Klotho, a key aging regulator, is predominantly expressed in the kidney. Various methods now enable the measurement of soluble αKlotho blood levels in humans. Limited studies have explored the renal origin of circulating αKlotho in humans.
MethodsSoluble αKlotho in the inferior vena cava blood was measured using an enzyme-linked immunosorbent assay kit using blood samples from patients undergoing adrenal venous catheterization for close examination of primary aldosteronism.
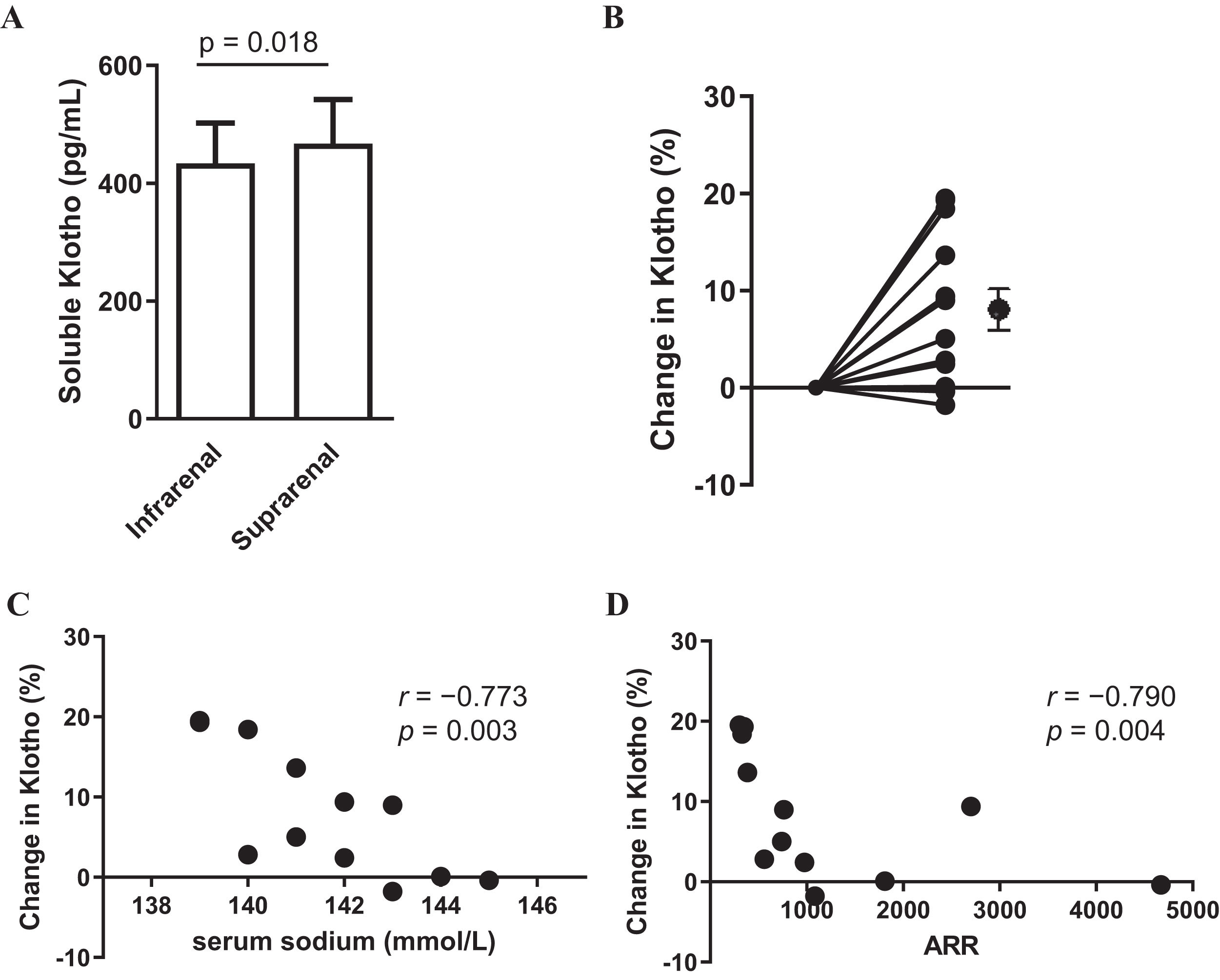
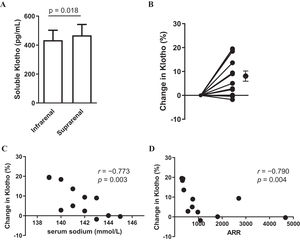
ResultsThe concentration at the suprarenal inferior vena cava (476±68.2) was significantly higher than that at the infrarenal inferior vena cava (434±74.8) (p=0.018), with a rate of change of 8.12 (2.3)%.
