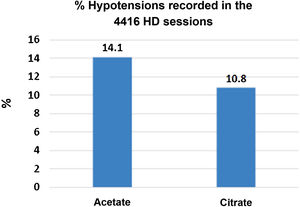
Hemodialysis (HD) with bicarbonate dialysis fluid (DF) requires the presence of an acid to prevent the precipitation of calcium and magnesium carbonate. The most used acid is acetic acid, with it several complications have been described. In a previous work we described the acute changes during an HD session with a DF with citrate instead of acetate. Now we report the results in the medium term, 16 weeks. It is a prospective, multicenter, crossover and randomized study, where 56 HD patients with bicarbonate three times a week were dialysed for 16 weeks with 3 mmol/L acetate and 16 weeks with 1 mmol/L citrate. Patients older than 18 years with a previous stay on HD of more than 3 months and with a normal functioning arteriovenous fistula were included. Epidemiological data, dialysis, bioimpedance, biochemistry before and after HD, as well as hypotensive episodes, were collected monthly. After 16 weeks of citrate treatment, preHD ionic calcium and magnesium were significantly lower and PTH higher than in the acetate period. No differences were observed in the effectiveness of dialysis. Hypotensive episodes were significantly more frequent with acetate than with citrate: 311 (14.1%) vs 238 (10.8%) sessions. The lean mass index increased by 0.96 ± 2.33 kg/m2 when patients switched from LD with acetate to citrate.
HD with citrate modifies several parameters of bone mineral metabolism, not only acutely as previously described, but also in the long term. The substitution of acetate for citrate improves hemodynamic stability, producing less hypotension and can improve nutritional status.
La hemodiálisis (HD) con líquido de diálisis (LD) con bicarbonato requiere la presencia de un acido para prevenir la precipitación del carbonato de calcio y magnesio. El acido más usado es el acido acético, con él se han descrito diversas complicaciones. En un trabajo previo describimos los cambios agudos, durante una sesión, en los pacientes en HD con un LD con citrato en lugar de acetato, en este referimos los resultados a medio plazo, 16 semanas.
Es un estudio prospectivo, multicéntrico, cruzado y randomizado, donde 56 pacientes en HD con bicarbonato tres veces a la semana se dializaron 16 semanas con 3 mmol/L acetato y 16 semanas con 1 mmol/L de citrato. Se incluyeron pacientes mayores de 18 años con una estancia en HD previa superior a 3 meses y con fistula arteriovenosa normofuncionante. Se recogieron mensualmente datos epidemiológicos, de diálisis, bioimpedancia, bioquímica pre y postHD, así como los episodios de hipotensión.
Después de 16 semanas de tratamiento con citrato el calcio iónico y el magnesio preHD eran significativamente inferiores y la PTH mas alta, que en el periodo con acetato. No se observo diferencias en la eficacia de la diálisis. Los episodios de hipotensión fueron significativamente más frecuentes con acetato que con citrato: 311 (14.1%) vs 238 (10.8%) sesiones. El índice de masa magra se incremento en 0.96 ± 2.33 kg/m2 cuando los pacientes pasaron de LD con acetato a citrato.
La HD con citrato modifica varios parámetros del metabolismo óseo-mineral, no solo de forma aguda como se había descrito, sino también a medio plazo. La sustitución del acetato por el citrato mejora la estabilidad hemodinámica, produciendo menos hipotensiones y puede mejorar el estado nutricional.
ClinicalTrials.gov NCT03319680.
In hemodialysis (HD), the composition of the dialysis fluid (DF) is key to optimize the balance of fluids, electrolytes and acid-base.1 HD machines produce the DF by mixing its three components: purified water, concentrated acid and bicarbonate. Acid concentrate receives this name because it must contain an acid which, when mixed with bicarbonate in the hydraulic circuit of the machine, prevents the precipitation of calcium and magnesium carbonate. The acid most commonly used is acetic acid, in concentrations ranging from 3 to 10 mmol/L, most often 3−4 mmol/L. This concentration is about 30 times higher than the observed in plasma, 0.1 mmol/L, which results in a net transfer of acetate from the DF to plasma during the HD session, increasing the plasma concentration of acetate.2 The concentration of acetate during and after HD are higher in those patients with a lower capacity to metabolize acetate, generally with decreased muscle mass, and will produce adverse effects.
The DF with citrate (CDF) was developed to improve the biocompatibility of the dialysis with bicarbonate plus acetate.3 Citrate is a calcium chelating agent and as such it has anticoagulant properties, by reducing ionic calcium. The benefits that have been reported with CDF include a reduction of thrombogenicity,4 inflammation, an increased clearance of low and medium molecular weight molecules,5,6 improvement in nutrition,7 tolerance8 and acid-base control, with less predialysis acidosis9 and without severe complications even with the hemodiafiltration.10
The clinical evidence of these advantages in randomized studies is scarce. The use of DF with citrate has increased slowly in daily clinical practice.
In 2019 we published the first part of the ABC-treat study11 (NCT03319680), which demonstrated that the use of citrate instead of acetate has an acute effect on electrolytes, reducing the levels of bicarbonate, calcium and magnesium post-dialysis and increasing PTH. A lower frequency of hypotension was also observed, 10.8% with CDF as compared to 14.1% with ADF.
This second part of the ABC-treat study investigates the chronic effect of HD with citrate, without acetate, compared with the standard DF with respect to changes in acid-base status, parameters of mineral-bone metabolism, dialysis efficacy, tolerance, nutrition and inflammation.
