The adequate control of phosphorus levels is a major concern for professionals involved in the care of patients with chronic kidney disease (CKD), since high phosphorus levels are directly related to an increase in mortality.
ObjectivesTo know the perception and involvement of Spanish nephrologists on the control of phosphorus levels, the so-called 'Phosphorus Week' was organized (November 13–17, 2017).
MethodsAll members of the Spanish Society of Nephrology were invited to participate in an online survey, which included questions on aspects related to phosphorus control in patients with advanced CKD (aCKD) (glomerular filtration rate <30 ml/min/1, 73 m2) and in the different modalities of renal replacement therapies [peritoneal dialysis (PD), hemodialysis (HD) and renal transplantation (KT)].
Results72 data entries were obtained in the survey with an inclusion of 7463 patients. Of them, 35.4% were on HD, 34.8% were KT, 24.2% had aCKD and 5.5% were on PD. The serum phosphorus level target for the four groups of patients was 4.5 mg/dl, with minimal variations depending on the area of the national territory. The patients with better control of phosphataemia were patients with KT (93.3% had phosphorus values <4.5 mg/dl), followed by patients with aCKD (65.6% with phosphorus <4.5 mg/dl). Only 53.6% of the patients on HD and 39.4% of those on PD reached the phosphorus goal <4.5 mg/dl. The group of patients on dialysis was the one in whom phosphorus binders prescribed the most (73.5% and 75.6% in HD and PD, respectively), being less frequent in patients with patients with aCKD (39.9%) and only 4.5 % in KT.
ConclusionsThe objectives of the Spanish nephrologists are in line with those recommended by the national and international clinical guidelines; however, there is still a wide room for improvement to achieve these goals, especially in HD and PD patients.
El adecuado control de la fosfatemia es objeto de importante preocupación por los profesionales involucrados en el cuidado de los pacientes con enfermedad renal crónica (ERC), ya que los valores elevados de fósforo se encuentran directamente relacionados con un aumento de la mortalidad.
ObjetivosCon el objetivo de conocer la percepción e implicación que los nefrólogos españoles tienen de la necesidad de controlar el fósforo sérico, así como lograr una muestra lo más representativa posible de los valores séricos actuales, se organizó la denominada ‘Semana del Fósforo’ (13–17 de noviembre de 2017).
MétodosSe invitó a participar en una encuesta “on-line” a todos los socios de la Sociedad Española de Nefrología, que incluía preguntas sobre aspectos relacionados con el control del fósforo en pacientes con ERC avanzada (ERCA) (filtrado glomerular <30 ml/min/1.73 m2) y en las distintas modalidades de tratamiento renal sustitutivo [diálisis peritoneal (DP), hemodiálisis (HD) y trasplante renal (TR)].
ResultadosSe obtuvieron 72 entradas de datos con 7463 pacientes incluidos, de los cuales el 35.4% de ellos estaban en HD, el 34.8% eran TR, el 24.2% tenían ERCA y el 5.5% estaban en DP. El objetivo de fósforo sérico para los cuatro grupos de pacientes fue de 4.5 mg/dl, con mínimas variaciones en función del área del territorio nacional. Los pacientes con mejor control de la fosfatemia fueron los pacientes con TR (el 93.3% presentaban valores de fósforo <4.5 mg/dL), seguidos por los pacientes en ERCA (65.6% con fósforo <4.5 mg/dL). Sólo un 53.6% de los pacientes en HD y el 39.4% de los que estaban en DP cumplieron el objetivo de fósforo <4.5 mg/dL. El grupo de pacientes en diálisis fue en el que más se prescribían captores de fósforo (73.5% y 75.6% en los pacientes en HD y DP, respectivamente), siendo menos frecuente en los pacientes en ERCA (39.9%) y sólo un 4.5% en los TR.
ConclusionesLos resultados nos indican que los objetivos de los profesionales españoles están en consonancia con lo que recomiendan las guías clínicas nacionales e internacionales; sin embargo, aún hay un amplio margen de mejora para lograr esos objetivos, especialmente en los pacientes en HD y DP.
Key points
- -
The phosphorus objectives of Spanish nephrologists are in line with the recommendations of the clinical guidelines.
- -
Patients on HD and PD are those with the greatest margin for improvement, as they have serum phosphorus values higher than ACKD patients and especially those on KT.
- -
Almost two thirds of dialysis patients received treatment with phosphorus binders, 40% of patients in ACKD and only 4.5% of patients on KT.
The control of phosphatemia is a major concern for health professionals involved in the care of patients with chronic kidney disease (CKD). Hyperphosphatemia is a clinical condition directly associated with impaired renal function.1 A direct association between elevated serum phosphorus level and mortality has been demonstrated in numerous observational studies performed in patients with CKD, before or under dialysis treatment.2 Therefore, the use of phosphorus binders to reduce plasma phosphorus levels could lead to a reduction in the risk of mortality.3 Numerous studies, trials, meta-analyses and guidelines have helped us to establish phosphatemia targets in recent years4–12 although it is also true that they do not always coincide and may condition different therapeutic attitudes.
