Introducción: la parathormona (PTH) presenta buena correlación con los parámetros histomorfométricos y bioquímicos de remodelado óseo, aunque su cuantificación presenta limitaciones por la variabilidad según el método empleado. La PTH circulante es una mezcla de péptidos, y solo la PTH 1-84 es responsable de su actividad. Los fragmentos carboxiterminales tienen acción antagónica y, al aclararse por el riñón, varía su proporción según el estadio de enfermedad renal crónica. Nos planteamos estudiar posibles diferencias en su proporción en función del tipo de diálisis: hemodiálisis (HD) o diálisis peritoneal (DP). Material y métodos: En 73 pacientes en DP (46 varones y 27 mujeres entre 22 y 82 años) se cuantificó calcio total (Ca) y iónico (Cai), fosforo (P), telopéptidos carboxiterminales del colágeno tipo I (BCTx) y PTH mediante seis métodos de segunda generación (uno isotópico (IRMA) y cinco inmunoquimioluminescentes (ECLIA) y por el único método PTH de tercera generación (IRMA) disponible en ese momento. Resultados: Las concentraciones medias de Ca, Cai, P y BCTx fueron respectivamente, 9.03, 4.76, 4.73 mg/dl y 1181 pmol/l. Se observaron diferencias significativas en la PTH dependiendo del método de segunda generación utilizado. Los valores de PTH ajustados al rango equivalente a 150-300 de la PTH Allegro de Nichols en los pacientes en DP fueron superiores a los obtenidos en el estudio previo de HD. El porcentaje de PTH 1-84 biológicamente activa y el ratio PTH 1-84/7-84 fueron significativamente menores, indicando que, a igualdad de valor de PTH intacta, el porcentaje de fragmentos 7-84 circulantes es mayor en los pacientes en DP. Conclusión: Los pacientes en DP tienen una mayor proporción de fragmentos 7-84 PTH. Por este motivo las fórmulas de corrección inter-método utilizadas en los pacientes en HD no son aplicables en DP. En este estudio sugerimos otras fórmulas para aplicar en DP.
Introduction: Parathyroid hormone (PTH) shows a strong correlation with histomorphometric and biochemical parameters of bone turnover, however its measurement presents limitations due to inter-method variability. Circulating PTH is a mixture of peptides, but only on its whole form (1-84 PTH) is responsible of PTH biological activity. Carboxyl-terminal fragments exhibit antagonist actions and their proportion differs at each stage of chronic kidney disease, as consequence of differences on their renal clearance. The aim of this study is to evaluate possible differences in the proportion of these fragments according to dialysis type: haemodialysis (HD) or peritoneal dialysis (PD). Material and methods: Serum total (Ca) and ionized calcium (iCa), phosphate (P), carboxyl-terminal telopeptides of collagen type I (BCTx) were measured in 73 patients on PD (46 men and 27 women with an age between 22 and 82 years). PTH was quantified by six second generation assays (one isotopic and five chemiluminescence assays) and by one third generation PTH method. Results: Mean serum levels of Ca, iCa, P and BCTx were 9.03, 4.76, 4.73 mg/dl and 1181 pmol/l, respectively. Significant differences were observed in PTH values according to the method used. Adjustment of PTH results to PTH Allegro (Nichols) range of 150-300 nmol/l in PD patients showed higher values than those assessed previously for HD population. The percentage of biologically active 1-84 PTH as the 1-84 PTH/ 7-84 PTH ratio in PD were significantly lower than in HD patients, reflecting the higher proportion of 7-84 PTH circulating fragments for a given intact PTH result in PD. Conclusions: PD patients have a higher proportion of 7-84 PTH circulating fragments. Consequently, the inter-method adjustment algorithms proposed for HD patients are not useful for PD patients. This study proposes alternative algorithms for PTH inter-method adjustment to be applied in PD.
INTRODUCTION
Mineral and bone disorders triggered by chronic kidney disease (CKD) are a major cardiovascular mortality risk factor. The association between these disorders and an increase in cardiovascular disease has also been reported in the general population1, which explains their diagnosis and early correction.