ConclusionsWe demonstrate a step-up in αKlotho concentration from the infrarenal to suprarenal vena cava in humans, supporting the kidney's origin of soluble αKlotho in the bloodstream.
Klotho, un regulador clave del envejecimiento, se expresa predominantemente en el riñón. En la actualidad, diversos métodos permiten medir el nivel sanguíneo de α-klotho soluble en humanos. Son escasos los estudios que han explorado el origen renal de la α-klotho circulante en humanos.
MétodosSe midió la α-klotho soluble en la sangre de la vena cava inferior mediante un kit de ensayo inmunoabsorbente ligado a enzimas en muestras de sangre de pacientes con cateterismo venoso suprarrenal para un examen detallado del aldosteronismo primario.
ResultadosLa concentración en la vena cava inferior suprarrenal (476±68,2) fue significativamente superior a la de la vena cava inferior infrarrenal (434±74,8) (p = 0,018), con una tasa de variación del 8,12% (2,3%).
ConclusionesDemostramos un aumento de la concentración de α-klotho desde la vena cava infrarrenal a la suprarrenal en humanos, lo que respalda el origen renal de α-klotho soluble en el torrente sanguíneo.
The αKlotho gene, initially discovered in mice, exhibits a deficiency-induced phenotype akin to human aging.1 Highly expressed in the kidney, the Klotho protein functions as a receptor for fibroblast growth factor 23 (FGF23).2 Klotho forms the FGFR-Klotho complex by binding to the FGF receptor (FGFR) via the receptor binding arm, serving as a physiological receptor for FGF23.2 Known as a single-pass transmembrane protein, the Klotho protein undergoes cleavage by proteolytic enzymes, such as secretases, resulting in the secretion of its extracellular domain (ectodomain shedding).3 In patients with chronic kidney disease, renal Klotho transcript significantly decreases, and soluble αKlotho emerges as a promising early-stage kidney disease biomarker.4 Current methods enable the measurement of soluble αKlotho (sKlotho) levels. Although the origin of sKlotho was identified as αKlotho highly expressed in the kidney,5 measuring sKlotho in healthy humans through invasive tests like catheterization proves challenging. Consequently, few clinical studies have reported the renal origin of blood sKlotho in humans via catheter sampling. To address this, we opted to measure sKlotho in blood samples from patients undergoing adrenal venous sampling (AVS) for primary aldosteronism (PA) diagnosis. This approach aims to ascertain whether the kidneys secrete sKlotho and identify the clinical parameters associated with blood sKlotho.
Patients and methodsPatientsEligible participants comprised patients with PA undergoing AVS for unilateral aldosterone-producing adenoma diagnosis at Jichi Medical University Saitama Medical Center during the 2019–2020 enrollment period. A total of 12 consecutive patients were enrolled, and PA was diagnosed following the clinical practice guidelines of the Japan Endocrine Society.6 All AVS procedures were conducted by interventional radiologists, with 12 cases confirming successful AVS outcomes. This study, approved by the ethics committee of Jichi Medical University, Saitama Medical Center (no. S17-006), adhered to the ethical guidelines of the Declaration of Helsinki, and written informed consent was obtained from all subjects.
Patient data collectionBasic clinical data, including age, sex, body mass index, and systolic or diastolic blood pressure before AVS, were collected. Additionally, serum sodium, potassium, estimated glomerular filtration rate (eGFR), hemoglobin (Hb), plasma renin activity (PRA), and plasma aldosterone concentrations (PAC) were measured. PAC and PRA were assessed using radioimmunoassay and enzyme immunoassay, respectively. Post-AVS, blood samples were promptly transported to the in-hospital laboratory, with the remaining samples frozen at −80° after aldosterone concentration measurement. Soluble αKlotho was measured using an enzyme-linked immunosorbent assay (ELISA) kit (soluble α-Klotho Assay Kit, IBL, Japan). This ELISA kit, featuring intra-assay and inter-assay coefficients of variation (2.7–3.5% and 2.9–11.4%, respectively), showed no cross-reaction to βKlotho. Soluble αKlotho was measured using triplicate blood samples collected from the infrarenal and suprarenal inferior vena cava (IVC).