The so-called "Phosphorus Week" was organized with the aim of finding out the perception and involvement of Spanish nephrologists regarding the need to control phosphatemia, the range of desired values, the degree of compliance with these values, as well as the therapeutic measures adopted for this purpose.
Methods"Phosphorus Week" was designed to invite participation in an online survey to all members of the Spanish Society of Nephrology (SEN). The survey included questions on aspects related to phosphorus control in patients with advanced CKD (aCKD) (glomerular filtration rate <30 ml/min/1.73 m2) and in the different modalities of renal replacement therapy, which are, peritoneal dialysis (PD), hemodialysis (HD) and renal transplantation (KT). It was conducted between November 13 and 17, 2017, and during its development the participants had the option of including the patients visited in their work unit. Access was through the SEN website. Hospitals from all over Spain participated in the survey.
The questions of the survey included the opinion on what serum phosphorus target they had for their patients [Question: "What is your serum phosphorus target for your patients?"]; questions also asked about the percentage of patients in different ranges of phosphatemia [Question: "How many patients have a serum phosphorus value according to the following ranges: <4.5, 4.5–4.9, 5.0–5.4 and ≥5.5 mg/dl?"]; finally, it was asked how many of these patients were on phosphate binders [Question: "Of the patients referred in each of these ranges, how many are on phosphate binders?"].
