Elderly patients on haemodialysis (HD) are a steadily increasing group. They show a high complexity, dependency and comorbidity. Multiple benefits from exercise in HD patients have been reported; however, they have not been specifically evaluated in an elderly population.
ObjectiveTo assess the effect of an adapted low intensity intradialytic exercise programme on muscle strength, functional capacity and health-related quality of life in our elderly patients (>80 years) on HD.
Materials and methodsHD patients were non-randomly assigned to an exercise training group (E) or a control group (C) in a 12-week single-centre prospective study. E included a combined exercise programme using balls, weights, elastic bands and cycle movements in the first 2h of HD sessions. C group patients received standard HD care. Endpoints were: (1) main biochemical data; (2) maximum quadriceps length strength (MQLS) and hand-grip (HG); (3) functional capacity tests: “Sit to stand to sit” (STS10) and “6-min walking test” (6MWT); (4) Beck Depressive Inventory (BDI); and (5) health-related quality of life questionnaire: EuroQol-5D (EQ-5D).
ResultsA total of 22 patients were included (50% men). Mean age was 83.2 years; patients had received HD for 44.1 months. Charlson index was 9.5. Main aetiology was diabetes mellitus (36.4%). Eleven patients were assigned to E group and 11 to C group. No related adverse effects were observed. At the end of the study, E group showed an overall improvement in tests (*p<0.05): MQLS 10.5±7.6 vs. 12.9±10.1kg, HG* 16.6±8.7 vs. 18.2±8.9kg, STS10* 29.9±10.6 vs. 25±7.87s, 6MWT* 14.6%, 234.4 vs. 274.7m, BDI* 14.4±11.5 vs. 11.7±10.8 and EQ-5D 49±19.1 vs. 59.5±20.3. No similar changes were observed in C group. Significant differences between groups were also found for HG, MQLS, STS10, 6MWT, BDI and EQ-5D. No significant changes were found in biochemical and anthropometric data, antidepressant treatment or suitable dialysis parameters at the end of the study.
Conclusions(1) An adapted low intensity exercise programme improved muscle strength, functional capacity and health-related quality of life in our elderly patients on HD. (2) Our results highlight the benefits from exercise in HD patients even in this elderly population. (3) In elderly patients on HD, it is worth considering an adapted low intensity intradialytic exercise programme as a part of a comprehensive care.
Los pacientes ancianos constituyen un grupo en continuo crecimiento en los programas de hemodiálisis (HD). Estos se caracterizan por su elevada complejidad, dependencia y comorbilidad asociada. Múltiples beneficios del ejercicio físico en los pacientes en HD han sido descritos, si bien no han sido completamente evaluados en la población anciana.
ObjetivosAnalizar el efecto de un programa adaptado de ejercicio físico intradiálisis sobre la fuerza muscular, la capacidad funcional y la calidad de vida relacionada con la salud en nuestros pacientes ancianos (>80 años) en HD.
Material y métodosEstudio prospectivo unicéntrico no aleatorizado (12 semanas) con 2 grupos comparativos. El grupo ejercicio (E) incluía un programa de ejercicio físico adaptado mediante pelotas medicinales, pesas, bandas elásticas y cicloergómetros en las primeras 2h de HD. El grupo control (C) recibía el cuidado habitual en HD. Analizamos: (1) Parámetros bioquímicos. (2) Fuerza de extensión máxima de cuádriceps (FEMQ) y “hand-grip” (HG). (3) Tests de capacidad funcional: “sit to stand to sit” (STS10) y “six-min walking test” (6MWT). (4) Sintomatología depresiva: inventario Beck (BDI). (5) Calidad de vida: EuroQol-5D (EQ-5D).
ResultadosUn total de 22 pacientes incluidos: 50% hombres. Edad media 83,2 años y 44,1 meses en HD. Charlson medio: 9,5. Principal etiología: DM (36,4%). Un total de 11 pacientes asignados al grupo E y 11 al grupo C. No se observaron efectos adversos relacionados. Al final del estudio, el grupo E presentó de forma global una mejoría en las pruebas realizadas (*p<0,05): FEMQ 10,5±7,6 vs. 12,9±10,1kg; HG* 16,6±8,7 vs. 18,2±8,9kg; STS10* 29,9±10,6 vs. 25±7,87s; 6MWT* 14,6%, 234,4 vs. 274,7m; BDI* 14,4±11,5 vs. 11,7±10,8 y EQ-5D 49±19,1 vs. 59,5±20,3. Estos cambios no se observaron en el grupo C al final del estudio. Del mismo modo, el análisis entre grupos mostró una diferencia significativa para HG, FEMQ, STS10, 6MWT, BDI y EQ-5D. No observamos cambios relevantes en los datos bioquímicos ni antropométricos, en la medicación antidepresiva ni en los parámetros de adecuación dialítica a la finalización.
