The correction of metabolic acidosis caused by renal failure is achieved by adding bicarbonate during dialysis. In order to avoid the precipitation of calcium carbonate and magnesium carbonate that takes place in the dialysis fluid (DF) when adding bicarbonate, it is necessary to add an acid, usually acetate, which is not free of side effects. Thus, citrate appears as an advantageous alternative to acetate, despite the fact that its acute effects are not accurately known.
ObjectiveTo assess the acute effect of a dialysis fluid containing citrate instead of acetate on acid-base balance and calcium-phosphorus metabolism parameters.
Material and methodsA prospective crossover study was conducted with twenty-four patients (15 male subjects and 9 female subjects). All patients underwent dialysis with AK-200-Ultra-S monitor with SoftPac® dialysis fluid, made with 3 mmol/L of acetate and SelectBag Citrate®, with 1 mmol/L of citrate and free of acetate. The following were measured before and after dialysis: venous blood gas monitoring, calcium (Ca), ionic calcium (Cai), phosphorus (P) and parathyroid hormone (PTH).
ResultsDifferences (p<0.05) were found when using the citrate bath (C) compared to acetate (A) in the postdialysis values of: pH, C: 7.43 (0.04) vs. A: 7.47 (0.05); bicarbonate, C: 24.7 (2.7) vs. A: 27.3 (2.1) mmol/L; base excess (BEecf), C: 0.4 (3.1) vs. A: 3.7 (2.4) mmol/L; corrected calcium (Cac), C: 9.8 (0.8) vs. A: 10.1 (0.7) mg/dL; and Cai, C: 1.16 (0.05) vs. A: 1.27 (0.06) mmol/L. No differences were found in either of the parameters measured before dialysis.
ConclusionDialysis with citrate provides better control of postdialysis acid-base balance, decreases/avoids postdialysis alkalaemia, and lowers the increase in Cac and Cai. This finding is of special interest in patients with predisposing factors for arrhythmia and patients with respiratory failure, carbon dioxide retention, calcifications and advanced liver disease.
La corrección de la acidosis metabólica provocada por la insuficiencia renal se consigue aportando bicarbonato durante la diálisis. Para evitar la precipitación de carbonato cálcico y magnésico que se produce en el líquido de diálisis (LD) al añadir bicarbonato, es necesario añadir un ácido, habitualmente acetato, que no está exento de efectos secundarios. Así, el citrato se presenta como una alternativa ventajosa al acetato, aunque sus efectos agudos no se conocen con precisión.
ObjetivoEvaluar el efecto agudo sobre los parámetros del equilibrio ácido base y del metabolismo calcio-fósforo con la utilización de un líquido de diálisis con citrato en lugar de acetato.
Material y métodosEstudio prospectivo y cruzado realizado en veinticuatro pacientes (15 hombres y 9 mujeres). Todos los pacientes se dializaron con monitor AK-200-Ultra-S con líquido de diálisis SoftPac®, elaborado con 3 mmol/l de acetato y con SelectBag Citrate®, con 1 mmol/l de citrato, libre de acetato. Se extrajeron pre y post-diálisis: gasometría venosa, calcio (Ca), calcio iónico (Cai), fósforo (P) y hormona paratiroidea (PTH).
ResultadosEncontramos diferencias (p < 0,05) cuando utilizamos el baño con citrato (C) frente a acetato (A) en los valores postdiálisis de: pH (C: 7,43 (0,04) vs. A: 7,47 (0,05)), bicarbonato (C: 24,7 (2,7) vs. A: 27,3 (2,1) mmol/L), exceso de base (BEecf) (C: 0,4 (3,1) vs A: 3,7 (2,4) mmol/L), calcio corregido (Cac) (C: 9,8 (0,8) vs A: 10,1 (0,7) mg/dl) y Cai (C: 1,16 (0,05) vs A: 1,27 (0,06) mmol/L). No encontramos diferencias en ninguno de los parámetros medidos prediálisis. Conclusión La diálisis con citrato consigue un mejor control de equilibrio ácido base postdiálisis disminuyendo/evitando la alcalemia postdiálisis y un menor aumento de Cac y Cai. Este hallazgo es de especial interés en pacientes con factores predisponentes a arritmias, pacientes con insuficiencia respiratoria, retención de carbónico, calcificaciones y hepatopatía avanzada.