The results were anonymous, and the dissemination of the results was done as a whole (global data). The data were analyzed according to the clinical situation of the patient (ACKD, PD, HD or KT). A sub-analysis was also performed by dividing the national territory into five areas that included a similar number of responses: Northwest (Galicia, Asturias, Cantabria, Basque Country, Navarra, La Rioja and Castilla y León), Northeast (Aragón and Catalonia), Levante (Valencian Community and Balearic Islands), South (Extremadura, Castilla-La Mancha, Murcia, Andalusia and the Canary Islands) and Center (Community of Madrid).
SPSS v.20 software (SPSS Inc., Chicago, IL, USA) was used for data analysis. Continuous variables are described as mean or median with standard deviation or interquartile range. The survey was approved by the Ethics Committee of the Hospital Clínic de Barcelona.
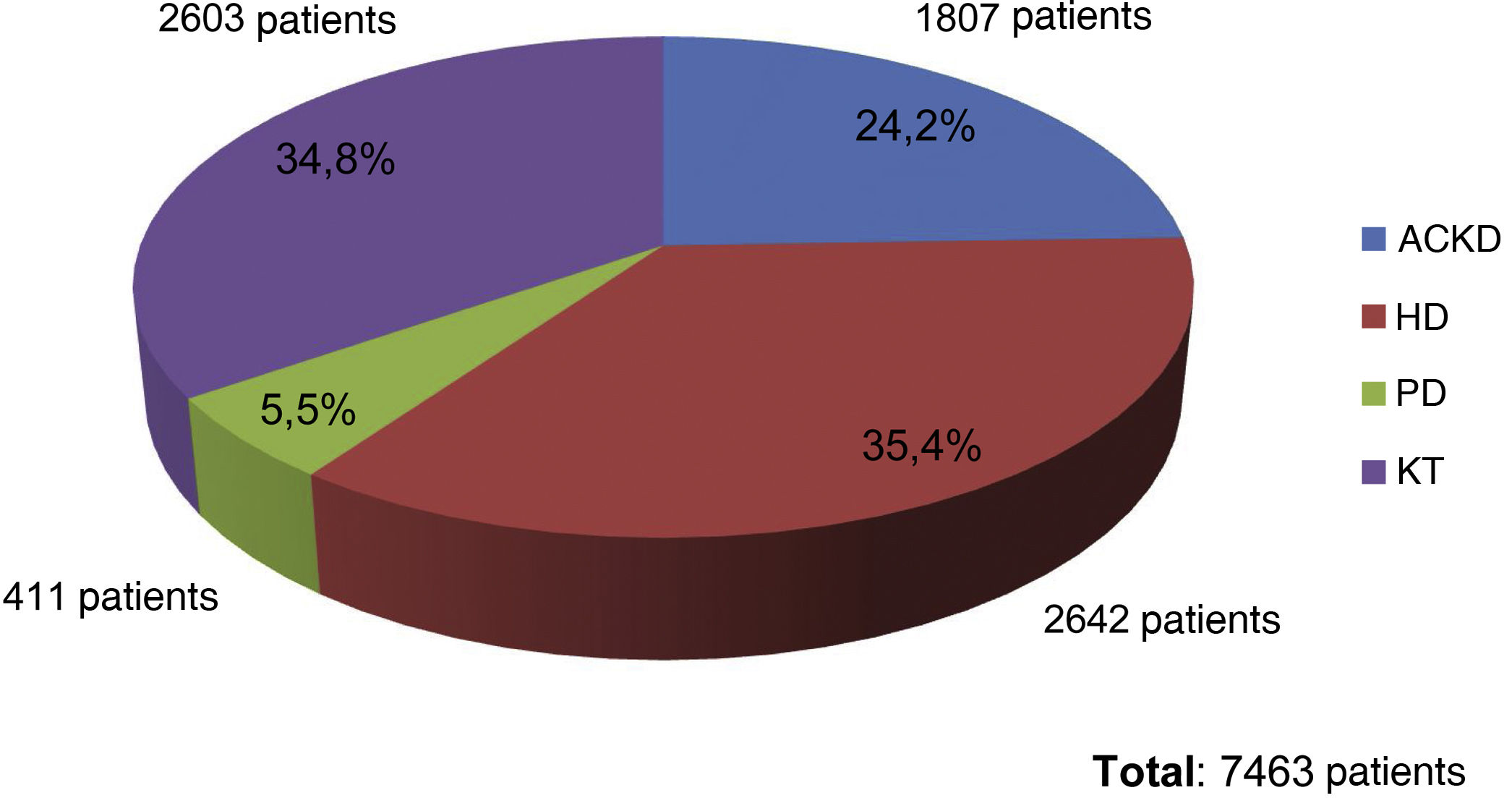
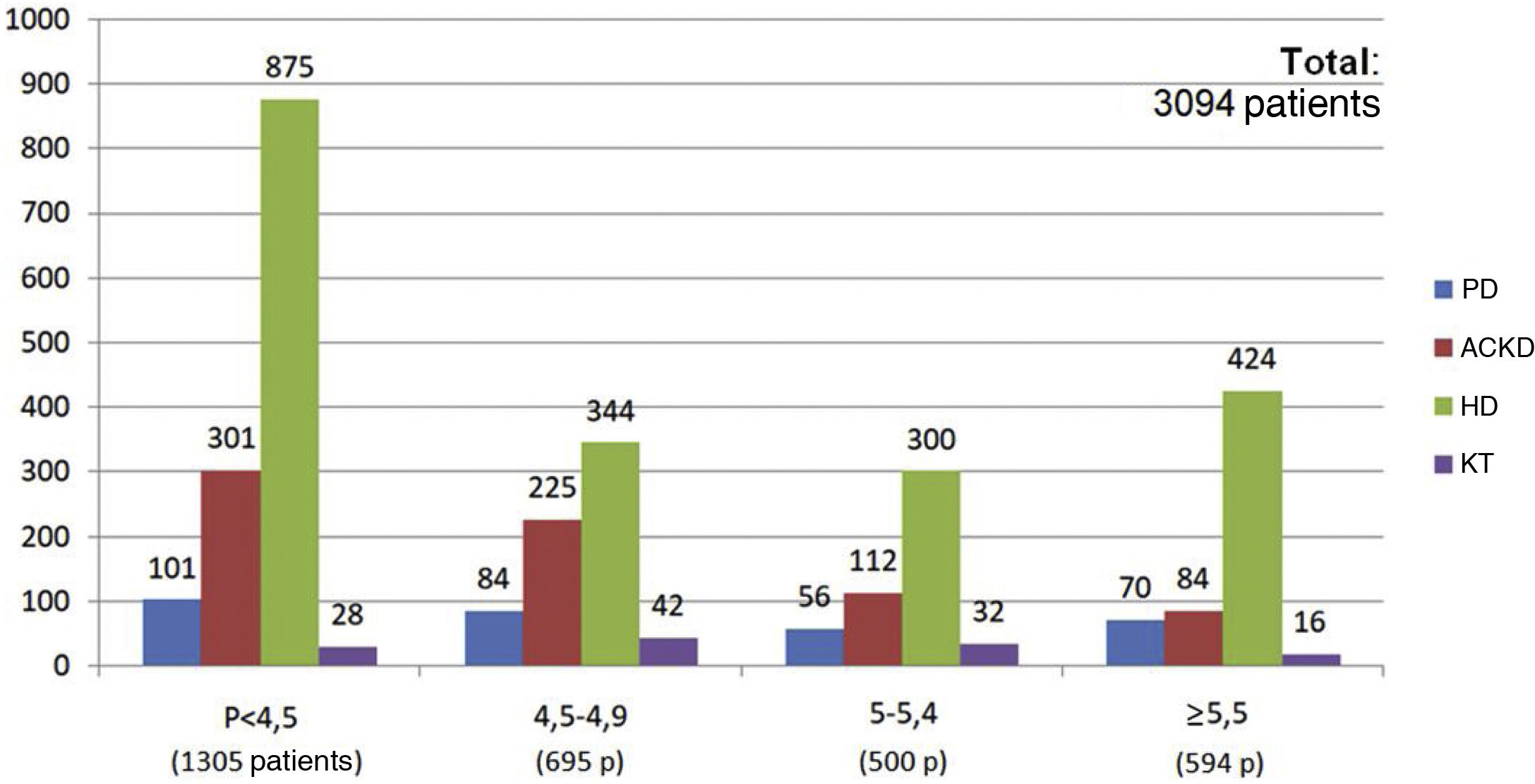
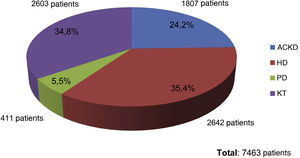
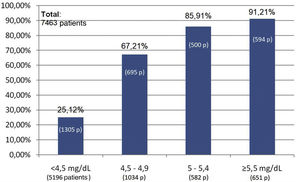
At the end of the "Phosphorus Week", 72 data entries had been obtained, corresponding to entries from professionals mainly from HD units (60%), followed by ACKD consultations (17%), PD (16%) and finally TR (7%). The total number of patients included was 7,463, of whom 35.4% were on HD (2,642 patients), 34.8% were on KT (2,603 patients), 24.2% had ACKD (1,807 patients) and 5.5% were on PD (411 patients) (Fig. 1).