Conclusiones(1) El programa adaptado de ejercicio físico intradiálisis mejoró la fuerza muscular, la capacidad funcional y la calidad de vida relacionada con la salud de nuestros pacientes ancianos en HD. (2) Aun en población anciana, nuestros resultados realzan los beneficios del ejercicio físico en los pacientes en HD. (3) Ante un paciente anciano en HD, merece la pena considerar la realización de ejercicio físico adaptado intradiálisis como una parte más del cuidado integral en HD.
Due in part to advances in medical science and improved quality of life, today's longer life expectancies and low birth rates in many developed countries have contributed towards the ageing of the worldwide population and continuous recent growth in the percentage of seniors.1 This ageing trend of the population is no different among nephrology patients.2–5 Our better understanding and prevention of renal disease, the correction of anaemia, advances in the management of secondary hyperparathyroidism, new therapeutic alternatives as well as the rapid and continuous technological development in haemodialysis (HD) techniques are some of the factors that have led to improved symptoms of uraemic patients and even increased survival in recent years.6–9 Thus, while in the 1970s a patient on dialysis was considered elderly at the age of 65, this term is currently used for patients over the ages of 75 or 80.4,10,11 In this situation, it is not difficult to understand the progressive growth of the senior population in HD programmes, which is a patient group characterised by greater complexity, poor response to prescribed treatments, high degree of dependence and elevated associated comorbidities, among other factors.12–15
An extraordinarily important aspect in patients with renal replacement therapy is the declining physical capacity with the progression towards permanent HD use. Older age, elevated associated comorbidities, uraemic myopathy and neuropathy, altered protein catabolism, anaemia as well as the sedentarism required during renal replacement therapy all lead to the appearance of complaints about muscle symptoms that limit daily physical capacity and quality of life.14,16,17 Therefore, one of the fundamental aspects of renal patient management should be to provide adequate physical therapy in order to preserve functional capacity and avoid dependence, which involves the need for assistance to carry out daily activities.18–20
In recent decades, several studies have been published about different physical exercise programmes for renal patients on HD; most of these programs have resulted in physiological, functional and psychological benefits.21,22 Elderly HD patients are sometimes unable to participate in exercise programmes safely or satisfactorily, resulting in muscle injuries and a high drop-out rate. Recently, there has been growing interest on low-intensity physical exercise programmes adapted to the characteristics of each patient, which have provided beneficial results similar to those reported in standard programmes. Nevertheless, the number of related studies published in the literature is still limited.23–26
Given the progressive increase in the senior population in HD programmes and the fact that no routine physical exercise programmes have been established for HD patients in our country, we believed that it would be of interest to evaluate muscle strength, functional capacity and health-related quality of life after the implementation of a low-intensity physical exercise programme adapted to elderly patients (>80 years) in renal replacement therapy at our HD unit. Our intention was to show that such a programme would slow the adverse effects of replacement therapy on the muscle tissue of these patients.
Materials and methodsFrom November 2012 and January 2013, we proceeded with a 12-week single-centre prospective study that was approved by the Ethics Committee at our institution and followed the regulations of the Declaration de Helsinki. We evaluated the effects of an intradialytic specifically adapted physical exercise programme on muscle strength, functional capacity, depressive symptoms and health-related quality of life of our elderly HD patients.
Our HD programme included 63 patients distributed into 6 groups that were similar in size, all of which underwent a 4-h sessions on alternate days on a morning, midday or afternoon schedule. Because no specific resources had been designated for the study, our nursing staff led the adapted physical exercise programme. As this activity meant an increase in the daily workload and in order to guarantee safe, adequate HD sessions, the midday patients were assigned to the control group. The reason behind this decision was that the midday session also included most of the hospitalised patients who required therapy (patients in acute clinical situation, HD for critical care, vascular catheterisation, etc.). The patients included in the morning and afternoon sessions were assigned to the exercise group (E) because we considered that the daily hospital workload was not as high and that the exercise programme could be led by the nursing staff at those times.