The correction of metabolic acidosis is one of the treatment goals for chronic kidney disease. To achieve so in patients who are undergoing haemodialysis, bicarbonate is added during the sessions. The optimal bicarbonate concentration these patients should maintain is not accurately known. The KDOQI guidelines recommend maintaining a predialysis bicarbonate of 22 mEq/L in all patients1, while the UK Renal Association suggests different targets for patients undergoing peritoneal dialysis (25-29 mmol/L) and haemodialysis (HD) (20-26 mmol/L)2.
We could define the optimal bicarbonate concentration of DF as that which prevents interdialysis metabolic acidosis and avoids intradialysis and postdialysis alkalosis. Achieving this goal is not an easy task, as patients who are undergoing haemodialysis have progressive bicarbonate depletion in the interdialysis period and a sudden bicarbonate overload takes place during dialysis.
From a technical point of view, in order to avoid precipitation of calcium carbonate and magnesium carbonate that takes place in the DF when adding bicarbonate, it is necessary to add an acid. Thus, a DF generation system is used with 2 concentrates: one with bicarbonate and the other with acid. Acetic acid is most generally used, at concentrations ranging from 3 to 10 mmol/L. This small amount causes an acetate transfer to the patient during HD, increasing its blood concentration, since the DF has concentrations which are 30 to 40 times greater than the normal blood values (0.1 mmol/L). This exposure to acetate increases in online haemodiafiltration (HDF) techniques3. due to the higher amount of infused fluid. Among the side effects described with acetate, hemodynamic instability caused by vasodilation mediated by nitric oxide release4 and the activation of proinflammatory cytokines by hypoxia5 are worth mentioning due to their importance dur ing HD. Even compared to a DF w ith low concentrations of acetate (3 mmol/L), a lower risk of haemodynamic complications has been described when patients undergo dialysis with an acetate-free DF6.
Therefore, other acids have been researched for years as DF stabilisers. The first acetate substitution attempts involved hydrochloric acid. With this acetate-free DF, it was possible to observe that the usual increase in acetatemia shown by patients undergoing dialysis with DF containing bicarbonate and 4 mmol/L of acetate could be corrected by using a concentrate containing hydrochloric acid7,8. The problem with this DF with high chlorine content is that it modifies the sodium concentration-conductivity correlation, thus producing changes in serum ions so that it is necessary to change the total and partial conductivities of bicarbonate, despite the fact that its use has not been clearly standardised.
At present, we have a DF containing citrate, which appears as an alternative to acidification without using acetate. Citrate is a calcium (Ca) chelating agent that is used due to its anticoagulant effect by reducing ionic calcium (Cai). It is estimated to cause a 10% decrease in Cai; therefore, most authors recommend supplementing calcium contained in the DF when citrate is used as an acid to correct these differences. As shown by Steckiph et al.9, per each citrate mmol, the calcium concentration has to be increased 0.15 mmol/L to maintain the calcium balance during treatment and to prevent hypocalcaemia. Several long-term beneficial effects related to citrate have been described, such as a lower thrombogenicity10, improvement in clearance11,12, inflammation13, nutrition14, tolerance15,16 and acid-base control with a lower predialysis acidosis17.
ObjectiveTo assess the acute effect on acid-base balance and calcium-phosphorus metabolism parameters with the use of a DF containing citrate instead of acetate in patients with chronic HD.
Material and methodsThe study followed a prospective, crossover design and was conducted at single hospital dialysis site.
PatientsTwenty-four clinically stable patients were enrolled (15 male and 9 female subjects). The inclusion criteria were age older than 18, having received dialysis treatment for more than three months, being clinically stable and giving an informed consent.