The target serum phosphorus for the four patient groups was 4.5 mg/dl (median and mode). This target, however, differed minimally depending on the area of the national territory, with values in the Central area of 4.4 mg/dl, in the Southern area of 4.5 mg/dl, in the Northeast area of 4.6 mg/dl, in the Northwest area of 4.6 mg/dl and in the Levant area of 4.7 mg/dl.
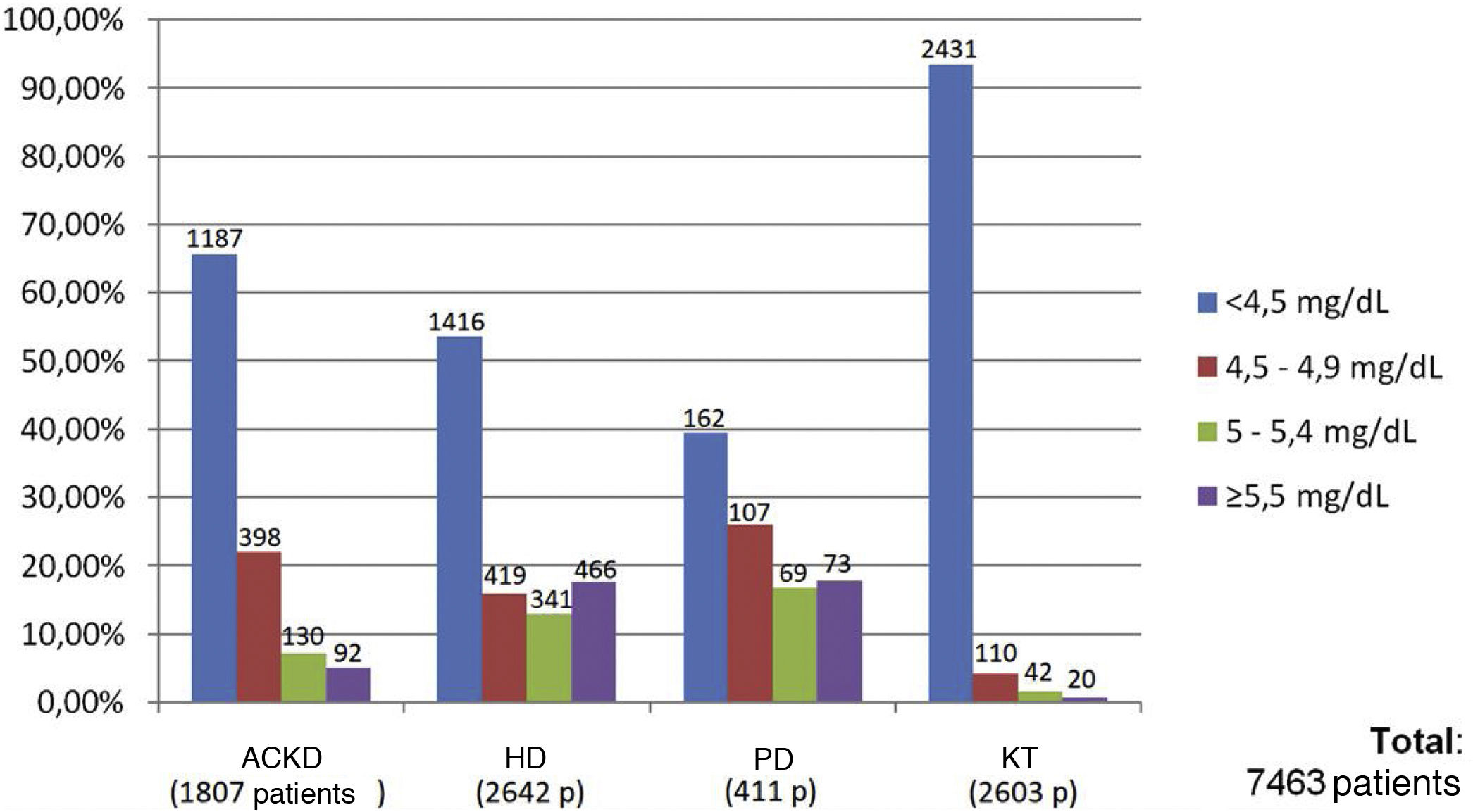
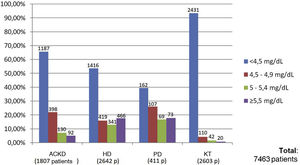
Of the total of 7,463 patients, 93.3% of patients with KT (2,431 of 2,603 patients), 65.6% of patients with ACKD (1,187 of 1,807 patients), 53.6% of patients on HD (1,416 of 2,642 patients) and 39.4% of those on PD (162 of 411 patients) met the target phosphorus values <4.5 mg/dl. In contrast, plasma phosphorus values ≥5.5 mg/dl were present in 17.6% of patients on HD (466 of 2,642 patients), 17.7% on PD (73 of 411 patients), 5% on ACKD (92 of 1,807 patients) and only 1% of KT (20 of 2,603 patients) (Fig. 2).