Monitoring circulating levels of parathyroid hormone (PTH) in kidney patients is key to the non-invasive diagnosis of bone disease2. Although the high or low values of circulating PTH have a good correlation with histomorphometric and biochemical bone turnover parameters3, PTH quantification has its limitations and often results in diagnostic doubts4. The first KDOQI (Kidney Disease Outcomes Quality Initiative) guidelines established a reference range for PTH in stage 5D CKD of between 150 and 300nmol/l5 in accordance with the results of bone biopsies existing at that time, taking into account that most patients with PTH of less than 150nmol/l (quantified by a second generation method: Nichols Allegro) displayed adynamic bone, and those with a PTH greater than 300nmol/l displayed high bone turnover.
Subsequent studies have demonstrated the enormous variability of PTH results in accordance with the method used for its quantification6, due to: 1) the heterogeneity of circulating forms of PTH, 2) the differences in antigenic configuration between the different methods, meaning that they do not all identify the same circulating forms of PTH, and 3) differences in method standardisation. Circulating PTH is a mixture of peptides of which intact PTH 1-84 is responsible for the classic biological action on bones and kidneys. A series of carboxy-terminal fragments circulate alongside this molecule, which are the result of intraparathyroid and hepatic degradation. Around 10% these are long fragments cleaved between amino acids 4 and 19 and are known as PTH 7-84 or PTH non-1-847. These 7-84 fragments are not inactive and, upon activating a carboxy-terminal receptor, they exercise an antagonistic effect to that of the intact PTH8-10. There is a problem because not all methods identify the same circulating forms of PTH; as such, the “intact PTH” or second generation methods quantify PTH 1-84 and the long PTH 7-84 carboxy-terminal fragments, while the “bio-PTH”, “whole PTH” or third generation methods avoid the interference of the antagonistic effect of PTH 7-84. This difference in the antigenic configuration explains why the PTH results obtained with the third generation methods are approximately half those obtained with a second generation method, but it does not explain the discrepancies that exist among the second generation methods. The absence of a universal synthetic human PTH standard would explain this variability, because each method is calibrated with synthetic PTH standards of different origins6.
Awareness of this variability between methods has created concern due to its clinical implications6,11, since the NKF (National Kidney Foundation)/KDOQI recommendations were established with a second generation method (Nichols Allegro PTH), considered the gold standard because it was validated by bone histomorphometry. In a comparative study that considered this method as a reference, deviations from -40 to 120% were observed6. This meant that the 150-300nmol/l recommended by the KDOQI guidelines for stage 5 CKD was not applicable whenever other PTH methods were used and it led to erroneous therapeutic decisions if a specific correction factor was not applied for each PTH method12. This variability between methods, along with the current tendency to identify extreme levels associated with the risk of mortality13-17, is the reason for the current KDIGO guidelines to advise the use of extreme risk intervals calculated between <2 and >9 times the upper reference limit of the PTH method used in each hospital18.
Given this situation, the Spanish Society of Nephology (S.E.N.) designed a cross-sectional study to assess the variability between PTH methods and establish a potential equivalence between the methods most used in our country19. Based on this study, cards with algorithms were issued to nephrologists to facilitate their interpretation of PTH results and an application was uploaded to the S.E.N. website that allowed calculation of the adjustment according to the method. However, these adjustments were made based on a haemodialysis- (HD) prevalent CKD population and, therefore, they are only applicable to HD patients. Within the group of patients with stage 5D CKD, there may be variations in the percentage of circulating PTH forms in accordance with the type of dialysis: HD or peritoneal dialysis (PD). We therefore proposed a second study with an identical design for stage 5 CKD patients on PD, in order to assess potential differences and, if there were any, have the adjustments between PTH methods for this population.