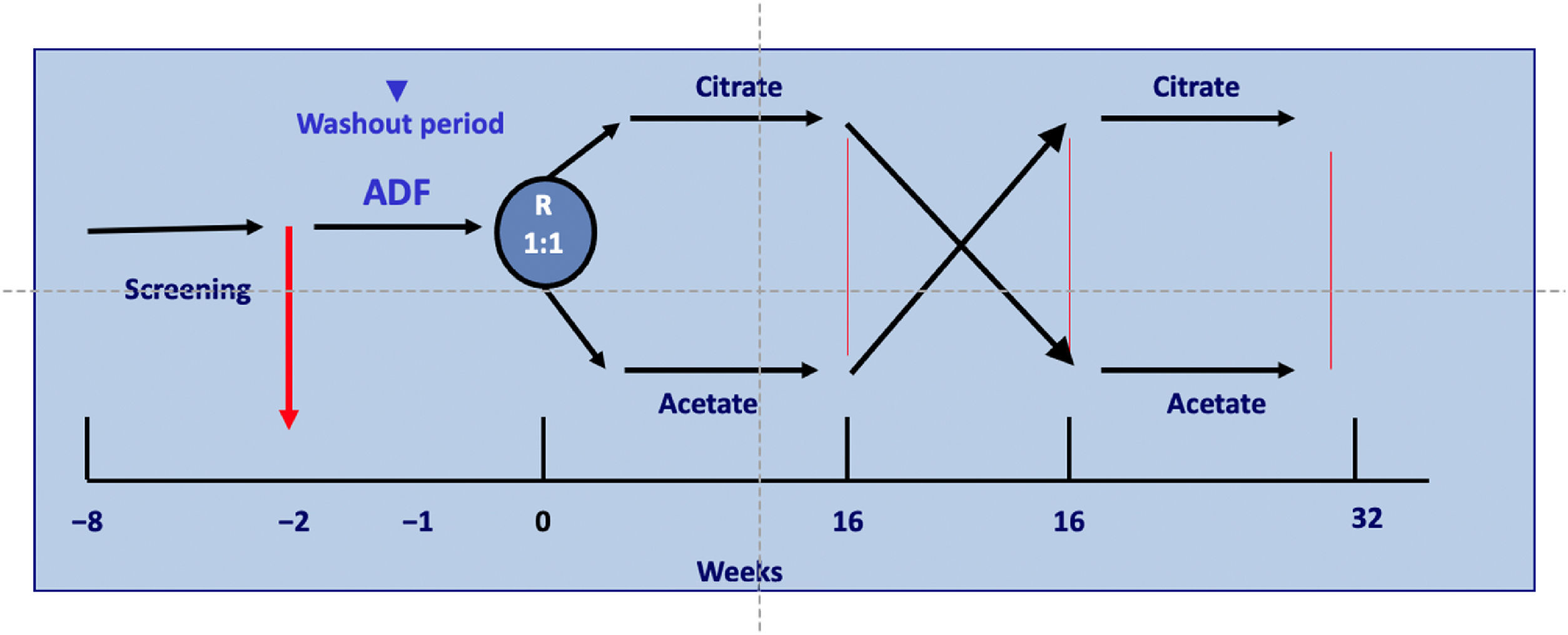
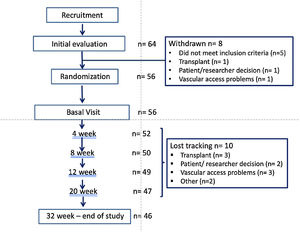
Material and methodsStudy designThis is an open, randomized, prospective, multicenter and crossover study of 32 weeks duration in patients on regular HD three times weekly. Patients received HD for 16 weeks using a DF with acetate (ADF) and another 16 weeks with citrate in the DF (CDF). The initial DF was determined by randomization with a 1:1 ratio and stratified by centers, see Fig. 1.
The acute effect of the HD session, pre and post HD values, of CDF versus ADF has been previously described in detail.11 A summary, at the end of an HD session, the serum bicarbonate, ionized calcium and magnesium were lower with CDF than with ADF but the PTH level was higher after CDF than ADF, Table 2. These results of the acute effect have been confirmed in the long-term study (32 weeks).
Patients were dialyzed with AK 200 Ultra S or Artis monitors, using 3 mmol/L SoftPac® acetate or 1 mmol/L SelectBag Citrate® (Baxter Healthcare, Deerfield, USA).
More details are provided in the study by Sequera et al. 2019.11
The Ethics Committee approved the study, which was conducted in accordance with the Helsinki statement. (ClinicalTrials.gov: NCT03319680).
The composition of the ADF and CDF is shown in Table 1. We used two different calcium concentrations in the DF; these were concentrations that each patient used before entering the study, 1.25 or 1.50 mmol/L. With ADF, the calcium concentration was maintained. If previous calcium concentration in ADF was 1.25 or 1.50 mmol/L, the concentration in CDF was increased to 1.50 and 1.65 mmol/L, respectively. The degree of increase in calcium was not the same for both groups, from 1.25 to 1.50 (0.25) and from 1.5 to 1.65 (0.15) because these were the calcium concentration available on the market.