Statistical analysisContinuous variables were presented as means (± standard error of the mean, SEM) or medians with interquartile range, while categorical variables were expressed as numbers or percentages. Linear correlations between soluble αKlotho and clinical parameters were determined using Pearson's or Spearman's correlation coefficient analysis. The change in αKlotho (%) at the suprarenal IVC level relative to the infrarenal IVC level (baseline, 0%) was calculated. Paired t-tests were employed to compare soluble αKlotho (pg/mL) between the two groups. All analyses were conducted using EZR (Jichi Medical University Saitama Medical Center), a graphical user interface for R (The R Foundation for Statistical Computing, ver. 2.13.0), and a modified version of the R commander (ver. 1.6-3) with added statistical functions frequently used in biostatistics.7 A significance level of p<0.05 was applied based on two-tailed calculation.
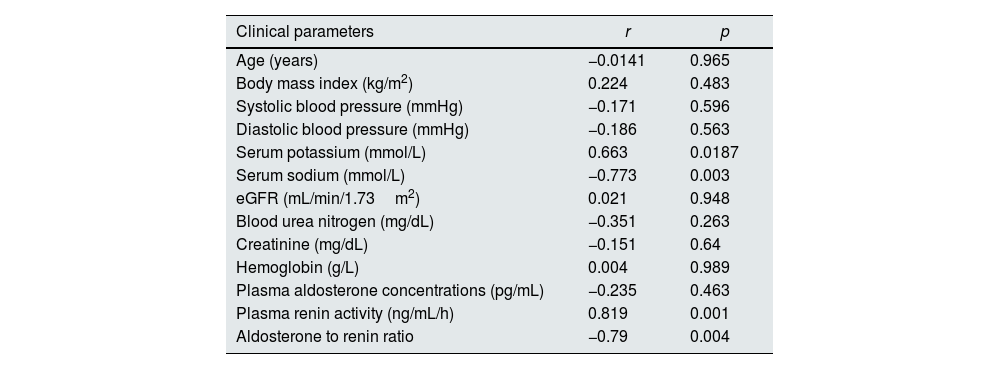
ResultsTable 1 presents the baseline characteristics of the 12 patients. In summary, the median age was 44.5 [43.8–56.0] years, with 50% (n=6) being male. The mean eGFR was 78 (±22) (mL/min/1.73m2). For all 12 cases, the PAC/PRA ratio (ARR) exceeded 200(pg/mL/ng/mL/h). All patients were on calcium channel blockers and three of them on concomitant alpha blockers. The patients were not taking any medications (mineralocorticoid receptor antagonists, beta blockers and diuretics) that could significantly affect ARR. The sKlotho concentration at the suprarenal IVC (476±74.8) was significantly higher than that at the infrarenal IVC (434±68.2) (p=0.018) (Fig. 1A), with a change rate of 8.12±2.3% (Fig. 1B). Correlations were observed between serum sodium level and the change in sKlotho (r=−0.773, p=0.003) and between ARR and the change in sKlotho (r=−0.790, p=0.004) (Fig. 1C and D). Table 2 shows the association between other clinical parameters and the rate of change in sKlotho. Renal function indices, such as BUN, Cr, and eGFR, did not exhibit significant associations with the rate of change in soluble αKlotho.
Baseline characteristics of the 12 patients.
| Clinical parameters | |
|---|---|
| Age (years) | 44.5 [43.8–56] |
| Male, n (%) | 6 (50) |
| Body mass index (kg/m2) | 27.5±7.1 |
| Systolic blood pressure (mmHg) | 136±14 |
| Diastolic blood pressure (mmHg) | 83±12 |
| Serum potassium (mmol/L) | 3.4±0.56 |
| Serum sodium (mmol/L) | 142±1.9 |
| eGFR (mL/min/1.73m2) | 78±22 |
| Blood urea nitrogen (mg/dL) | 13.5±5.2 |
| Creatinine (mg/dL) | 0.80±0.31 |
| Hemoglobin (g/L) | 13.7±1.36 |
| Plasma aldosterone concentrations (pg/mL) | 261±128 |
| Plasma renin activity (ng/mL/h) | 0.3 [0.2–0.4] |
| Aldosterone to renin ratio | 751 [375–1266] |
Data are presented as numbers (%) for categorical variables and as means (±standard error of the mean, SEM) or median with interquartile range (IQR) for continuous variables.
Blood soluble αKlotho in the infrarenal and suprarenal vena cava (A). Change in αKlotho (%) from the infrarenal to suprarenal vena cava (B). Relationship between serum sodium (mmol/L) levels and change in αKlotho (%) (C). Relationship between PAC/PRA ratio (ARR) and change in Klotho (%) (D). Data are presented as mean with standard error of the mean (SEM). Black circles and bars indicate the mean and SEM of Klotho.