MATERIAL AND METHOD
This assessment of PTH methods in the context of CKD was designed as a cross-sectional study. After obtaining informed consent, a total of 73 CKD patients on PD from the same hospital were recruited. All of them underwent a clinical assessment, recording their age, sex, anthropometric data and medication, particularly with regard to drugs with a special influence on PTH synthesis and release, such as vitamin D analogues and/or calcimimetics. All patients received dialysis with three or four daily exchanges of 2l with solutions containing 1.75mmoles/l of calcium.
Blood samples were collected in the morning and after its identification using a numeric code, the serum was separated and it was frozen in aliquots at -80ºC to be subsequently defrosted and analysed. Ionised calcium was also quantified in whole blood using an ion-selective electrode (Rapidpoint 400; Siemens) and the serum values of calcium and phosphate were quantified using the standard method in a Cobas Modular autoanalyser (Roche). One of the serum aliquots was used to quantify the circulating values of carboxy-terminal telopeptide of type I collagen (β-CTx) using an automatic electrochemiluminescent (ECLIA) method (Elecsys CrossLaps, Roche), as a bone turnover marker in all patients. In the remaining aliquots, we determined the serum PTH concentration by different methods, using the same batch and processing all samples in the same assay to minimise the effect of analytical variability.
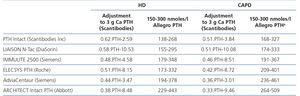
The selection of PTH methods for this study was identical to that designed in the previous study on 129 HD patients, which was based on the assessment of a previous questionnaire on PTH quantification sent to all dialysis centres in our country. Using these results as a basis and considering not only the number of centres but also the number of patients treated by each, we decided to carry out this study on serum, since it is the biological matrix that is recommended in clinical practice20 and that most commonly used in our survey. We did so using a second generation isotopic immunoradiometric (IRMA) method, five electrochemiluminescent (ECLIA) methods, and the only third generation PTH (IRMA) method available at that time (Table 1):
Total intact PTH (Scantibodies Laboratory Inc). Non-automatic IRMA that uses polyclonal anti-PTH 39-84-coated beads and a second specific I125-labelled antibody against PTH region 1-34. The intra- and inter-assay coefficients of variation assigned by the manufacturer are lower than 4.9% and 6.75%, respectively. It quantifies intact PTH 1-84 with the interference of PTH 7-84.
ELECSYS intact PTH (Roche). Automatic ECLIA based on a double antibody sandwich system: the first of them is directed against a distal amino-terminal region (26-32) and it immobilises the circulating forms of PTH that contain that amino acid sequence (PTH 1-84, PTH non-1-84 and amino-PTH), and the second is ruthenium-labelled and recognises the sequence 55-64 and quantifies it. The reference range indicated by the manufacturers is 15-65nmol/l. It is the only second generation method that quantifies amino-PTH, along with intact PTH 1-84 and PTH non-1-84.
AdviaCentaur PTH (Siemens). ECLIA based on a double antibody system: the first recognises the sequence 39-84, and the second is directed against the amino-terminal region 1-34, which is acridinium ester-labelled to allow quantification. The reference range indicated by the manufacturer is 14-72nmol/l. As with the other second generation methods (with the exception of the PTH Elecsys), it measures the intact hormone 1-84, with the crossed interference of PTH truncated at the amino-terminal level.
IMMULITE 2500 Intact PTH (Siemens). Automatic ECLIA that uses a double antibody system. The first of them, in coated beads, is a polyclonal murine anti-PTH 44-84 antibody and the second is a goat polyclonal anti-PTH 1-34 antibody labelled with an enzyme (alkaline phosphatase). The guideline reference range indicated by the manufacturer is 12-65nmol/l, and the maximum intra- and inter-assay imprecision coefficients are 5.7% and 8.6%, respectively.
ARCHITECT Intact PTH (Abbott). It is an automatic sandwich ECLIA that uses two goat polyclonal anti-PTH antibodies: the first, connected to paramagnetic microparticles, binds to PTH and the second is an anti-PTH acridinium-labelled conjugate. The regions of the PTH molecule against which both antibodies are directed are not specified in the protocol. The reference interval indicated by the manufacturer for healthy adults is 15-68.3nmol/l, and the maximum intra- and inter-series variability is 6.1% and 8.7%.