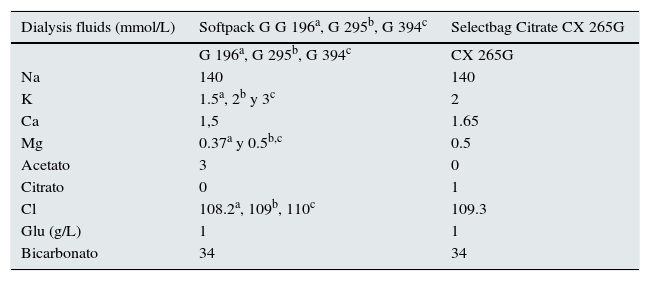
MethodsA prospective, crossover study was used to compare a single dialysis session with DF containing acetate to another dialysis session with citrate. All patients underwent dialysis with AK-200-Ultra-S monitor with SoftPac® dialysis fluid, made with 3 mmol/L of acetate and with SelectBag Citrate®, with 1 mmol/L of citrate and free of acetate. The composition of the used dialysis fluids is shown in Table 1.
Calculated Electrolyte Composition of DF containing Acetate and DF containing Citrate.
| Dialysis fluids (mmol/L) | Softpack G G 196a, G 295b, G 394c | Selectbag Citrate CX 265G |
|---|---|---|
| G 196a, G 295b, G 394c | CX 265G | |
| Na | 140 | 140 |
| K | 1.5a, 2b y 3c | 2 |
| Ca | 1,5 | 1.65 |
| Mg | 0.37a y 0.5b,c | 0.5 |
| Acetato | 3 | 0 |
| Citrato | 0 | 1 |
| Cl | 108.2a, 109b, 110c | 109.3 |
| Glu (g/L) | 1 | 1 |
| Bicarbonato | 34 | 34 |
Na: sodium; K: potassium; Ca: calcium; Mg: magnesium; Cl: chloride; Glu: glucose
Each patient was his/her own control, and no modifications were made in the dialysis schedule or pharmacological treatment during the study; the standard work schedule was followed. The fluid calcium concentration used was higher in the DF containing citrate: 1.5 mmol/L in acetate fluid and 1.65 mmol/L in the citrate fluid. Three K concentrations were used in the DF containing acetate (1.5, 2 and 3 mmol/L), which were the same the patients had before their enrolment in the study, and 2 mmol/L of K in the DF containing citrate.
Collected dialysis and demographic parametersA set of demographic parameters were collected: age, underlying condition, weight, dialysis technique (HD or HDF), the type of vascular access (fistula [AVF] or catheter [CT]), and residual renal function (RRF), measured as mean urea and creatinine clearance ((CCr+CU)/2) in 24-hour urine (if this was < 1mL/min or diuresis < 100mL/day, absence of RRF was considered).
Dialysis parameters included: time, blood flow (Qb), dialysis fluid flow (Qd), sodium and bicarbonate conductivities, fluid temperature, dialyser, heparin type and dose, HDF infusion volumes, Kt automatically measured by the Diascan® biosensor, ultrafiltration (UF) per session, and blood pressure (BP) before and after HD. The number of hypotensive episodes was also recorded, which was defined as every acute decrease in blood pressure perceived by the patient which required the intervention of nursing personnel.
Laboratory testsAll blood samples were collected through the arterial line of the vascular access. The predialysis samples were collected immediately before starting the technique, and postdialysis samples were taken after reducing the Qb to 50mL/min for 60seconds upon finishing the session.
Blood tests included- •
Acid-base parameters by means of venous blood gas monitoring that included pH, partial pressure of carbon dioxide (PCO2), partial pressure of oxygen (PO2), bicarbonate, base excess of the extracellular fluid (BEecf), measured oxygen saturation (sO2m).
- •
Biochemical parameters: sodium (Na), potassium (K), magnesium (Mg), Ca, Cai, phosphorus (P), and parathyroid hormone (PTH)
The pH was determined by potentiometry, pCO2 by Severinghaus electrode, pO2 by amperometry and Cai by ion-selective electrode (ISE). Biochemical determinations were made with an autoanalyser (ADVIA® 2400 Chemistry System, Bayer). PTH determinations were made by chemiluminescence with the Bayer ADVIA CENTAUR system.
Ca concentration was corrected for pH (Cac) using the following formula:
Statistical analysisThe statistical analysis was conducted via the SPSS 15.0 programme (SPSS INC., Chicago IL, USA). Descriptive data were expressed as arithmetic mean and standard deviation (SD).