Inclusion criteria were: informed consent, age 80 or older, on regular HD at our hospital for more than 3 months, and clinical and haemodynamic stable during the last 3 months. Exclusion criteria were: recent cardiovascular event (ischaemic heart disease, CVA, coronary bypass, etc.), evident physical inability, frequent symptomatic hypotension (BP<90/70) at HD sessions, or no written consent.
At office visits scheduled for each trimester (on non-dialysis days), we analysed a series of variables both at the start and at the conclusion of the study.
Demographic variables, anthropometric measurements and biochemistryDemographic variables included age, sex, renal aetiology, Charlson comorbidity index and time on HD. Likewise, we collected the main biochemical data and HD adjustment parameters (Kt/V Daugirdas, 2nd generation).
Together with these variables, we obtained measurements of the muscle tone of the biceps and quadriceps muscle groups of both extremities. A flexible, inextensible tape was used to measure the anatomical reference areas without compressing the soft tissue, and muscle diameter was estimated in centimetres.27
Muscle strength and functional capacityFor the assessment of arm muscle strength, an approved Jamar dynamometre (Handgrip dynamometre) (HG) was used on the dominant arm. This was done with the subject sitting, the shoulder at neutral rotation, elbow flexed at 90° and the forearm in a neutral position. The dynamometre was used on both arms, while instructing the patient to make the greatest effort possible with the arms separated from the body. The arm that demonstrated the greatest strength was considered dominant.28
In order to assess the muscle strength of the lower extremities, a Kern traction dynamometre was used (Kern CH50 50KG dynamometre). The maximum strength of the quadriceps muscle (QMS) of the left leg was estimated with the patient sitting upright in a chair with his/her hips and knees forming 90° angles. In this position, an inextensible strap was placed on the distal third of the tibia and the patient was asked to extend the leg as much as possible without grabbing the arms of the chair.29
The results obtained both in anthropometric variables and muscle strength represent the mean of 3 consecutive measurements at intervals of 15s done by the same professional in order to avoid possible errors in measurement.
The tests used to assess functional capacity were the 6-min walk test (6MWT) and the sit to stand to sit 10 (STS10).30,31 The 6MWT test was done while monitoring vital signs and oxygen saturation by pulse-oximetry. This involved evaluating the maximum distance walked during a 6-min period at an active pace. After the 6min, the total distance measured by an approved odometer was recorded. The STS10 test involved getting up and sitting down 10 consecutive times as fast as possible, starting from a sitting position with the arms against the chest. The time it took to perform the exercise was recorded.
Symptoms of depression and quality of lifeSymptoms of depression were assessed by the Beck Depression Inventory (BDI).32 This is a self-administered questionnaire of 21 multiple-answer questions created to detect the presence of depression and to estimate its severity by evaluating a wide spectrum of depressive symptoms (psychological, cognitive and somatic). The score ranges from 0 to 63 points. Values up to 10 points were considered normal. In general, the higher the score is, the greater the severity of the depression.
Quality of life was estimated with a EuroQol-5D health questionnaire (EQ-5D) because of its simplicity and easy application.33 The first part contained 5 healthcare dimensions (mobility, personal care, daily activities, pain/malaise and anxiety/depression) and each had 3 levels of severity. In this part of the questionnaire, the patient had to define the level of severity of their condition for each of the dimensions on the same day the questionnaire was completed. Levels of severity were scored as 1 (no problems), 2 (some or moderate problems) and 3 (many problems). The second part of the EQ-5D was a visual scale from 0 (poorest state of health) to 100 (best state of health). On this scale, the patient had to define the point that best reflected the state of his/her overall state of health on the day the questionnaire was completed.
Adapted intradialytic physical exercise programmeThe adapted physical exercise programme, which had been previously developed with the Physical Rehabilitation Department at our centre, was supervised and led by our nursing staff. Exercise was done twice weekly in the first 2h of the HD session and lasted for approximately 45–50min. Before and after exercise, the basic vital signs of all patients were monitored (blood pressure, temperature, heart rate and baseline oxygen saturation). During the HD session and after a brief warm-up period, the physical exercises specifically targeted anaerobic capacity, coordination and flexibility of different muscle groups of the extremities that did not have a vascular access. For this purpose, patients used elastic resistance bands, medicine balls, elastic balls, ankle weights, dumbbells and different free weights. For aerobic capacity, patients used electric cycloergometers (Jocca® model) that had been placed at their feet. The intensity (40–50–60rpm) and duration (3–6–9–12–15min) of the cycloergometer exercises were adapted to each individual. We recorded the mean number of revolutions per minute (rpm), the number of revolutions and the mean cycloergometer exercise time.