LIAISON N-Tac PTH (DiaSorin). Direct automatic sandwich ECLIA with two binding sites: the solid phase with an antibody against PTH region 39-84 and the second antibody against the 1-34 region conjugated with an isoluminol derivative, inducing the chemiluminescent reaction. The reference range indicated by the manufacturer is 12.3-73nmol/l.
Whole PTH (CA-PTH) (Scantibodies Laboratory Inc). Non-automatic third generation IRMA that immobilises the PTH using the same polyclonal anti-PTH 39-84-coated bead described in the foregoing Scantibodies method, but, unlike the latter, the second specific I125-labelled antibody is only directed against PTH region 1-4. The intra- and inter-assay coefficients of variation assigned by the manufacturer are lower than 4.83% and 7.75%, respectively. Since it is a third generation method, it only quantifies intact molecule 1-84, without the crossed interference of PTH 7-84, but with the amino-PTH minority circulating fraction.
The results obtained were recorded on a data sheet specifically designed for this study and the statistical analysis was carried out using the General + Clinical Laboratory Statistics Analyse-it (vsn 1.73) and SPSS IT software. After the analysis of frequency distribution and Spearman’s correlation, the comparative analysis and the similarity study between the different PTH methods were carried out, applying the Passing and Bablok fits. Lastly, we compared these results with those obtained in the previous variability between PTH methods study on HD patients, through Student’s t-test and χ2. The level of significance was established for P at <.05.
RESULTS
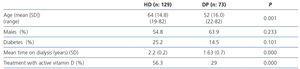
The study population included 46 males and 27 females aged between 22 and 82 (mean ± standard deviation [SD]: 52±16 years old) and a mean time of 1.63±0.7 years on dialysis. The diabetes percentage in this population was 14.5%. 29% were treated with a vitamin D active metabolite and none received treatment with paricalcitol or calcimimetics. We did not analyse residual function. Table 2 compares the characteristics of this study population with those of the HD prevalent population on whom we performed the previous inter-method adjustment study, with there being significant differences in age and time on dialysis, which was lower in the PD group, as well as in the percentage of patients treated with the active from of vitamin D, which was also lower in this group.
The mean ionised calcium and total circulating calcium concentrations were 4.76mg/dl (95% confidence interval [CI]: 4.66-4.84mg/dl) and 9.03mg/dl (95% CI: 8.8-9.26mg/dl) respectively, and the mean serum phosphate value was 4.73mg/dl (95% CI: 4.38-5.08mg/dl). The mean serum concentration of βCTx was 1181pmol/l (95% CI: 946-1393pmol/l), a range that was much higher than that of the general population (300pmol/l, SD: 142pmol/l).
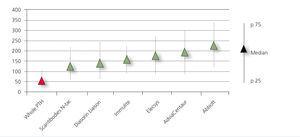
The distribution of serum PTH concentration was similar for each PTH method assessed. However, when comparing the circulating levels of intact PTH, we observed major differences in the mean concentrations obtained by all second generation methods assessed (Figure 1), with the isotope method showing mean values that were lower than those estimated using all the ECLIA, with the exception of the DiaSorin method. Considering that the third generation method does not have crossed interference with the long carboxy-terminal fragments, which account for approximately 50% in kidney patients, the results were significantly lower than those obtained using second generation methods (107.2nmol/l [SD: 154nmol/l; 95% CI: 65.5-148]). The PTH 1-84/PTH 7-84 ratio calculated using the Scantibodies methods was 0.88 (95% CI: 0.7-1.1).
