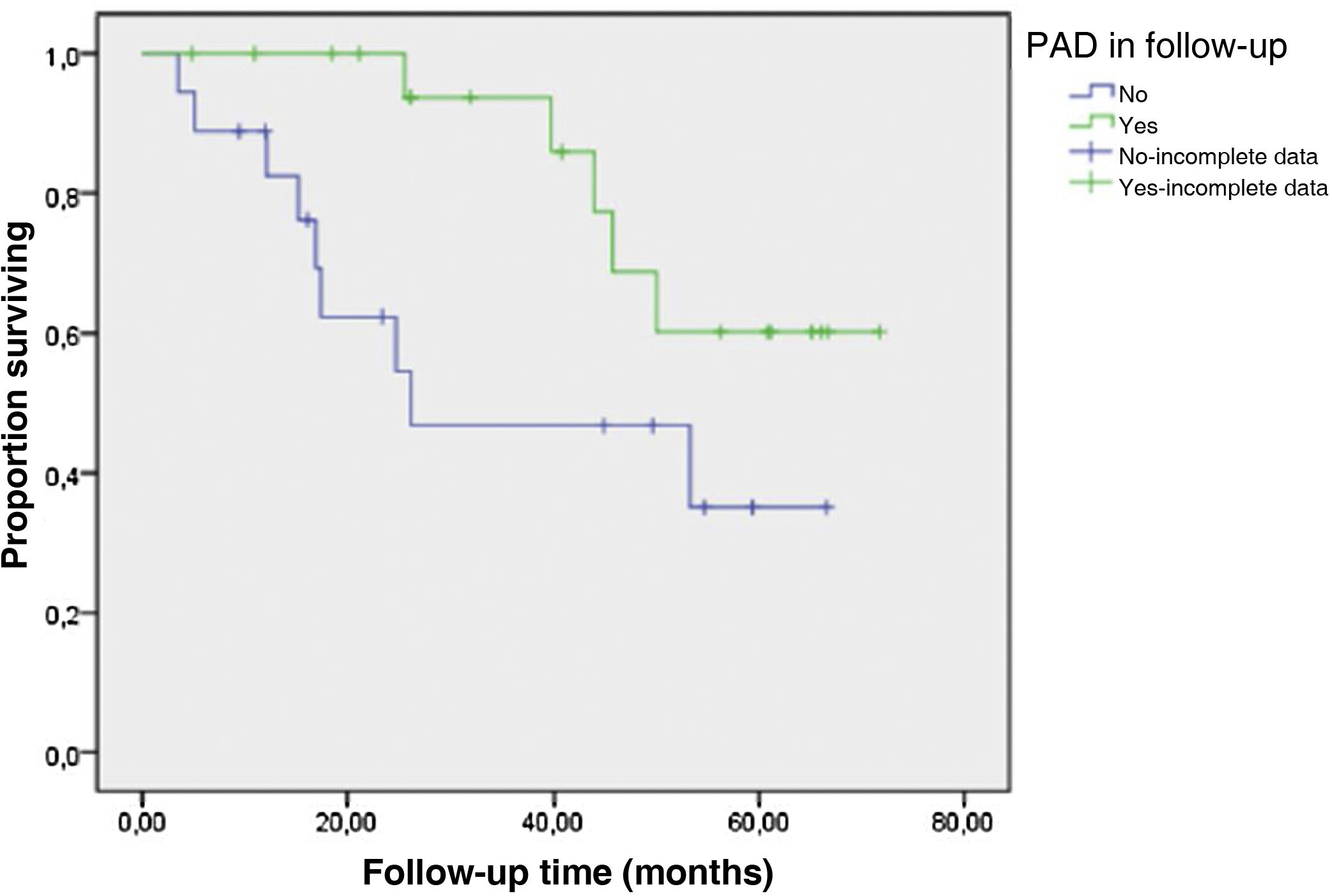
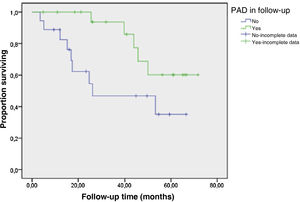
With respect to cardiovascular mortality, in the Cox multivariate analysis, only PAD was related to mortality of cardiovascular origin: Exp (β): 1.73; p = 0.006; CI: 1.17–2.56 (Fig. 1).