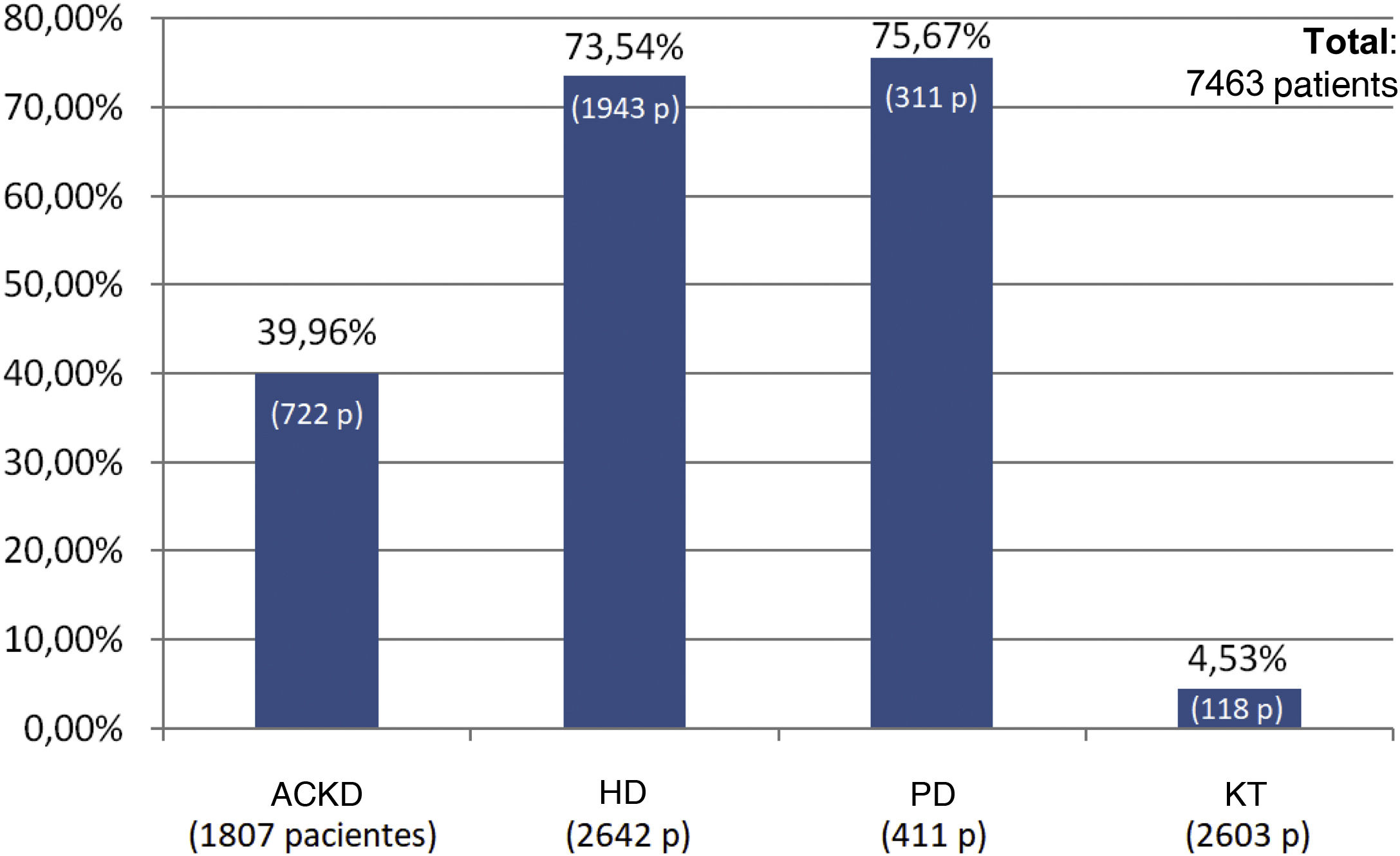
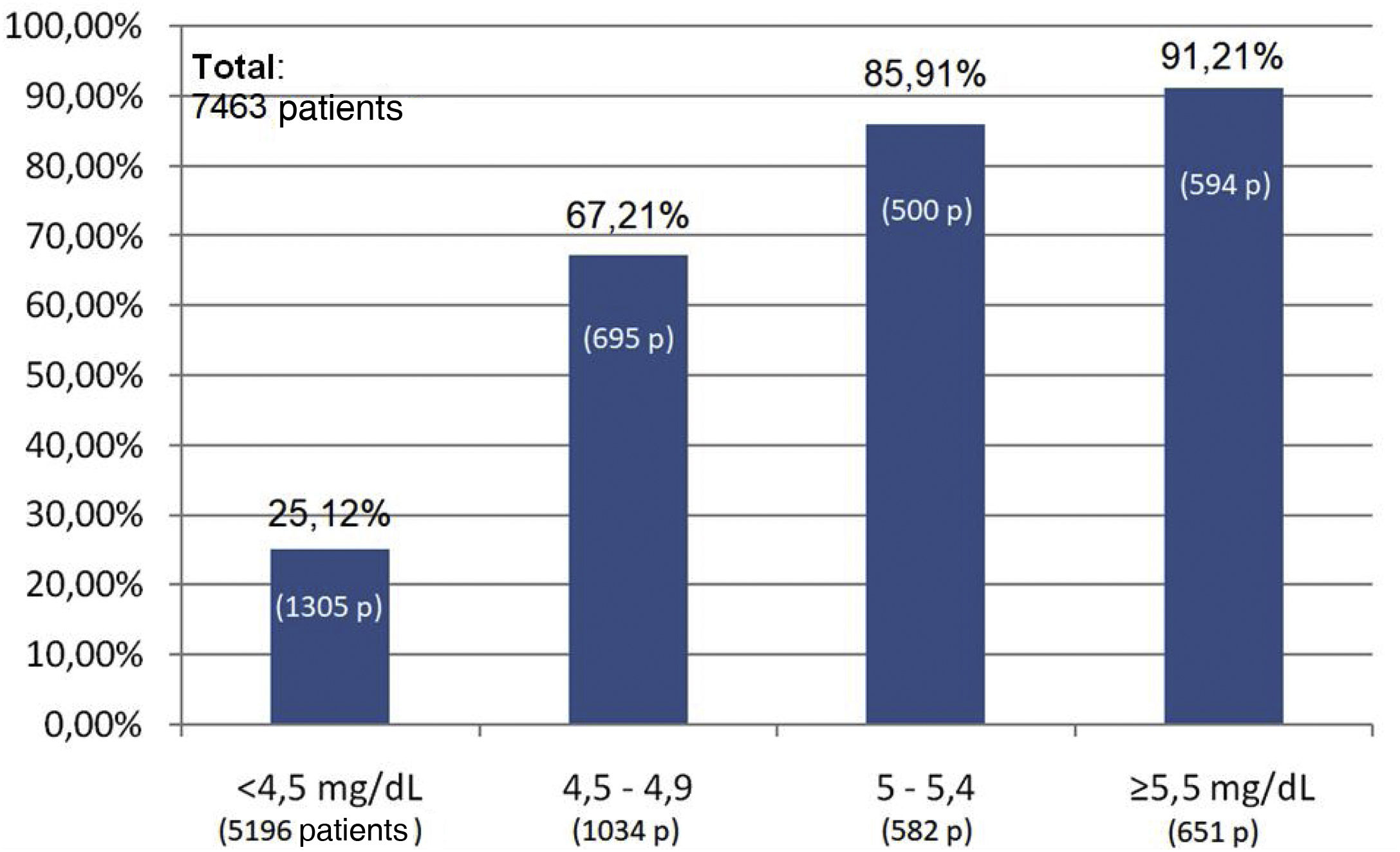
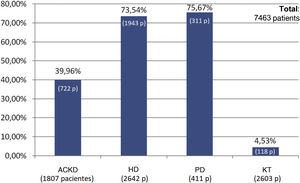
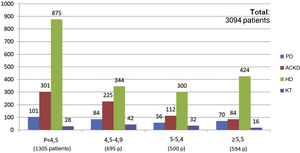
The percentage use of phosphorus binders prescribed to reduce serum phosphorus was 73.5% (1,943 of 2,642 patients) and 75.6% (311 of 411 patients) in the groups of patients on HD and PD, respectively; in the group of KT patients it was 4.5% (118 of 2,603 patients), and 39.9% in patients on ACKD (722 of 1,807) (Fig. 3). Globally, the vast majority of patients with phosphorus values ≥5.5 were on treatment with phosphorus binders. In addition, 25.1% of patients with a phosphorus <4.5 mg/dl were under treatment with phosphorus binders (Figs. 4 and 5).