All exercises were adapted to each patient according to their complexity, dependence and associated comorbidity and were adjusted according to the position of the patient during the HD session. Our objective was for them to perform as many repetitions as possible and the greatest variety of exercises during each HD session in order to avoid monotony and maintain constant motivation throughout the study. Exercise intensity was determined by the nursing staff according to the number of complete flex-extension repetitions with free weights on the dominant arm and complete leg extensions with resistance bands during 1min, which was evaluated monthly. Likewise, a datasheet was created to monitor exercise types, duration and intensity as well as the appearance of adverse effects related with exercise, such as symptomatic clinical hypotension, severe muscle symptoms (pain, fatigue or muscle cramps), cardiac rhythm disorders or cardiovascular events (acute coronary syndrome or cerebrovascular accident) and the number of drop-outs.
The statistical analysis was carried out with the SPSS programme version 18.0 (SPSS Inc., Chicago, IL, USA). The quantitative variables were expressed with their means and standard deviation. The qualitative variables were expressed as percentages. The comparison of the quantitative data of the same group was done with the Wilcoxon test for non-parametric related variables and the qualitative data using the McNemar test; a p value ≤0.05 was considered statistically significant. The comparison of the main quantitative variables among the study groups was done with the Mann Whitney non-parametric U, and those correlations with a p value ≤0.05 were considered statistically significant.
ResultsSixty-three patients from our HD programme unit were analysed. From this total, 22 patients who met the established study criteria were selected: 11 were assigned to group E and 11 to group C. Fifty percent were men, with a mean age of 83.2±4.2 years and a mean HD treatment time of 44.1±59.6 months. Mean Charlson index was 9.5±1.2. The aetiologies of chronic kidney failure were: diabetes mellitus (36.4%), kidney disease of unknown origin (18.2%), hypertension (13.6%), polycystic kidney disease (9.1%), chronic pyelonephritis (4.5%), glomerular disease (4.5%) and others (13.6%).