Moreover, (second generation) serum intact PTH levels quantified by the different methods showed a very good correlation with each other and with the third generation whole PTH, showing R values between 0.924 (Elecsys versus DiaSorin) and 0.993 (Centaur versus Whole and Centaur versus Scantibodies). Likewise, all methods were significantly associated with phosphataemia and carboxy-terminal telopeptides of collagen type I (β-CTx), and inversely with ionised calcium (Table 3). The very good correlation between the different PTH methods allowed the similarity study and the inter-method adjustment (Table 4) to be carried out.
Upon stratifying patients into three groups according to second generation circulating PTH levels, in accordance with the criteria established in the KDOQI guidelines for stage 5D CKD5: (A) low turnover with PTH values <150nmoles/l, (B) PTH 150-300nmoles/l and (C) high turnover with PTH >300 nmoles/l, without carrying out the inter-method adjustment, we observed major differences in their distribution according to the PTH method used, especially for Abbott’s Architect method, which, unlike the others, displayed a lower percentage of adynamic patients (30.1%) in relation to group C, which represented patients with high turnover (38.4%) (Figure 2A). After transforming the PTH values estimated by the different second generation methods to whole PTH, in accordance with the Passing and Bablok analysis (Table 3) and adjusting to the range equivalent to 150 and 300nmoles/l of Allegro (Nichols) PTH with that established in the KDOQI guidelines (corresponding to 84 and 165nmoles/l of whole PTH [Scantibodies], according to Souberbielle et al.6), the distribution pattern for these three groups was made even and we observed a higher patient percentage in the adynamic range (53.4 [Abbott]-65.8 [Elecsys]%) (Figure 2B).
Once we carried out the regression study, we compared the results obtained in the PD population with those estimated in the previous study carried out on HD patients (Table 4) and observed that, after calculating the range equivalent to 150-300 of Nichols Allegro PTH, the PTH levels were higher in PD patients, and if we had adjusted them for PD patients using the algorithms calculated in HD patients, patient distribution for turnover activity according to the KDOQI guidelines would be significantly different (χ2 Scantibodies: 94.78, P <.001, χ2 DiaSorin: 71.8, P <.001, χ2 Imulite: 122.9, P <.001, χ2 Elecsys: 94.3, P <.001, AdviaCentaur: 110.6, P <.001, χ2 Abbott: 117.2, P <.001) with a lower proportion of patients in the adynamic range (49.3-55.6%) (Figure 2C).
This observation prompted us to study the possible differences in PTH according to the type of dialysis. As is recorded in Table 5, although there were no differences in mean PTH concentrations quantified by both second and third generation methods between the two dialysis groups, the PD group displayed a lower percentage of biologically active H 1-84 (CaPTH Scantibodies) and also a significantly lower PTH 1-84/PTH 7-84 ratio and as such, at an equal intact PTH value, the percentage of circulating 7-84 fragments is higher in PD patients. As confirmation of the above, the PD patient group also displayed lower mean β-CTx values than those observed in the HD group (1181 versus 2084pmol/l, p=.001), reflecting lower bone activity in PD patients. Likewise, we also observed significant differences in blood concentration of ionised calcium, whose circulating values were higher in PD patients.
DISCUSSION
It is seemingly true that PTH is no longer the focus of attention in mineral metabolism disorders in CKD, giving way to recently discovered molecules such as fibroblast growth factor 23 and Klotho. However, it continues to play a key role in the daily control of these disorders in normal clinical practice, particularly in the assessment of renal osteodystrophy. PTH, along with calcium and phosphorus, are the values used for providing treatment almost daily. Therefore, the metabolism group of the S.E.N. gives great importance to an adequate knowledge of what PTH represents in reality and how we can improve daily practice with its correct interpretation.
The first KDOQI guidelines established reference ranges for PTH in different CKD stages without specifying the PTH method, since they did not consider that there could be a significant variability between methods, let alone possible differences between dialysis techniques. Despite their limitations, especially in diagnosing low turnover situations21, the S.E.N. guidelines continue to recommend similar values, although they require values between 100 and 500nmoles/l22, when considering variability between methods. Additionally, and in order to partially alleviate this problem, the S.E.N. has proposed adjustment formulas that would allow homogenisation of PTH values independently of the method used19 after carrying out a variability study on HD-prevalent stage 5 CKD patients. To date, we have used these algorithms to adjust the results of PTH in patients on dialysis without considering the dialysis technique.