DiscussionThe results of the "Phosphorus Week" provide information on the objectives, the degree of control of serum phosphorus and the pharmacological treatment used to achieve phosphate control in a large population of patients with CKD in Spain, including patients with aCKD, on HD/PD and KT.
In recent years, the control of phosphorus levels has acquired great relevance in the field of abnormalities of bone and mineral metabolism. The latest SEN Guidelines4 were published in 2011, and recommended achieving normal phosphorus values (between 2.5 and 4.5 mg/dl) in all stages of CKD, accepting up to 5 mg/dl in dialysis patients. Something similar is advised by the 2017 Kidney Disease: Improving Global Outcomes (KDIGO) Guidelines, although these focus primarily not so much on achieving normal phosphorus values but on having a tendency toward the normal range in those with hyperphosphatemia.
However, the KDIGO Guidelines generate some uncertainty in that they do not support active measures, such as dietary phosphorus control or the use of phosphorus binders when plasma phosphorus levels are in the normal range, because of the risk of malnutrition associated with hypophosphatemic diets or the side effects associated with the use of binders. This measure, however, could be in line with the findings of Block et al.13 in which treatment with phosphate binders, especially calcium, in patients with moderate to advanced CKD and normal or near-normal serum phosphorus concentrations significantly reduced serum and urinary phosphorus and attenuated the progression of secondary hyperparathyroidism, but resulted in progression of calcification.
The publication of the new SEN recommendations on the management of bone and mineral metabolism in patients with CKD is imminent. It was presented at the 50th Congress of the Spanish Society of Nephrology and it maintains the same objective of achieving a serum phosphorus concentration within the normal range at any stage of CKD. The enormous number of studies evaluating the impact of alterations in bone and mineral metabolism and the publication of the different guidelines (among them, the KDIGO 2017) should influence the arguments of nephrologists when implementing measures for the control of phosphate. Knowing the results of the "Phosphorus Week" carried out then and comparing them with the perception we have today of the management of hyperphosphatemia allows us to see how all these recommendations impact on Spanish nephrology.
The COSMOS study, a multicenter, open cohort, prospective, observational, European multicenter study with 3 years of follow-up, with data from more than 6,500 HD patients from all over Europe14 showed the lowest relative risk of mortality in patients with phosphorus values of 4.4 mg/dl (range between 3.6 and 5.2 mg/dl).15 In addition, it assessed the impact of variations in these values on mortality. In patients with baseline phosphorus values within this range, increases or decreases in phosphorus were associated with an increased risk of mortality. In contrast, in patients with baseline phosphorus values above this range (>5.2 mg/dl) reduction of serum phosphorus toward the safer range was associated with a lower relative risk of mortality.
According to the results of the "Phosphorus Week", the objectives of Spanish nephrologists are in line with the recommendations of national and international clinical guidelines. The mean target value (4.5 mg/dl) is in the range that these guidelines consider normal (2.5–4.5 mg/dl) and in the range that the COSMOS study15 observed a lower relative risk of mortality (although this work only includes patients on HD). There were no differences in target phosphorus control between patients seen in ACKD, PD, HD or RR, despite the fact that some guidelines are more permissive in phosphorus control in dialysis patients. The differences in the objectives of serum phosphorus control among the different regions within the Spanish territory are striking. Unfortunately, the analysis of the reasons for these differences cannot be carried out with the information provided by the "Phosphorus Week".
Patients on dialysis (HD and PD) show values of serum phosphorus higher than patients with ACKD and especially those on KT, where all the possibilities of renal function exist. This observation should not be surprising, given the close relationship between renal function and serum phosphorus clearance.16
In relation to the comparison between PD and HD, classically it has been commented that serum phosphorus control is slightly better in PD. Ansell17 found a difference of almost 0.3 mg/dl in serum phosphorus concentration when comparing the means of a large dialysis population (8,327 patients on HD and 3,084 patients on PD). In addition, data from the British Kidney Disease Registry18 reveal that more HD patients have hyperphosphatemia (defined as a serum phosphorus value above 5.3 mg/dl), as compared to the PD population. They estimate that 38% of patients on PD have hyperphosphatemia, compared to 43% of those on HD. This observation could be explained by the better preservation of renal function in PD19 and the characteristics of the technique itself (continuous in PD versus discontinuous in HD).20 However, all these observations are not found in our survey, which shows that the percentage of patients with serum phosphorus below 4.5 mg/dL is significantly higher in HD than in PD (53% versus 39%), although there is no striking difference when accounting for patients with serum phosphorus above 5.5 mg/dL (17% in both cases). To interpret this data correctly, other variables should be incorporated, such as the age of the patients in each group, therapeutic adherence to phosphorus binders, as well as the degree of malnutrition, among others. These variables should be taken into consideration in order to avoid population biases.