For the comparison of two independent continuous variables, the Student's t-test was used for paired samples. For the comparison of more than two quantitative variables, the ANOVA test was used. A p <0.05 was considered statistically significant.
ResultsMean age of the twenty-four patients was 68.13 (19.2) (range 19-92) years. Dry weight was 72.7 (20.5) Kg. Renal failure aetiologies were as follows: glomerulonephritis (n = 8), interstitial nephritis (n = 3), polycystosis (n = 1), vascular (n = 3), diabetes mellitus (n = 2), and unknown (n = 7). Ten (41.7%) patients had RRF, with (CCr+CU)/2 of 6.5 (3.2) [2.7-14.4] mL/min. Six of them underwent dialysis twice weekly (25%). This is the frequency with which we usually administer dialysis if the patient meets the following requirements: (CCr+CU)/2 ≥ 5mL/min, normal blood pressure, adequate P and volume control. Eighteen (75%) patients underwent dialysis through an arteriovenous fistula and 6 (25%) by catheter. Thirteen (54.2%) patients underwent dialysis with high-flow HD and 11 (45.8%) with HDF. Mean dialysis time was 250 (16) [210-270] minutes. All patients had individualised bicarbonate concentrations: 30.8 (2.4) [26-34] mmol/L, sodium conductivity: 13.8 (0.1) [13.7-14.1] mS/cm and DF temperature: 35.8 (0.4) [35-36.5] °C.
All patients were informed about the study characteristics and gave their consent to participate in the study.
Dialysers were the same and were distributed as follows: 5 (21%) polyethersulfone of 1.8 m2 (Xenium®), 8 (33%) helixone of 1.8 m2 ([FX-class]® 80 and 800) and 11 (46%) polyamide of 2.1 m2 (Poliflux® 210H). The HDF technique was implemented in 11 (45.8%) patients, with a mean infusion volume of 27.7 (3.8) [20.8-33.9] litres.
Potassium concentrations used in DF containing acetate were 1.5, 2 and 3 mmol/L in 9 (37.5%), 12 (50%) and 3 (12.5%) patients, respectively.
Anticoagulation therapy was performed with heparin sodium at 1% in all patients except for one who used enoxaparin, with a mean heparin dose of 51.6 (18.5) [20-90] units.
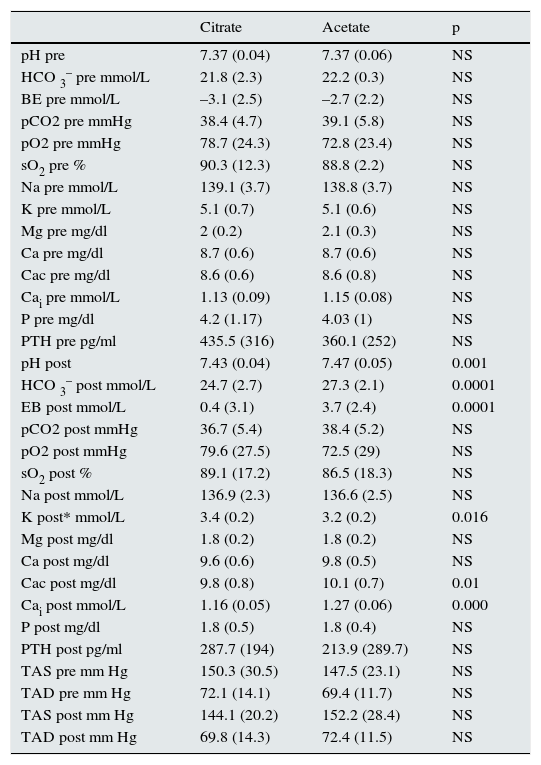
The mean values of the analysed parameters are shown in Table 2. Statistically significant differences were found when using the citrate bath compared to acetate in the following postdialysis values: pH, bicarbonate, BEef, K, Cac and Cai. No differences were found in any of the predialysis values, or in the rest of the analysed postdialysis values. Despite increasing Ca concentration in the bath, the postHD Cai values were lower in patients who underwent dialysis with citrate, with no changes in PTH.