Baseline, pre and post-hemodialysis (HD) laboratory data (n = 56).
| Pre - Hemodialysis | P | Post - Hemodialysis | P | |||
|---|---|---|---|---|---|---|
| ADF | CDF | ADF | CDF | |||
| Bicarbonate (mmol/L) | 23.0 (1.87) | 22.8 (2.20) | .668 | 28.5 (3.0) | 26.9 (1.9) | .032 |
| Delta Bicarbonate | −5.4 (2.7) | −4.1 (2.4) | .073 | |||
| pH | 7.37 (0.05) | 7.38 (0.03) | .448 | 7.45 (0.045) | 7.45 (0.036) | ,883 |
| pCO2 | 40.6 (4.3) | 38.9 (4.3) | .153 | 40.2 (6.1) | 39.0 (4.4) | .424 |
| Ca++(mmol/L) | 1.11 (0.06) | 1.12 (0.08) | .573 | 1.23 (0.086) | 1.11 (0.05) | ,000 |
| Total Ca (mg/dl) | 9.04 (0.52) | 8.92 (0.63) | .452 | 10.0 (0.62) | 9.6 (0.69) | ,117 |
| Magnesium (mg/dl) | 2.29 (0.39) | 2.23 (0.32) | .505 | 1.98 (0.20) | 1.85 (0.018) | .035 |
| Phosphate (mg/dl) | 4.32 (1.07) | 4.30 (0.86) | .918 | 1.9 (0.52) | 2.0 (0.72) | ,531 |
| iPTH (pg/mL) | 276.8 (154.2) | 311.1 (214.2) | .510 | 148.4 (148.8) | 255.1 (171.9) | .040 |
ADF: acetate dialysis fluid; CDF: citrate dialysis fluid; pCO2: partial pressure carbon dioxide; iPTH: intact parathyroid hormone.
*Significant P's are highlighted in bold.
Data are expressed as the mean (standard deviation).
Additional explanation: all blood samples were drawn from the arterial line in the midweek session. PreHD blood was drawn just before starting the session and post-HD 60 s after having reduced blood flow to 50 mL/min at the end of the session.
These results correspond to the first HD session after randomization. Previously, all the patients had been dialyzed with ADF for 2 weeks, so the patients had preHD values correspond to 2 weeks with acetate and postHD values to assigned ADF or CDF.
Before randomization, all patients were dialyzed with ADF for two weeks. Each patient maintained the same dialysis schedule throughout the study and served as his self-control. The only change made in the HD procedure was the use of CDF. The design of study is represented in Fig. 1.
Study populationThis study included prevalent patients with chronic kidney disease (CKD) on HD over 18 years of age and who had been on HD for a minimum of three months and using an arteriovenous fistula (AVF) as vascular access (Table 3). All patients signed an informed consent.
Baseline characteristics of the patients according to the group that has been randomized.
| Acetate to Citrate (n = 29) | Citrate to Acetate (n = 27) | P | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (year) | 63.5 (18.3) | 67.1 (14.1) | .409 |
| Gender (males/females) | 22/7 | 25/2 | .089 |
| Weight (kg) | 67.1 (12.5) | 72.1 (11.9) | .133 |
| Height (cm) | 163.6 (8.3) | 167.8 (8.2) | .056 |
| Diabetes (n) | 4 | 6 | |
| Adjusted Charlson Index | 6.2 (2.6) | 6.2 (2.4) | .934 |
| Time on Dialysis (months) | 100.0 (134.9) | 95.7 (101.1) | .894 |
| Characteristics of Hemodialysis | |||
| HF-HD/OL- HDF | 10/19 | 10/17 | .531 |
| SBP (mm HG) pre HD session | 137.1 (24.1) | 132.9 (24.7) | .524 |
| DBP (mm HG) pre HD session | 73.7 (14.4) | 68.2 (11.4) | .122 |
| Kt (urea) | 53.0 (7.6) | 53.9 (8.8) | .689 |
| Blood flow (ml/min) | 394.7 (47.1) | 387.4 (52.3) | .587 |
| DF (dialysate) flow (ml/min) | 514.8 (45.6) | 514.8 (36.2) | 1 |
| Bioimpedance data | N = 20 | N = 18 | |
| LTI (Kg/m2) | 12.1 (2.8) | 13.9 (3.2) | .055 |
| FTI(Kg/m2) | 12.6 (5.5) | 12.7 (4.7) | .96 |
| TBW (L) | 37.3 (4.2) | 37.1 (4.3) | .92 |
| ECW (L) | 15.5 (3.9) | 16.0 (5.0) | .73 |
HF-HD: high flux hemodialysis; OL-HDF: online hemodiafiltration; PreHD PAS: systolic blood pressure preHD; PreHD PAD: preHD diastolic blood pressure; LTI: lean mass index; FTI: fat mass index; TBW: total body water; ECW: water extracellular. Data are expressed as the mean (standard deviation) or as specified.
The exclusion criteria were: allergy or intolerance to citrate; intercurrent inflammatory disease; permanent catheter and cognitive impairment.
Study protocolThe objectives of the study were to evaluate and compare the effects of CDF versus ADF on bone mineral metabolism; acid-base state; efficacy of HD; inflammation; nutritional status, coagulation, hemodynamic stability and tolerance.
We collected epidemiological and dialysis data, laboratory tests, body composition, coagulation parameters and hypotensive episodes monthly during the 8 months of the study, as previously described.11 Blood samples were obtained pre and post HD session, and the following parameters were measured: pH, bicarbonate, pCO2, ionized and total calcium, phosphorus, magnesium, albumin, c-reactive protein (CRP) and parathyroid hormone (PTH). These parameters were measured by standard laboratory methods that were similar in all centers. No modifications to the methodology were allowed during the study.
The Kt was automatically measured by the Diascan® ionic dialysance biosensor. Assessment of nutritional status was based on the laboratory data, and the measurement of body composition by multi-frequency electrical bioimpedance (BIA), using the Body Composition Monitor [(BCM®) Fresenius Medical Care, Bad Homburg vor der Höhe, Germany]. The body composition was measured in 29 patients, since some centers did not have equipment for BIA. The measurements were performed on the dominant side or the opposed to the AVF. It was done before starting the midweek HD session after 5 min of resting in the decubitus position using the standard electrode configuration. The hydration (OH) and body composition data collected included the fat mass index (FTI) and lean mass index (LTI) adjusted to body surface (kg/m2). The state of inflammation was assessed by measuring blood values of C-reactive protein (CRP).