Relationship between change in αKlotho (%) and clinical parameters.
| Clinical parameters | r | p |
|---|---|---|
| Age (years) | −0.0141 | 0.965 |
| Body mass index (kg/m2) | 0.224 | 0.483 |
| Systolic blood pressure (mmHg) | −0.171 | 0.596 |
| Diastolic blood pressure (mmHg) | −0.186 | 0.563 |
| Serum potassium (mmol/L) | 0.663 | 0.0187 |
| Serum sodium (mmol/L) | −0.773 | 0.003 |
| eGFR (mL/min/1.73m2) | 0.021 | 0.948 |
| Blood urea nitrogen (mg/dL) | −0.351 | 0.263 |
| Creatinine (mg/dL) | −0.151 | 0.64 |
| Hemoglobin (g/L) | 0.004 | 0.989 |
| Plasma aldosterone concentrations (pg/mL) | −0.235 | 0.463 |
| Plasma renin activity (ng/mL/h) | 0.819 | 0.001 |
| Aldosterone to renin ratio | −0.79 | 0.004 |
eGFR, estimated glomerular filtration rate.
In this study, we utilized ELISA to demonstrate in humans that the concentration of sKlotho at the suprarenal IVC surpasses that at the infrarenal IVC, affirming the kidney as the organ of origin for soluble αKlotho. Hu et al. conducted a study with blood from catheterized patients (n=9) evaluating heart failure and other conditions, revealing a step-up in sKlotho concentration from the infrarenal to suprarenal vena cava.8 They measured sKlotho concentrations as protein levels using Western blot.8 While catheterization is highly invasive and challenging in normal subjects, our study and theirs consistently depict a step-up in sKlotho concentration from the infrarenal to suprarenal vena cava, despite differing pathological conditions in the sampled patients.
Picciotto et al. extensively examined renal Klotho release parameters in patients undergoing cardiac catheterization (n=22), identifying oxygen uptake as a predictor of renal αKlotho release.9 Although our study did not assess oxygenation, we found a correlation between the change in sKlotho (%) and serum sodium and ARR. In PA, excessive aldosterone secretion induces sodium reabsorption, leading to increased fluid volume and hypertension.10 Klotho(+/−) mice with elevated aldosterone levels develop hypertension,11 suggesting a potential link between strong aldosterone production and impaired αKlotho secretion. However, no relationship was observed between baseline blood pressure and the extent of sKlotho change. A recent large-scale study reported no difference in serum sKlotho levels measured by ELISA between individuals with and without hypertension.12 On the other hand, partial correlation analysis, controlling for serum potassium levels, showed no correlation between changes in sKlotho and serum sodium (r=0.162, p=0.677) in this study. This could be a result of the pathophysiology of primary aldosteronism affecting serum potassium levels, but the small sample size precluded detailed statistical analysis. Further investigation is needed to explore the relationship between hyperaldosteronism severity and the extent of sKlotho change.
In our study, the change in sKlotho did not correlate with eGFR. Only two patients had an eGFR below 60mL/min/1.73m2, and none exhibited advanced renal impairment. Given aldosterone's renal damage effects13 and the potential decrease in blood sKlotho levels from the early stages of renal damage,4,14,15 it cannot be ruled out that subjects in this study might have lower blood sKlotho levels than healthy individuals. Blood sKlotho levels in our study were measured by ELISA rather than protein levels. Another study utilizing an immunoprecipitation immunoblot (IP-IB) assay showed a decrease in sKlotho in people with renal impairment.16 Additionally, it has been reported that whether measured by ELISA or IP-IB, when a blood sample undergoes two or more freeze-thaw cycles, the value significantly decreases compared to zero or one thaw cycle.16 Our study used samples frozen only once, and we believe that sample degradation did not have a significant effect. IB could measure distinct full-length proteins but is labor-intensive, while ELISA is a simple method, but the results are highly variable and less accurate than IB. In this study, the number of blood samples obtained was small and klotho could not be measured in IB.
However, this study has several limitations. First, as a small-scale study, it was not feasible to thoroughly investigate the patient background under which the sKlotho step-up disappears. Other factors relevant to the FGF23-Klotho endocrine system, such as FGF23, serum calcium, phosphate, and parathyroid hormone, were also not evaluated. Analyzing whether these parameters affect serum sKlotho levels or the change in sKlotho should be considered. Second, sKlotho was not measured using any method other than ELISA to verify if a similar αKlotho step-up exists.
ConclusionsIn conclusion, Klotho was measured using ELISA, demonstrating the step-up of αKlotho concentration from infrarenal to suprarenal vena cava in humans. This supports the kidney's origin of circulating soluble αKlotho.
Conflicts of interestNone.
The authors thank Enago for English editing.