DiscussionWe present the results of our study on incident patients on HD in which we have detected that 10.7% initiated renal replacement therapy with PAD, and during the 35 months of follow-up an additional 14.3% developed PAD. Thus, a quarter of our HD population (25%) develops PAD. Therefore, this pathology continues to be frequent in dialysis.
The HD population is at a greater risk of vascular calcification and PAD. The frequency in prevalent dialysis patients shows a variable range that can be partly explained by a tendency to underdiagnosis, ranging from 25 to 40% with implications in morbidity, mortality and quality of life.4,19
In a previously published study by our group in 220 prevalent HD patients followed retrospectively from 2001 to 2005, the development of PAD was associated with older age, diabetes mellitus, Charlson index, prealbumin, and CRP. The prevalence of PAD in that study was 39.5% (87/220) and was independently associated with mortality at the end of follow-up, in which 55.2% (48/87) had died. Five major amputations were performed, 4 of them died.20
Despite the high prevalence, at that time the percentage of patients receiving some type of interventional treatment was only 5% and it consisted mostly of major amputation. The remaining 95% received medical treatment only, and not always from peripheral vascular surgery, but often limited to management at the dialysis center or health center.20
Compared to our previous work on prevalent HD patients with PAD, the current work focuses on incident patients. Taking this into account we observed a somewhat lower prevalence of 25%. In contrast, in the current cohort, 9/21(42.8%) required initial surgical intervention compared to only 5% in the previous work and most patients continued to be followed up for peripheral vascular surgery. In our current study, there was only one major amputation, compared to 5 in the previous study. Neither minor nor major re-amputations had a negative impact on survival in the current cohort.
Previous studies have related worse outcomes of PAD in HD after surgical revascularization and/or amputation.18,20 Probably the main explanation for this association is due not to the type of treatment, but to the delay in diagnosis. In our opinion, the fact that in the present study PAD was not associated with overall mortality but with cardiovascular mortality could be related to the close follow-up by peripheral vascular surgery of practically all patients, which allows early detection of the disease, application of various treatments and subsequent evaluation of their effectiveness. Many of our patients received interventional treatment instead of medical treatment, and yet major amputation was necessary on only one occasion.
In the Rutherford classification used to grade symptoms or presence of trophic lesions at the time of onset of symptomatic PAD, almost half of the patients scored above 3, corresponding to the more severe stages. During the follow-up time, PAD remained stable. No case of worsening of the Rutherford category was observed; this is due to the early detection of infections or ulcers amenable to treatment in the follow-up by peripheral vascular surgery.
As for interventional treatments, there were initially performed in 9 cases and symptoms recurred in 56%, and the total number of interventions was 18. The most frequent intervention, both de novo and reinterventions, was minor amputation, and only one case required major amputation.
The need for reintervention such as percutaneous revascularization or angioplasty was not associated with mortality, so it does not seem to have a negative outcome in terms of survival. It could allow a benefit of intermediate treatments with improvement in their quality of life and functionality. However, we believe that the limited sample size does not allow us to correlate reintervention with mortality.
The profile of patients who develop PAD has not changed substantially in recent years; in our series it was significantly associated with being male, with a history of ischemic heart disease, the presence of diabetes mellitus and a high Charlson index.
Only elevated CRP was significantly associated with overall mortality, with no association observed with any type of cardiovascular event, so that it was basically the elevated inflammatory state that influenced mortality. Neither was it associated with age, probably because we studied an incident sample with a mean age that was not excessively high. However, although we did not observe that PAD significantly influenced overall mortality, PAD did show a clear and independent association with cardiovascular mortality.
As a limitation of our study, cases with symptomatic PAD were included due to the difficulty of detecting asymptomatic PAD, since ABI, which would have provided complementary information, was only available in 7 cases. However, the main strength is that we included all incident patients on HD for a period of 12 months, with a follow-up of 5 years and with the collection of all the events that occurred during this period.
Therefore, we can conclude that early intervention in patients with symptomatic PAD has increased compared to 10 years ago. In our cohort, a significant percentage of patients developed PAD, requiring intervention by peripheral vascular surgery. Thus, new treatments and follow-up by vascular surgeons could improve the prognosis of these patients.
FundingThis research has not received specific support from public sector agencies, commercial sector or non-profit entities.
Conflicts of interestThe authors has no conflicts of interest to declare.