Statistical analysisContinuous normal variables are expressed as the mean and standard deviation.
The only variable with a non-normal distribution was CRP, Z of Kolmogorov-Smirnov and by using the Log values it has been possible to transform it into normal distribution. Paired or unpaired Student t-test was used to compare the normal variables. More than two normal variables were compared by MANOVA. Given the study design, a multivariate adjustment analysis was not used.12,13 A P < .05 has been considered significant. Statistical analysis was performed using the SPSS 15.0 program (SPSS INC., Chicago IL, USA).
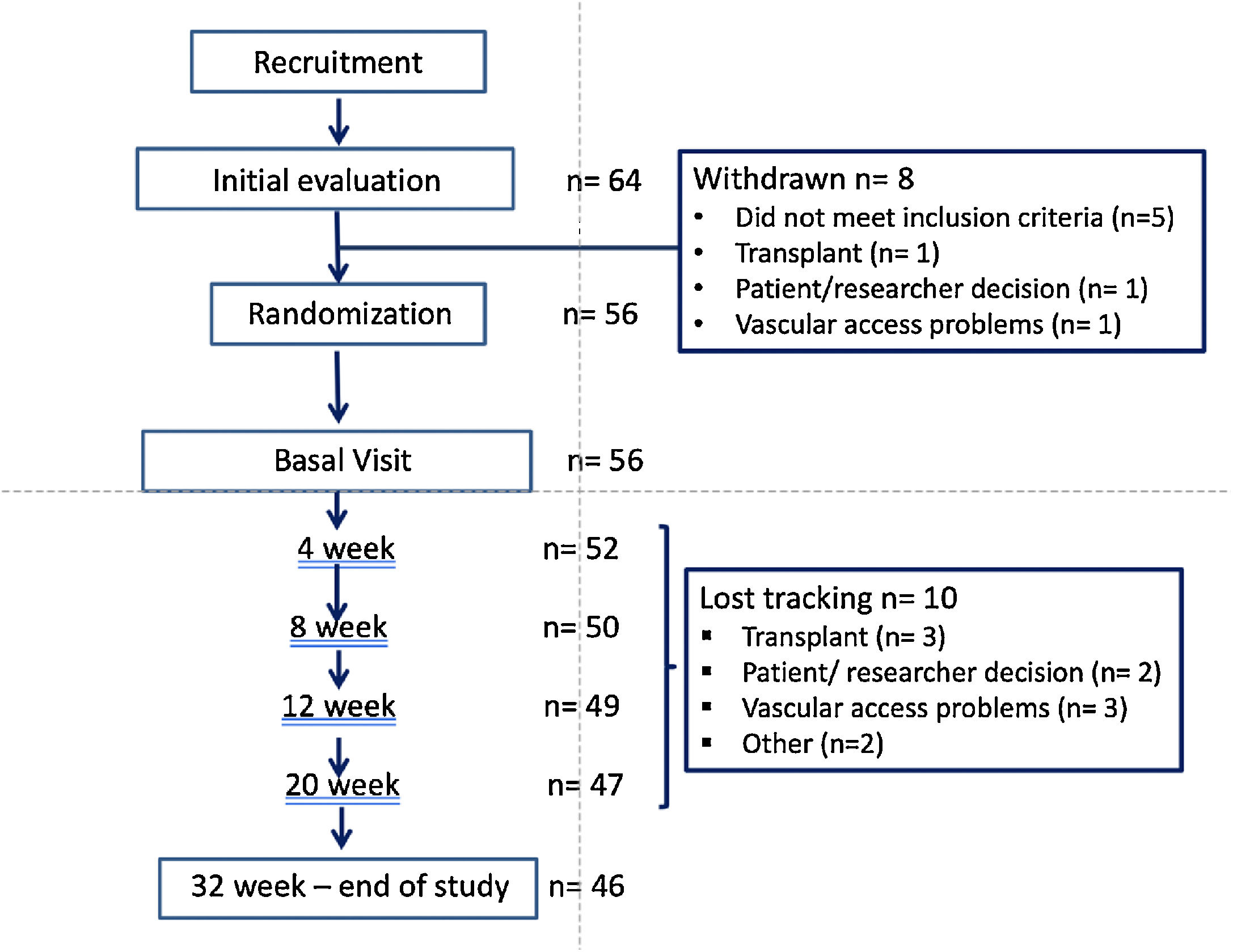
ResultsStudy populationPatients flow chart is shown in Fig. 2. Out of the 64 patients initially recruited (from 8 Hemodialysis Units), 8 drop out the study and 10 were lost the follow-up. The final sample includes 56 of the initial patients and the 46 who completed the study.
The mean age was 65.3 (16.4) {range 23–93} years, 47 men and 9 women, with a Adjusted Charlson index of 6.2 (2.5). The etiology of CKD was: 16 glomerulonephritis, 10 diabetes, 9 vascular disease, 4 inherited disease, 4 interstitial nephritis, 9 other causes and 4 of unknown cause.
There were 36 patients on online hemodiafiltration (OL-HDF) and 20 on high flux HD (HD-HF).
By randomization 29 patients were assigned to start with ADF and 27 with CDF, after 16 weeks the patients were switched to the other type of DF for and additional period of 16 weeks. Table 2 describes the baseline characteristics of the patients according to the group assigned and no significant differences were observed between the two groups. The proportion of men predominated among our study patients, and it was more accentuated in the group that started with CDF however the difference was not statistically significant.