Results of mean values of parameters analysed with citrate and acetate.
| Citrate | Acetate | p | |
|---|---|---|---|
| pH pre | 7.37 (0.04) | 7.37 (0.06) | NS |
| HCO 3– pre mmol/L | 21.8 (2.3) | 22.2 (0.3) | NS |
| BE pre mmol/L | –3.1 (2.5) | –2.7 (2.2) | NS |
| pCO2 pre mmHg | 38.4 (4.7) | 39.1 (5.8) | NS |
| pO2 pre mmHg | 78.7 (24.3) | 72.8 (23.4) | NS |
| sO2 pre % | 90.3 (12.3) | 88.8 (2.2) | NS |
| Na pre mmol/L | 139.1 (3.7) | 138.8 (3.7) | NS |
| K pre mmol/L | 5.1 (0.7) | 5.1 (0.6) | NS |
| Mg pre mg/dl | 2 (0.2) | 2.1 (0.3) | NS |
| Ca pre mg/dl | 8.7 (0.6) | 8.7 (0.6) | NS |
| Cac pre mg/dl | 8.6 (0.6) | 8.6 (0.8) | NS |
| Cai pre mmol/L | 1.13 (0.09) | 1.15 (0.08) | NS |
| P pre mg/dl | 4.2 (1.17) | 4.03 (1) | NS |
| PTH pre pg/ml | 435.5 (316) | 360.1 (252) | NS |
| pH post | 7.43 (0.04) | 7.47 (0.05) | 0.001 |
| HCO 3– post mmol/L | 24.7 (2.7) | 27.3 (2.1) | 0.0001 |
| EB post mmol/L | 0.4 (3.1) | 3.7 (2.4) | 0.0001 |
| pCO2 post mmHg | 36.7 (5.4) | 38.4 (5.2) | NS |
| pO2 post mmHg | 79.6 (27.5) | 72.5 (29) | NS |
| sO2 post % | 89.1 (17.2) | 86.5 (18.3) | NS |
| Na post mmol/L | 136.9 (2.3) | 136.6 (2.5) | NS |
| K post* mmol/L | 3.4 (0.2) | 3.2 (0.2) | 0.016 |
| Mg post mg/dl | 1.8 (0.2) | 1.8 (0.2) | NS |
| Ca post mg/dl | 9.6 (0.6) | 9.8 (0.5) | NS |
| Cac post mg/dl | 9.8 (0.8) | 10.1 (0.7) | 0.01 |
| Cai post mmol/L | 1.16 (0.05) | 1.27 (0.06) | 0.000 |
| P post mg/dl | 1.8 (0.5) | 1.8 (0.4) | NS |
| PTH post pg/ml | 287.7 (194) | 213.9 (289.7) | NS |
| TAS pre mm Hg | 150.3 (30.5) | 147.5 (23.1) | NS |
| TAD pre mm Hg | 72.1 (14.1) | 69.4 (11.7) | NS |
| TAS post mm Hg | 144.1 (20.2) | 152.2 (28.4) | NS |
| TAD post mm Hg | 69.8 (14.3) | 72.4 (11.5) | NS |
HCO3–: bicarbonate; BE base excess; pCO: partial pressure of carbon dioxide; pO2: partial pressure of oxygen (PO2), sO2: oxygen saturation; Na: sodium; K: potassium; Mg: magnesium; Ca: calcium; Cac: corrected calcium; Cai: ionic calcium; SBP: systolic blood pressure; DBP: diastolic blood pressure
* Mean of total patients where a DF containing acetate was used with three K concentrations (1.5, 2 and 3 mmol/L) and DF containing citrate only 2 mmol/L. In the 12 (50%) patients with the same concentration (2 mmol/L), no differences were found in postdialysis K concentrations: 3.2 (0.2) and 3.4 (0.3) for acetate and citrate, respectively. For DF containing acetate with 1.5 and 3 mmol/L of K, there were differences: 3.02 (0.1) vs. 3.4 (0.1) and 3.6 (0.1) vs. 3.1 (0.1).