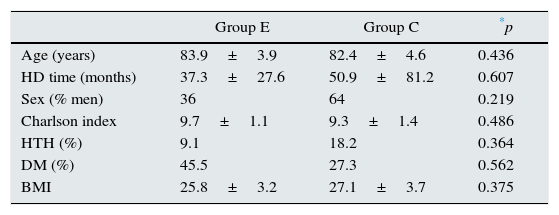
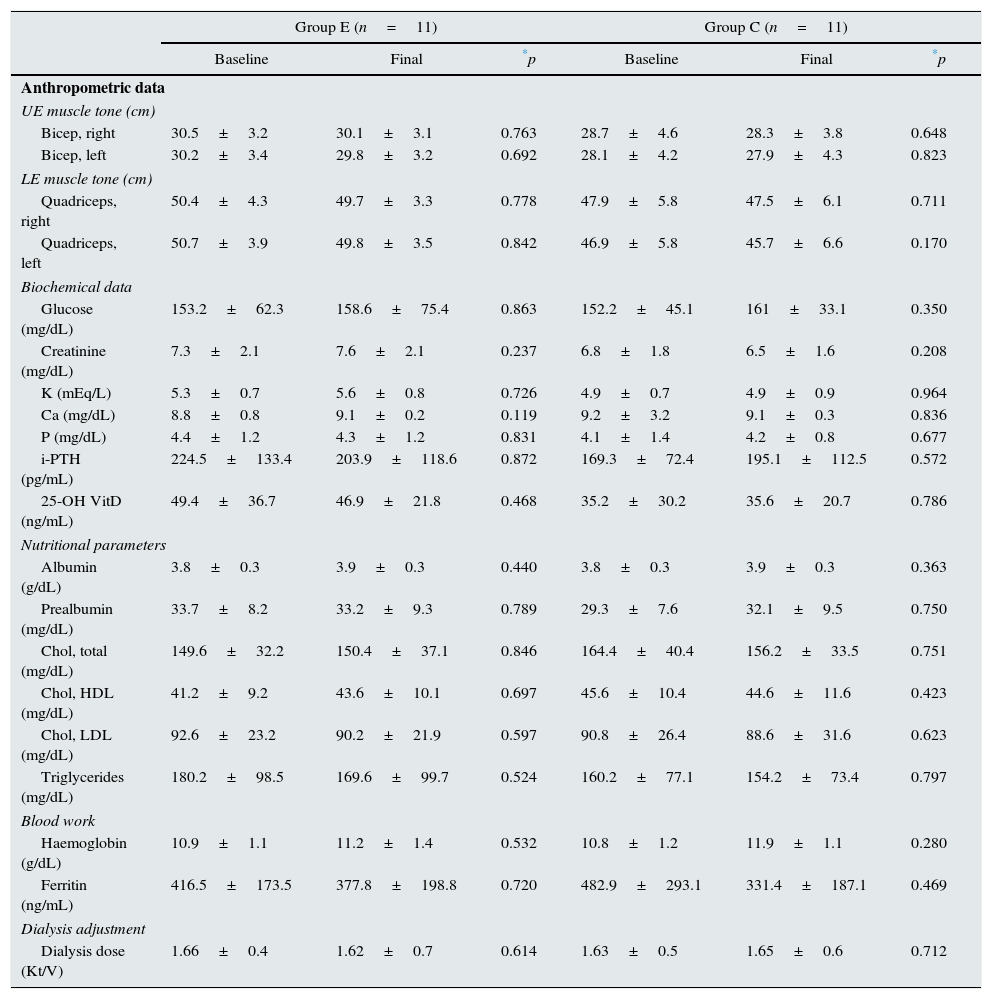
No significant differences were found between the study groups for demographic variables, comorbidity and main aetiology of the kidney disease (Table 1) at the start of the study. Moreover, no significant differences were found between the groups studied for the variables of muscle tone, main biochemical data and dialysis adjustment parameters (Table 2) after physical exercise. No modifications were made in the mean dose of erythropoietic agents or in the treatment with native vitamin D (calcifediol 0.266mg/month) usually prescribed in our patients during the study (30.9±11.6 vs. 28.8±10.4mcg darbepoetin/week; 58.7 vs. 56.4% patients treated with vitamin D for groups E and C, respectively, during the study).
Demographic data, comorbidity and main aetiology of the kidney disease.
| Group E | Group C | *p | |
|---|---|---|---|
| Age (years) | 83.9±3.9 | 82.4±4.6 | 0.436 |
| HD time (months) | 37.3±27.6 | 50.9±81.2 | 0.607 |
| Sex (% men) | 36 | 64 | 0.219 |
| Charlson index | 9.7±1.1 | 9.3±1.4 | 0.486 |
| HTH (%) | 9.1 | 18.2 | 0.364 |
| DM (%) | 45.5 | 27.3 | 0.562 |
| BMI | 25.8±3.2 | 27.1±3.7 | 0.375 |
Group E: n=11 and C n=11 at start of study.
C: control; DM: diabetes mellitus; E: exercise; HTN: arterial hypertension; BMI: body mass index.
Anthropometric data, biochemical parameters and adjustment of dialysis.
| Group E (n=11) | Group C (n=11) | |||||
|---|---|---|---|---|---|---|
| Baseline | Final | *p | Baseline | Final | *p | |
| Anthropometric data | ||||||
| UE muscle tone (cm) | ||||||
| Bicep, right | 30.5±3.2 | 30.1±3.1 | 0.763 | 28.7±4.6 | 28.3±3.8 | 0.648 |
| Bicep, left | 30.2±3.4 | 29.8±3.2 | 0.692 | 28.1±4.2 | 27.9±4.3 | 0.823 |
| LE muscle tone (cm) | ||||||
| Quadriceps, right | 50.4±4.3 | 49.7±3.3 | 0.778 | 47.9±5.8 | 47.5±6.1 | 0.711 |
| Quadriceps, left | 50.7±3.9 | 49.8±3.5 | 0.842 | 46.9±5.8 | 45.7±6.6 | 0.170 |
| Biochemical data | ||||||
| Glucose (mg/dL) | 153.2±62.3 | 158.6±75.4 | 0.863 | 152.2±45.1 | 161±33.1 | 0.350 |