Acid-base and bone-mineral metabolism parametersThe acute effect of the HD session, pre and post HD values, of the CDF versus the ADF has been previously described in detail.11 Briefly, at the end of the HD session, the serum bicarbonate, ionized serum calcium and magnesium levels are lower with CDF than with ADF and the concentration of PTH is higher after CDF that after ADF, Table 3.
Table 4 shows the chronic effect (16 weeks, n = 46) of ADF and CDF on the acid-base status and parameters of the bone mineral metabolism preHD. No significant differences were observed in the level of bicarbonate however after CDF the serum concentrations the ionized calcium and magnesium were lower and the values of iPTH were significantly increased. It was analyzed the possible effect of ionized Calcium, phosphate and magnesium on the PTH increase. The ionized calcium and phosphate correlated with iPTH (P = .011 and P = .013 respectively). There was not a significant correlation between magnesium and iPTH levels. Multiple linear regression, shows that PTH (pg/mL), correlates with Phosphorus (mg/dL), Ionic Calcium (mg/dL), Magnesium (mg/dL) F = 4.257 and P = .012. The increase in PTH is mainly attributable to the change in ionic calcium. Low Mg would not be capable of stimulating PTH in the presence of low calcium.14 Using the PTH target range from 100 to 500 pg/mL. PTH was below the range in 13% of patients after CDF and in 8.7% after ADF; the opposed was observed for PTH above 500 pg/mL, it was present in 8.7% with ADF and in 13% with CDF.
Chronic effect of ADF or CDF (16 weeks) on acid-base and parameters of bone-mineral metabolism in the 46 patients who completed the study.
| ADF | CDF | Change | P | |
|---|---|---|---|---|
| Bicarbonate (mmol/l) | 23.2 (2.1) | 22.6 (2.5) | −0.65 (3.00) | .150 |
| Ionized calcium (mm/L) | 1.13 (0.09) | 1.10 (0.09) | −0.02 (0.07) | .023 |
| Total calcium (mg/dl) | 8.87 (0.86) | 8.84 (0.65) | −0.03 (0.76) | .767 |
| Magnesium (mg/dl) | 2.21 (0.31) | 1.90 (0.73) | −0.31 (0.71) | .010 |
| Phosphate (mg/dl) | 4.23 (1.28) | 4.57 (1.41) | 0.35 (1.40) | .099 |
| iPTH (pg/mL) | 296.7 (195.9) | 361.8 (209.0) | 65.03 (169.8) | .013 |
ADF: acetate dialysis fluid; CDF: citrate dialysis fluid; iPTH: intact parathyroid hormone. Significant differences are highlighted in bold.
The values of Kt were similar after ADF and CDF (53.06 (7.56) L and 53.97 (8.93) L, respectively). Likewise the Kt/V was not statistically different after ADF or CDF [1.41 (0.19) versus 1.39(0.29)].
Measurements at week 16 revealed no significant differences between the two groups in serum concentration of phosphorus, creatinine and beta-2-microglobulin (Tables 4 and 5).
PreHD laboratory data in the 46 patients after 16 weeks with ADF and CDF.
| ADF | CDF | Change | P | |
|---|---|---|---|---|
| Total Proteins (g/dl) | 6.45 (0.42) | 6.46 (0.46) | 0.014 (0.356) | .792 |
| Albumin (g/dl) | 3.71 (0.51) | 3.69 (0.55) | −0.02 (0.46) | .768 |
| Prealbumin (mg/dl) | 29.8 (8.7) | 29.0 (10.6) | −0.81 (8.82) | .558 |
| Cholesterol (mg/dl) | 140.0 (27.8) | 143.2 (30.3) | 3.17 (21.6) | .328 |
| Triglicerides (mg/dl) | 120.1 (47.7) | 122.9 (64.4) | 2.84 (51.1) | .710 |
| Creatinine (mg/dl) | 8.3 (2.4) | 8.4 (2.4) | 0.10 (0.96) | .492 |
| Urea (mg/dl) | 119.0 (39.4) | 122.0 (36.7) | 3.00 (23.2) | .385 |
| Glucose (mg /dl) | 117.1 (43.2) | 119.5 (40.1) | 2.45 (33.8) | .624 |
| B2-microglobulin (mg/L) | 25.9 (7.2) | 25.2 (9.2) | −0.53 (5.67) | .529 |
| Leucocytes (x 103/microL) | 6.6 (2.0) | 6.5 (1.9) | −0.10 (1.65) | .674 |
| Lymphocytes (%) | 18.48 (8.75) | 19.58 (9.13) | 1.10 (5.36) | .172 |
| Eosinophils % | 4.32 (3.71) | 3.24 (1.97) | −1.08 (3.03) | .020 |
| Platelets x103 /microL | 195.5 (63.2) | 190.2 (64.3) | −5.33 (46.95) | .446 |
| Hemoglobin (g/dl) | 11.3 (1.2) | 11.5 (1.2) | 0.18 (1.78) | .505 |
| IST % | 26.8 (12.3) | 27.0 (10.5) | 0.17 (12.05) | .923 |
| Ferritin (ng/mL) | 421.6 (266.5) | 403.7 (272.6) | −17.94 (240.3) | .619 |
| CRP (mg/L) n=42 | 6.22 (12.0) | 5.34 (8.25) | −0.87 (7.74) | .468 |
ADF: acetate dialysis fluid; CDF: citrate dialysis fluid; iPTH: hormone intact parathyroid; IST: Transferrin saturation index; CRP: C-reactive protein.
Bolds refers that this value is statistical significant.