According to the results of the study, the use of phosphorus binders in dialysis patients is common (approximately 3 out of 4 patients), with no differences found between both dialysis techniques, with a lower use in patients in ACKD outpatients clinics (39% of patients) and almost testimonial in the case of KT (only 4%). Despite the extensive use of phosphorus binders in the dialysis population, only 53% and 39% of HD and PD patients, respectively, achieved phosphorus values <4.5 mg/dl, so it is of paramount importance to identify the factors related to this noncompliance (intolerance/side effects of binders, unintentional nonadherence, number of tablets, etc.) and intensify measures to optimize these values (improve patient information, prescribing focused on patient preferences, motivational interviewing during follow-up, and avoid both therapeutic inertia and nihilism). The type of binder was not assessed, so we cannot know the pharmacological treatment profile of the groups. In addition, there are other factors not controlled for in this survey that could explain this insufficient control despite treatment with phosphorus binders, such as poor control of secondary hyperparathyroidism (where the phosphorus would come from the bone and not from the diet), or the use of high doses of vitamin D treatment (versus calcimimetics) in patients with poor phosphorus control.
Special mention should be made regarding of the situation of patients in ACKD, in which only 39.9% of patients are on phosphate binders (722 of 1,807 patients). Despite this, 87.7% of patients in ACKD had plasma phosphorus values <5 mg/dl (1,187 patients with P < 4.5 mg/dl and 398 patients with P values between 4.5−5 mg/dl). From the combined data in Figs. 2 and 5 we can extract that only 11.7% of patients with ACKD with P ≥ 5 mg/dl values (26 patients out of 222) were not on phosphorus binders. However, if we consider the threshold of patients with P ≥ 4.5 mg/dl this percentage would increase to 32%. This group of patients have a potential for improvement in this aspect, although this survey does not evaluate possible contraindications or treatment limitations that would advise against its use.
In conclusion, the results of the "Phosphorus Week" indicate that: (a) the objectives of Spanish professionals are in line with what is recommended by national and international clinical action guidelines; (b) patients on HD and PD have higher serum phosphorus values than patients with ACKD and especially KT. No differences between both dialysis modalities in terms of the use of phosphorus binders; (c) all this indicates to us that there is still room for improvement to achieve better phosphorus control in an attempt to reduce mortality in these patients. However, given that a survey offers low epidemiological evidence and that it was answered by a limited number of professionals, it is advisable to take these data with caution.
Conflict of interestThe authors declare that they have no conflicts of interest.
We thank the Nephrology Services of the hospitals listed below for their disinterested collaboration in the realization of Phosphorus Week.
Asyter Albacete, Hospital del Mar, Hospital Vithas Perpetuo Internacional, Hospital Universitario de La Ribera, Hospital Álvarez Buylla, Hospital Perpetuo Socorro, Centro de Diálisis Logroño, Centro de Diálisis Nevada, Hospital Lluís Alcanys, Hospital Clínic de Barcelona, Centro de Diálisis La Axarquía, Centro de Diálisis de Villagarcía, Sistemes Renals S.A., Hospital San Agustín, Hospital General Universitario de Alicante, Hospital de Zafra, Hospital San Francisco de Borja, Hospital General de Llerena, Centro de Diálisis de Pontevedra, Complejo Hospitalario Xeral-Calde, Clínica Universitaria de Navarra, Clínica de Diálisis Alcañiz, Hospital Clínico San Carlos, Hospital de Alcañiz, Hospital Universitario Nuestra Señora de Candelaria, Hospital de Jarrio, Hospital 12 de Octubre, Hospital del Tajo, Hospital Universitario Doctor Peset, Complejo Hospitalario de Pontevedra, Hospital Universitario de Getafe, Avericum Gran Canaria (Hospital Dr. Negrín), Hospital Universitario y Politécnico La Fe, Fundación Hospital Jove, Centro de Diálisis de Linares, Consorcio Hospital General Universitario de Valencia, Hospital Infanta Leonor, Unidad Vithas Perpetuo Elche, Hospital Universitario Central de Asturias, Fundació Puigvert, Hospital Arquitecto Marcide, Hospital Son Llàtzer, Clínica Benidorm, Complejo Asistencial de Zamora, Hospital da Costa, Hospital San Jorge, Hospital de Jaca, Unidad de Hemodiálisis de la Cruz Roja Española, Clínica Diálisis Mérida, Unidad de Diálisis de la Casa de la Salud (Hospital Católico), Centro de Diálisis Sagunto.