Results were also analysed according to technique (HD vs. HDF), and no statistically significant differences were found in the analysed parameters. No differences were found when we compared patients on HDF with infusion volumes higher or lower than 27 L either.
Regarding K, the concentration used was only the same with both DF in 12 (50%) patients (2 mmol/L), and no differences were found in postdialysis K concentrations: 3.2 (0.2) and 3.4 (0.3) mmol/L for acetate and citrate, respectively. Differences were found, however, in postdialysis potassium concentrations in total patients (Table 3) and, as expected, between the DF containing acetate with 1.5 mmol/L of K and the DF containing citrate with 2 mmol/L of K: 3.05 (0.1) vs. 3.48 (0.1), respectively.
As far as efficacy is concerned, no differences were found with DF containing citrate and acetate, respectively: Kt 58.8 (9.2) vs. 61.6 (6.8) L.
Finally, although no differences were found in postdialysis systolic and diastolic BP values with either DF (Table 2), there were differences in the number of symptomatic hypotension episodes. There were three symptomatic hypotension cases (6.2%) out of the total of sessions (48), and in the three cases a DF containing acetate was being used (12.5%). No hypotension episode was observed in the 24 sessions performed with citrate (0%). There were no differences in mean ultrafiltration rates in either group: 1849 (884) vs. 1904 (847) mL for citrate and acetate, respectively.
DiscussionThe main result from our study is that dialysis with citrate provides better control of postdialysis acid-base balance than acetate by decreasing/avoiding postdialysis alkalaemia.
Acute alkalaemia induced by the addition of bicarbonate during dialysis is an issue which has considerable clinical significance. It has been related to important adverse effects, such as hemodynamic instability18–20, cardiac arrhythmia, paraesthesia/cramps21, reduced cerebral blood flow, respiratory distress,22,23 headache, and a procalcifying effect. Finally, a higher bicarbonate concentration in the dialysis fluid has also been associated with increased mortality24.
The greater cardiac excitability is explained by several mechanisms, among which we could mention the effect metabolic alkalosis has on transcellular potassium redistribution. The increase in bicarbonate concentration favours potassium redistribution to the intracellular space, which may cause a more sudden decrease in the potassium levels during dialysis and precipitate cardiac arrhythmias25. The bicarbonate conductivity of DF and plasma bicarbonatemia have been associated with an increase in QT interval in postdialysis electrocardiographies, an effect which may probably be mediated by the sudden decrease in both K and Cai.
The acid-base balance also plays a relevant role in the regulation of calcium deposits in blood vessels, so alkalosis favours calcification26. The article by Solis et al.27 analyses the pH effect on calcification through two experimental approaches: the in vitro effect of uremic serum on plain muscle cell calcification at different pH levels, and the in vivo effect of treatment with bicarbonate on nephrectomised rats. Its results suggest that extracellular alkalinisation promotes vascular calcification while acidification prevents it, both in cell cultures and uremic rats. The authors of this study have reproduced the situation faced by our dialysis patients, who usually have moderate metabolic acidosis and experience repeated episodes of sudden alkalinisation in dialysis sessions.
There are certain situations in which preventing intradialysis and postdialysis alkalosis is of special interest. Examples of these instances include patients with advanced chronic liver disease28, respiratory failure with extreme carbon dioxide retention, or those with clinical events that may worsen due to small modifications in acid-base balance occurring during dialysis and may even be life-threatening for the patient. The increase in pH may precipitate hepatic encephalopathy with severe liver failure, both acute and chronic, since it increases the NH3/NH4+ ratio and the NH3 passes through the blood-brain barrier more easily 29. Respiratory failure and CO2 retention may worsen upon inducing metabolic alkalosis since respiratory compensation reduces the respiratory centre stimulation (lower respiratory amplitude and rate). These complications may be prevented by reducing bicarbonate concentration in the DF30 and/or using a DF such as citrate which produces less alkalinisation.