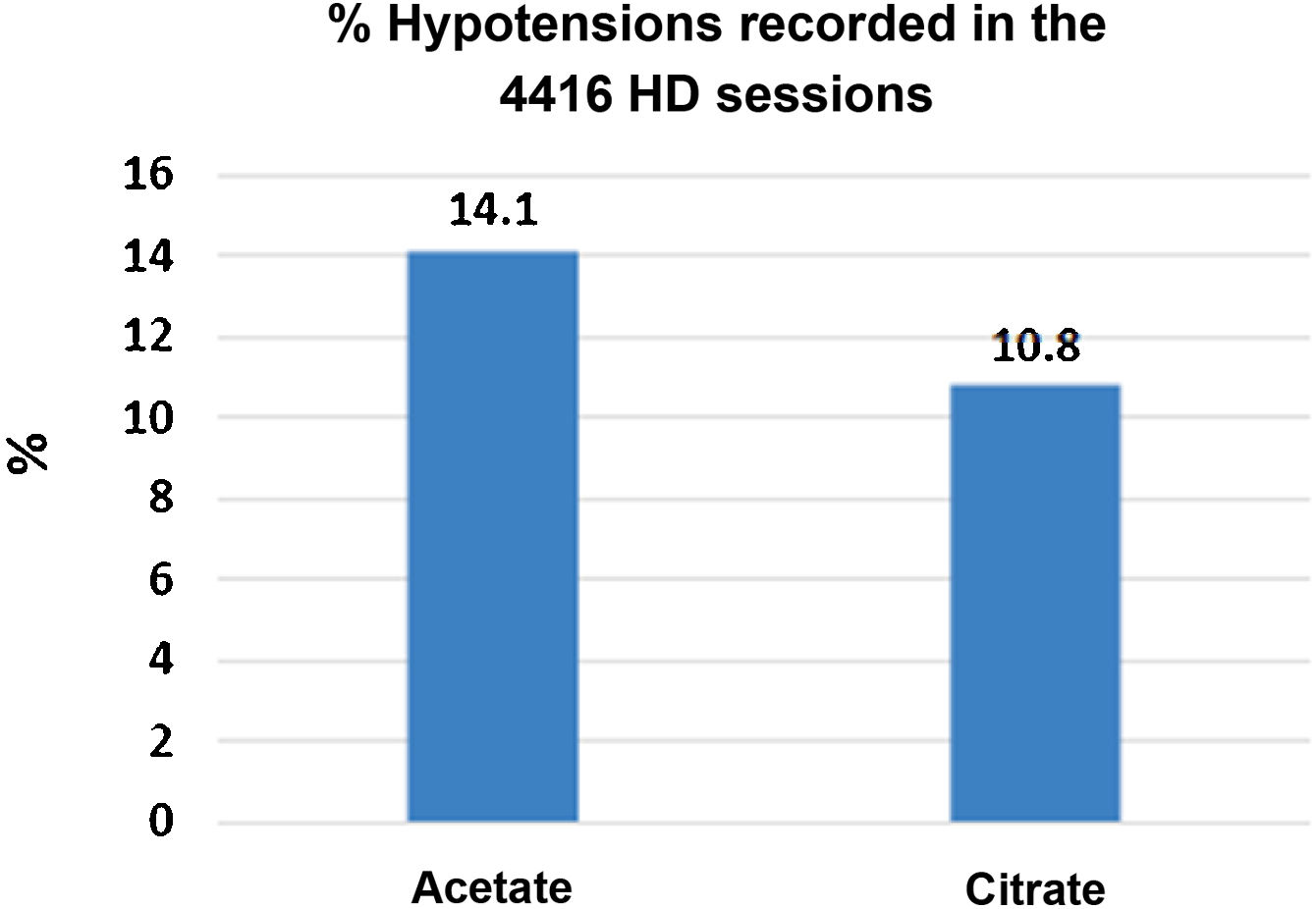
There were 4416 HD sessions performed in the 46 patients who completed the study, 50% of the sessions with ADF and another 50% with CDF. The number of hypotensive episodes was significantly greater in the period with acetate than in the citrate period: [311 (14.1%) versus 238 (10.8%) sessions, P < .01)] (Fig. 3). No differences were observed in the incidence of cramps.
Nutrition and inflammationAfter the 16 weeks there were no differences in the values of albumin or CRP between ADF or CDF (Talble5). However, the type of dialysis fluid made a difference in albumin concentration when the results were analyze separating the patients with a baseline serum albumin above or below 3.8 g/dl. After the 16 weeks with CDF, patients with a albumin less than 3.8 g/dl experienced a significant increase in the albumin concentration, from 3.37 to 3.49 g/dl, (P < .000).
The parameters of bioimpedance in the subgroup of 29 patients are shown in the Table 6. Lean mass index (LTI) and total body water (TBW) increased during the period with citrate, being at the end significantly higher than the period with acetate.
Body composition by bioimpedance at 16 weeks after changing the type of DF. The variables are compared in each patient at the end of the period on ADF with that on ADF.
| N = 29 | Acetate | Citrate | Change | P |
|---|---|---|---|---|
| Weight (kg) | 68.0 (13.6) | 68.5 (14.0) | + 0.48 (1.74) | .141 |
| TBW (L) | 37.4 (5.3) | 37.6 (5.4) | + 0.165 (0.47) | .024 |
| EBW (L) | 2.06 (1.25) | 1.70 (1.19) | −0.36 (1.36) | .159 |
| ECW (L) | 16.16 (3.76) | 15.25 (4.95) | + 0.92 (4.72) | .305 |
| FTI (kg/m2) | 13.23 (5.51) | 12.46 (5.72) | −0.77 (3.68) | .269 |
| LTI (Kg/m2) | 13.11 (3.27) | 14.08 (3.63) | + 0.96 (2.33) | .035 |
TBW: total body water; EBW: excess body water; ECW: extracellular water; FTI: fat mass index; LTI: lean mass index.