This study uses the same design employed by the S.E.N. in HD with PD patients. The distribution of mean PTH values between the different methods assessed was very similar to that found in the previous HD study, with automatic ECLIA methods in general yielding higher results, particularly that of Abbott. The concentration of PTH quantified by the third generation method was, as expected, notably lower in relation to intact PTH methods because it eliminates the interference of fragment 7-84. On comparing mean second and third generation PTH values in this PD population with those obtained using these same methods in HD patients, no significant differences were found. Given this situation, we interpret that the response of the parathyroid gland is consistent in both dialysis techniques and we do not consider that there are differences in the circulating forms of PTH. However, the adjustment of second generation methods to the third generation method, representing the reality of the biologically active molecule 1-84, was different from that reported in HD patients, with there being major differences in the proportion of fragments 7-84 between the two dialysis techniques. Years ago, differences were also reported in circulating intact PTH and PTH fragment values between HD and PD patients23, and although the findings of that study are not comparable with those of this study, since we are contrasting first generation PTH methods (which measure practically all circulating forms of PTH) with second generation methods, they showed evidence of these discrepancies, which were explained by differences in the clearance of PTH fragments in accordance with the type of dialysis. In this study, the major differences observed both in the PTH 1-84/7-84 ratio and in the percentage of PTH 1-84 between the two dialysis techniques showed that, for the same intact PTH value quantified using a second generation method, the proportion of biologically active PTH 1-84 is lower when the patient is on PD than when they are on HD.
Along with these differences in the proportion of circulating PTH fragments, calcium balance was also different between the two dialysis techniques. In fact, ionised calcium was significantly higher in our PD population, despite a smaller amount of active vitamin D being administered than in the HD group. This may be explained, at least in part, by calcium content in the dialysate24, since the PD dialysate has a calcium concentration of 1.75mmol/l, while in HD, it is 1.25-1.5mmol/l. Furthermore, PD is a continuous technique, and as such, this exchange is permanent, while in HD, it only occurs during the technique session25. Although ionised calcium is higher in PD, total calcium is identical in both techniques, and as such, the calcium normally used in clinical practice does not represent the reality. A possible explanation for this difference in ionised calcium may be a higher protein loss through the peritoneum in PD, which would mask a higher proportion of free calcium than protein-bound circulating calcium when total calcium is quantified. Unfortunately, we cannot demonstrate differences in serum protein concentration between the two groups since we do not have this information available.
PTH 1-84 catabolism starts in the gland itself and is regulated by extracellular ionised calcium concentration26; in fact, circulating levels of PTH are inversely associated with ionised calcium. An increase in serum ionised calcium leads to increased degradation within the parathyroid cell itself, increasing fragments 7-84, which would explain why PD patients have a higher proportion of PTH 7-84. In turn, this increase in PTH fragments may in part be responsible for the development of adynamic bone in PD patients27, which is more common in the latter than in HD patients28. In this regard and considering that there may be differences in clearance of circulating levels of β-CTx in accordance with the type of dialysis, the differences observed in the concentration of this turnover marker between HD and PD are very significant and PD patients have much lower values. Differences in age between the two groups would not explain these differences in turnover activity; on the contrary, the PD group has a mean age that is lower than that of HD patients, and as such, the PD group should include more high turnover situations than the HD group.
Apart from ionised calcium concentration, there are other differences between the two techniques that may influence the different proportion of fragments 1-84 and 7-84. Firstly, peritoneal loss. Protein loss via the peritoneum seems to clear molecules 7-84 more23. Residual renal function is usually higher in PD patients. Although we do not have renal function data for our patients, changes in the latter would likewise cause a decrease in PTH molecules 7-84 resulting from the catabolism of PTH 1-84.