We have found only one publication that analyses the acute effect of citrate on acid-base balance. Higher alkalinisation was found during dialysis with citrate compared to acetate (7.38 to 7.50 and 21 to 29.2 mmol/L vs. 7.39 to 7.45 and 22.4 to 24.3 for values of pH and bicarbonate with DF containing citrate and acetate, respectively)31. Our study shows that DF containing citrate produces less alkalaemia than acetate, shown by the differences in postdialysis gasometric parameters (pH, HCO3-and BE). It is important to consider that sodium and bicarbonate conductivities were kept stable. In addition, bicarbonate conductivity is individualised in our unit, and our goal is to achieve predialysis bicarbonate values > 18 mmol/L and postdialysis bicarbonate values < 28 mmol/L. We believe that the difference found may be even higher in units where bicarbonate does not meet each patient's needs. Therefore, in our study, where the bicarbonate conductivity range is between 26 and 34 mmol/L, patients on dialysis with acetate showed a slight postdialysis alkalaemia, only three (12.5%) patients showed HCO3-levels greater than 30 mmol/L and no alkalaemia was observed with DF containing citrate (mean values of pH 7.43, HCO3-24.7 mmol/L and BE 0.4). No patients showed HCO3-values greater than 30 mmol/L. Differences were kept in all used conductivity ranges, with no differences among them (results not shown).
The second result we would like to highlight is related to postdialysis calcaemia. Most studies analysing the effect of citrate on calcium-phosphorus metabolism have observed a reduction in Cai levels and an increase in PTH17. With the calcium concentration used in the DF containing citrate, which was 0.15 mmol/L greater than that of acetate, we observed no clinical or analytical hypocalcaemia and postdialysis values of both Cac and Cai were significantly lower using the DF containing citrate compared to acetate. Furthermore, with both DF at the used calcium concentrations, postdialysis values of Cac and Cai were greater than those obtained before dialysis. This result is even more important if we consider how changes in pH modify Cai32. Hydrogen ions displace Ca from albumin, so a reduction of 0.1 in pH increases the Cai concentration by 0.1 mEq/L, approximately, while alkalosis reduces free Ca and increases Ca binding to albumin. In our case, the postdialysis pH was higher in the dialysis conducted with acetate; had this not been true, the difference would have been even higher. In fact, no differences were found in postdialysis non-corrected Ca values, but there were differences in the corrected ones (9.8 and 10.1mg/dL with citrate and acetate, respectively).
We have not found higher clearance with the DF containing citrate, although other authors have16. This finding has been related to the local anticoagulant effect of citrate, which would imply a lower thrombogenicity and, therefore, a lower loss of useful surface area of the dialyser and, as a result, of dialysis efficacy. We have analysed the effect of both DF on coagulation in 12 patients by means of a visual classification of the status of the lines, chambers and dialyser (results not shown), and no differences were found either.
Finally, based on the higher hemodynamic stability described with citrate15, we have found no differences in blood pressure (BP), but we have found differences in the incidence of symptomatic hypotension episodes. Although the number of hypotension cases found was very low (in only 3 out of the 48 sessions was there hypotension [6.2%]), in the three cases it occurred using a DF containing acetate. No hypotensive episodes were observed in the 24 sessions performed with citrate (12.5 vs. 0%). It is worth mentioning that our incidence of hypotension is much lower than that reported in literature, ranging from 15 to 60%33,34. This result may also be explained by the known vasodilator effect of acetate, due to a lower bicarbonatemia found with citrate, since a direct relationship between bicarbonate concentration in DF and hemodynamic stability has been described19. The higher hemodynamic stability may also be the result of higher sodium [Na] concentration in the DF at an equal conductivity due to a modification in the relationship between the two (Na and conductivity). We have undisclosed data supporting this hypothesis.