Bolds refers that this value is statistical significant.
None of the other parameters analyzed was significantly different between the two groups, except the percentage of eosinophils that was significantly higher with acetate (Table 5), probably in relation to less biocompatibility.
DiscussionAlthough benefits of citrate dialysis fluid compared to that of acetate have been published, the information from randomized studies is scarce. This study was designed to compare the effect of citrate with acetate in a controlled and randomized manner. Dialysis with citrate versus acetate causes changes in parameters of bone-mineral metabolism, and acid-base, acutely and chronically, and also improves hemodynamic tolerance and probably, the nutritional status.
As previously described,11,15 citrate improves acid-base control with respect to Acetate; it decreases the peak of alkalemia post-HD while maintaining the same acid-base balance, as demonstrated by no change in preHD bicarbonate after 16 weeks.
Severe postHD alkalemia has been associated with hemodynamic instability16 and arrhythmias.17 HD with a high and inadequate concentration of bicarbonate can increase mortality.18 The metabolism of citrate19 is probably slower than that of acetate.10 About 25% of buffers transferred to the patient during HD depend on the metabolization of acetate or citrate.
The present study shows that, the CDF, as compared to the ADL, modifies some parameters of calcium-phosphorus metabolism both acutely, as previously described,4,5,9 and at a long term. Citrate is associated with lower levels ionized calcium and magnesium, pre and postHD and an increase in the serum concentration of PTH. All this despite the higher concentration of calcium used in the LDC as compared with ADF. Citrate has a high affinity for calcium and magnesium, which modifies the mass transfer through the dialyzer of these two elements. Low calcium concentrations in DF may cause hypocalcemia, that would stimulate PTH, bone turnover, and the appearance of cramps, arrhythmias and increasing the risk of hypotensive episodes. For all of the above, we and other authors,6,20 increase the calcium concentration in the DF when patients are dialyzed with citrate. As previously observed,6 the increase in calcium of 0.15 mmol/L does not prevent the decrease in ionic calcium. As described Gabutti et al.,20 the CDF, despite using a higher concentration of calcium, prevents the increase in post-HD ionic calcium that appears with ADF, which in turn inhibits the secretion of PTH. In this work we observe that the postHD values of ionic calcium and magnesium are slightly lower (∼0.1 mmol/L) with LDC than with LDA, which has been previously described.10 After 16 weeks with citrate, preHD PTH level is significantly increased.
Observational studies have shown that the risk of death is increased in HD patients with values of iPTH <2 or> 9 times the upper limit of normal for the assay.21 The spectrum of patients with CKD stage 5 has changed over the past 20 years, from a bone with high-remodeling to low-remodeling form of bone disease which is currently present in 40 and 70% of patients, and it is characterized by relatively low levels of iPTH. This change is due to a progressive aging of the HD population with more diabetics and a high prevalence adynamic bone disease, which is associated with an increased risk of fractures and vascular calcifications,22–24 that together with aging and diabetes are important risk factors for death.25,26 In this context, less alkalosis, the reduction of ionized calcium levels with an increase in PTH produced by the CDF could be an advantage to reduce vascular calcifications and adynamic bone disease. In addition, citrate would decrease the propensity to calcifications as measured by the T50 test at 3 months in a preliminary study,27 in a short study duration, multicenter, randomized and crossover28 and in another performed in rats with ex vivo cultured aortic rings.29
In our study, the serum magnesium concentration decreased when patients were on CDF.
This occurred despite that both CDF and ADF contained the same concentration of magnesium in the DF (0.5 mmol/L). This has been described in other studies,10 and it is not surprising since citrate chelates the magnesium in addition to calcium. Elevated serum magnesium levels are associated with a decrease in vascular calcifications.30,31 However, low serum magnesium on dialysis citrate does not adversely affect the propensity for calcification.27 Although optimal serum levels in HD patients remain controversial,32 patients with low levels of Mg have a worse prognosis than those with somewhat high levels, as we have recently published33; similar results have been observed in other studies.34–40 The type of DF could influence the concentration of magnesium and the risk of death.33 Jefferies et al.41 in a study based on DF with acetate, compares the usual concentration of magnesium, 0.5 mmol/L with a high, 1.0 mmol/L, without observing adverse effects with DF with high magnesium concentrations. It has been suggested that magnesium in the CDF should be increased, an idea that we share. The increase in magnesium concentration in dialysis fluid has been associated with improvement of vascular calcifications,42 a reduction in total and cardiovascular disease mortality,43 as well as a reduction in inflammation markers (TNF alpha and IL6).44
Although some authors have published an improvement in the effectiveness of dialysis with the CDF, as measured by Kt/V or Kt, we have not confirmed this finding. This improvement has been explained by the anticoagulant effect of citrate. In the present study, as previously described,11 we did not observe differences in the degree of coagulation of chambers or dialyzers, the degree of coagulation with both CDF and ADF was 0, absent, or 1, minimum in 80% of the sessions. Our explanation is that the differences in the coagulation of the dialyzer and therefore in the dialysis efficiency only becomes apparent when heparin doses are low.