The therapeutic implications of these differences in the proportion of circulating PTH fragments between the two dialysis techniques is important. Considering previous KDOQI guidelines, due to their histomorphometric validation, when grouping together PD patients with PTH lower than 150nmol/l and those with PTH higher than 300nmol/l, we observed that the proportion of patients in one or the other group varies according to the method used. This is the result of differences between PTH methods and, if therapeutic decisions were made based on these “raw” values, patients could be treated who would not be treated if another PTH method had been used. Aware of this, we applied the inter-method adjustment algorithms proposed by the S.E.N., in order to avoid potential errors and make appropriate therapeutic decisions, without considering the dialysis technique. Nevertheless, differences in the proportion of circulating fragments in accordance with the dialysis type indicates to us that the S.E.N. study conversion formula does not represent reality in PD patients. PD patients have a higher proportion of 7-84, with antagonistic effects on PTH 1-84. In fact, the percentage of patients with PTH in adynamic range is much higher on applying correction formulas calculated for PD compared with that obtained when using HD algorithms. By applying to PD patients the correction proposed for HD patients, we would overestimate bone turnover activity upon misinterpreting hyperthyroidism situations that are not such. In summary, we would unnecessarily treat a patient with normal or low bone activity, inducing even more adynamia in these patients and, with it, a higher risk of vascular calcification.
In an effort to minimise these diagnostic errors in turnover activity, other forms of expressing PTH results have been proposed, such as the PTH 1-84/PTH 7-84 ratio29 or the bioPTH/iPTH ratio30, in an attempt to relate the opposing effects of the various PTH fragments and the intact molecule, which are so variable in the kidney population. Herbert et al. found the predictive value of the PTH 1-84/PTH 7-84 ratio with bone turnover, such that a ratio <1 and iPTH <420pg/ml increases the diagnosis of low turnover in white patients, while a ratio >1.6 and iPTH of 340-790pg/ml increases the predictive value of high turnover. These ratios could be used with the Scantibodies method, with which histomorphometric studies in bone were compared, while bearing in mind that interference with fragment 7-84 is different for the other second generation methods. We feel that these ratios could not be extrapolated when using other methods for quantifying PTH, and their clinical applicability is uncertain. For these reasons, it seems logical, in the near future, to generalise the use of third generation methods for measuring PTH in dialysis patients with the aim of homogenising clinical protocols and research studies with more objective results for interpreting the value of PTH.
The main limitations of this study are that it lacks bone biopsies, and as such, the condition of bone disease cannot be concluded using PTH as the only variable. Furthermore, it lacks data on residual renal function, which is important in the reduction of PTH fragments.
CONCLUSION
PD patients have a different proportion of circulating forms of PTH from HD patients. For this reason, the formulae used for correcting variations in HD patients are not applicable. In this study, we suggested other formulas applicable to PD patients. The latter have a higher proportion of PTH 7-84 fragments. We believe that it is important to know these differences when considering hyperparathyroidism treatment in these patients, and third generation methods will be very useful in this regard.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Configuration and reference range of the different commercial methods used to quantify parathyroid hormone
Table 2. Differences between the peritoneal dialysis and haemodialysis study populations, for whom the inter-method adjustment of the Spanish Society of Nephrology parathyroid hormone study was carried out
Table 3. Correlations between the parathyroid hormone values determined by different methods and correlations with ionised calcium, serum phosphate and carboxy-terminal telopeptide of type I collagen, in peritoneal dialysis patients
Table 4. Comparison of adjustments (Passing & Bablok) of second generation methods with the third generation Scantibodies method between haemodialysis and peritoneal dialysis
Table 5. Differences between peritoneal dialysis and haemodialysis
Figure 1. Mean parathyroid hormone values (nmol/l), expressed as medians and percentiles (p25 and p75) obtained in patients with stage 5D chronic kidney disease on peritoneal dialysis.
Figure 2. Patient distribution by bone turnover activity established by the KDQI guidelines in accordance with circulating levels of parathyroid hormone.