There is no doubt that individualisation of the dialysis therapy should also include the bicarbonate concentration in the bath35. But, considering the results obtained in our study, should such individualisation be extended to the type of acid used? We should first consider that the bicarbonate concentration we set underestimates the actual concentration, since part of the acetate or citrate contained in the DF will then convert into bicarbonate. Both citrate and acetate transfer to plasma depend on the type of dialysis, clearance and treatment time, among other factors. As a result, the plasma citrate level increases <0.1 mmol/L at 0.2 to 0.3 mmol/L36 during dialysis with DF containing 1 mmol/L of citrate, while the acetate level increases from 0.2 to 0.5 mmol/L with a DF containing 4 mmol/L of acetate8. Once metabolised, acetate and citrate contribute to the increase in plasma bicarbonate concentration between 2 and 8 mEq/L, which may contribute to the development of postdialysis alkalosis. This fact led the Food and Drug Administration to write a communication warning physicians about the impact both acetate and other alkali sources have on bicarbonate prescription in dialysis37, and the risks of alkalosis induced by it. The way and period of time in which these acids are metabolised will have an impact on postdialysis acid-base balance values. The citrate-acetate concentration ratio we have used in the DF is 3/1 (3 mmol of acetate and 1 mmol of citrate per litre). Although it may be considered that this could partially explain the results, this is not the case because each metabolised citrate molecule is equivalent to the generation of three bicarbonate molecules. Meanwhile, in acetate metabolism there is a stoichiometric generation of bicarbonate, due to equimolar consumption of a proton when it is activated by the Acetyl CoA synthetase to make Acetyl CoA. Therefore, 1 mmol/L of citrate and 3 mmol/L of acetate, upon being metabolised by the body, should theoretically generate a similar amount of bicarbonate. For this reason, it is not considered necessary to modify the bicarbonate level prescribed when changing from a DF with 3-4 mmol/L to a DF with 1 mmol/L of citrate. So, we should resort to chemistry to explain this: citrate is the base of citric acid, a weak organic acid, whose dominating form at physiological pH is trivalent citrate C3H5 (COO)3-, of MW 189 D and a half-life of 30 to 60minutes. Its metabolism is mainly hepatic and also muscular: citrate enters the cell through transport proteins, then it moves into the mitochondrion, where it is converted into isocitrate during the Krebs cycle and, subsequently, into alpha-ketoglutarate, which is metabolised to generate bicarbonate and energy. Therefore, a fast conversion from citrate to bicarbonate takes place, but the main difference with acetate lies in the fact that this metabolism is incomplete during dialysis, since hepatic and muscular metabolism partly occurs after the technique is completed. Moreover, there are patients with fast metabolisms and others with slow metabolisms, depending on their liver function and muscle mass, which are factors that should also be taken into account. In patients who receive a citrate infusion as regional anticoagulant therapy, the plasma citrate levels are of 1 mmol/L, and patients on HD show similar levels to those having a normal renal function38. This is unlike patients with liver failure, in whom citrate clearance is reduced by 50%39. Another possible explanation for the lower alkalosis with citrate may be due to the different composition of both DF (Table 1), with components showing a different ionic strength.
Thus, to answer the foregoing question, the ideal bicarbonate conductivity with which patients should undergo dialysis has not been precisely determined. This value will depend on patient-related factors (predialysis bicarbonate concentration, hydrogen ion generation, bicarbonate distribution space, etc.) and dialysis-related factors (bath bicarbonate concentration, type of technique, frequency, ultrafiltration, blood flow and bath f low, etc.), among others. In light of our results, we should probably also add to these the type and concentration of acid used in the DF.
The main restriction of our study is that the number of patients included is small, but the crossover design enables the comparison between each patient and himself/herself, thus reinforcing the results. A second restriction is that the order of use of each acid in the DF has not been randomised. Even so, by analysing its acute effect, we think the impact of this randomisation would be minimal. Finally, the study has been conducted at a single hospital, but we believe the results may be valid for other centres with similar dialysis conditions.
It would have been interesting to measure citrate concentrations and acid-base balance parameters, not only postdialysis but also in the interdialysis period, in order to elaborate more on the metabolism mechanisms of both acids. This study opens the doors to future research analysing the clinically significant differences in patients with long-term treatment with DF containing citrate.
ConclusionsThe results of this article show that dialysis with citrate provides better control of postdialysis acid-base balance and decreases/avoids postdialysis alkalaemia. The lower postdialysis alkalaemia, together with a lower increase in calcaemia, support a less calcifying profile of the DF containing citrate.
This finding is of special interest in patients with factors predisposing to arrhythmia and those with respiratory failure, carbon dioxide retention, calcifications and advanced liver disease.