The most relevant effects of acetate in HD are hemodynamic instability secondary to the vasodilation it causes. This is mediated by nitric oxide45 and activation of pro-inflammatory cytokines.46,47 The plasma acetate increases above 1 mmol/L after HD with a DF containing 4 and 8 mmol/L of acetate.48 In patients with a slower rate of acetate metabolism, such as those with a reduced muscle mass, the concentrations of acetate would be higher and for a longer period of time. Previous studies found a lower frequency of hypotension in 44 patients in HD, especially the symptomatic and more severe episodes.8 With ADF there would be a greater fall in peripheral vascular resistance and systolic blood pressure.20 In the same line of these observations, we found that the hypotension episodes are more frequent during ADF than with CDF, our study which compile the information from 4416 HD sessions would be the largest evaluating this aspect.
In the present work there are no significant changes in CRP. The beneficial effect of citrate and the removal of acetate on inflammation49 could be counteracted, in some patients, due to the appearance of hypomagnesemia.50
One of the most interesting results of our study is the significant increase in Lean Mass Index (LTI) after 16 weeks with citrate, suggesting that CDF could improve nutritional status. In this sense, there is evidence previously published about an increased phase angle and body cell mass measured by monofrequency bioimpedance after 12 weeks of with citrate.51 The serum albumin would increase after 12 weeks with citrate,51 sometimes this effect would be limited to patients with hypoalbuminemia,52 as is the case in our patients with an albumin below 3.8 g/dl. These studies attribute the improvement in nutritional status to a reduction of inflammation or to the presence of citrate in the CDF.6 The anabolic impact suggested by the increase of the LTI could be related by the calories contributed by citrate53 and its incorporation into the Krebs cycle.54 Acetate to be metabolized in that precise way of needs Coenzyme A, pyruvate dehydrogenase and NAD+. The incorporation of citrate into cells do not require insulin.55 Mitochondria play a key role in the skeletal muscle function56 and citrate can be incorporated directly if other substrates are scarce.57 These data have been collected in patients with citrate as an anticoagulant, in which citrate is used in higher concentrations than in our study. However, the infusion of the CDF into OL-HDF provides a much greater amount of citrate to patients than conventional HD. Mass muscle and its function are adversely affected by several factors in patients with CKD in HD,58 the use of citrate instead of acetate could improve some of them.
Limitations of the studyThe sample of patients studied is small and includes only patients with an AVF. Because one of the objectives in the study design was to assess the efficacy of dialysis and the duration of the study was 32 weeks, it was decided to exclude patients with a catheter to avoid the possibility of dysfunction and influence on dialysance. For this reason, the etiology of CKD of the patients included in the study is not representative of the entire dialysis population, since the first cause was glomerulonephritis, followed by diabetes, while both in the Spanish population and in most countries, the main cause of CKD in dialysis patients is diabetes. A positive aspect of the study was the use of a crossover design: thus, we eliminate patient variation over time, and served as his own control. Although we did not analyze the impact of long-term use of CDF in key objectives, such as mortality,59–61 in our knowledge, this is the longest randomized trial using citrate, allowing the assessment and exploration of long-term consequences.
ConclusionsIn conclusion, DF with citrate modifies most of the metabolic parameters of bone mineral, not only acutely, but also in the long term. As far as we know, this it is the first time this has been addressed in a randomized controlled trial. This observation has two important practical implications: the magnesium concentration should be increased in DF with citrate in most cases, and citrate is the treatment of choice in patients with adynamic bone disease or low PTH levels. Compared to acetate, citrate offers greater hemodynamic stability reducing episodes of hypotension, and may improve nutritional status. Long-term randomized controlled trials are needed to confirm the potential nutritional benefits and their impact on the morbidity and mortality.
FundingThe sponsor of this study has been the Foundation of the Spanish Society of Nephrology (SEN) and an Independent Research grant from Baxter.
Conflicts of interestP. de S. has received consultancy or speaker fees or travel support from Vifor Pharma, Amgen, Fresenius, Astra Zeneca, Nipro, Alexion, Astellas, Braun and Baxter. R.P.G., M.M. have received fees for the participation as speakers at Fresenius and Baxter meetings and aids for travel to Scientific Congresses from Nipro, Fresenius and Baxter.
To the nursing staff of the Dialysis Units of the Hospitals participating in the study for their valuable collaboration.
P. de Sequera Ortiz (Nephrology Service, Infanta Leonor University Hospital, Madrid).
R. Pérez García (Nephrology Service, Infanta Leonor University Hospital, Madrid).
M. Molina Núñez (Hospital General Universitario Santa Lucía, Cartagena).
R.I. Muñoz González (Hospital Galdakao, Vizcaya).
G. Álvarez Fernández (Nephrology Service, Santa Lucía University Hospital, Cartagena).
E. Mérida Herrero (Doce de Octubre University Hospital, Madrid).
M.J. Camba Caride (University Hospital Complex of Ourense).
L.A. Blázquez Collado (Guadalajara University Hospital).
M.P. Alcaide Lara (Virgen del Rocío University Hospital, Seville).
R. Echarri Carrillo (Infanta Sofía University Hospital, Madrid).
I. Gallardo (Hospital Galdakao, Vizcaya).
E. Hernández Martínez (Doce de Octubre University Hospital, Madrid).
A. Otero González (Complexo Hospitalario Universitario de Ourense).
M. Sánchez Heras (University Hospital of Guadalajara).
G. de Arriba de la Fuente (University Hospital of Guadalajara).
L. Gil Sacaluga (Virgen del Rocío University Hospital, Seville).
A. Cirugeda García (Infanta Sofía University Hospital, Madrid).
V. Barrio Lucía (Infanta Sofía University Hospital, Madrid).
Please cite this article as: de Sequera P, Pérez-García R, Molina M, Álvarez-Fernández G, Muñoz-González RI, Mérida E, et al. Ventajas del uso de citrato respecto al acetato como estabilizante en el líquido de hemodiálisis: estudio randomizado ABC-treat. Nefrologia. 2022;42:327–337.