| Creatinine (mg/dL) | 7.3±2.1 | 7.6±2.1 | 0.237 | 6.8±1.8 | 6.5±1.6 | 0.208 |
| K (mEq/L) | 5.3±0.7 | 5.6±0.8 | 0.726 | 4.9±0.7 | 4.9±0.9 | 0.964 |
| Ca (mg/dL) | 8.8±0.8 | 9.1±0.2 | 0.119 | 9.2±3.2 | 9.1±0.3 | 0.836 |
| P (mg/dL) | 4.4±1.2 | 4.3±1.2 | 0.831 | 4.1±1.4 | 4.2±0.8 | 0.677 |
| i-PTH (pg/mL) | 224.5±133.4 | 203.9±118.6 | 0.872 | 169.3±72.4 | 195.1±112.5 | 0.572 |
| 25-OH VitD (ng/mL) | 49.4±36.7 | 46.9±21.8 | 0.468 | 35.2±30.2 | 35.6±20.7 | 0.786 |
| Nutritional parameters | ||||||
| Albumin (g/dL) | 3.8±0.3 | 3.9±0.3 | 0.440 | 3.8±0.3 | 3.9±0.3 | 0.363 |
| Prealbumin (mg/dL) | 33.7±8.2 | 33.2±9.3 | 0.789 | 29.3±7.6 | 32.1±9.5 | 0.750 |
| Chol, total (mg/dL) | 149.6±32.2 | 150.4±37.1 | 0.846 | 164.4±40.4 | 156.2±33.5 | 0.751 |
| Chol, HDL (mg/dL) | 41.2±9.2 | 43.6±10.1 | 0.697 | 45.6±10.4 | 44.6±11.6 | 0.423 |
| Chol, LDL (mg/dL) | 92.6±23.2 | 90.2±21.9 | 0.597 | 90.8±26.4 | 88.6±31.6 | 0.623 |
| Triglycerides (mg/dL) | 180.2±98.5 | 169.6±99.7 | 0.524 | 160.2±77.1 | 154.2±73.4 | 0.797 |
| Blood work | ||||||
| Haemoglobin (g/dL) | 10.9±1.1 | 11.2±1.4 | 0.532 | 10.8±1.2 | 11.9±1.1 | 0.280 |
| Ferritin (ng/mL) | 416.5±173.5 | 377.8±198.8 | 0.720 | 482.9±293.1 | 331.4±187.1 | 0.469 |
| Dialysis adjustment | ||||||
| Dialysis dose (Kt/V) | 1.66±0.4 | 1.62±0.7 | 0.614 | 1.63±0.5 | 1.65±0.6 | 0.712 |
Group E: n=11 and C: n=11; baseline vs. end of study.
C: control; Ca: calcium; Chol: cholesterol; E: exercise; LE: lower extremities; UE: upper extremities; HDL: high-density lipoproteins; i-PTH: intact parathyroid hormone; K: potassium; Kt/V: Daugirdas 2nd generation formula; LDL: low-density lipoproteins; P: phosphorus; VitD: vitamin D.
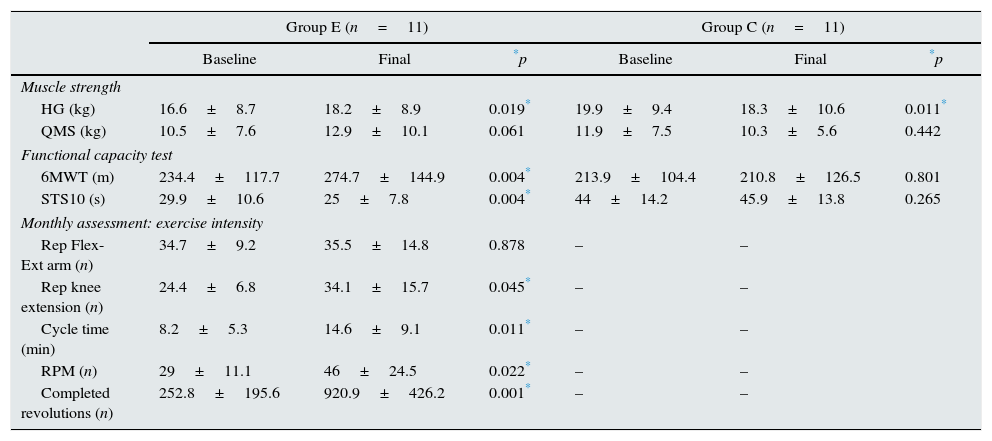
Table 3 shows the results from the assessment of muscle strength and functional capacity. After the adapted intradialytic physical exercise programme, we observed a significant improvement in muscle strength estimated by HG for group E (16.6±8.7 vs. 18.2±8.9kg; p=0.019) as well as a significant deterioration in group C (19.9±9.4 vs. 18.3±10.6kg; p=0.011). As for the muscle strength assessment of the lower extremities by QMS, we observed an improvement in group E at the conclusion of the study (10.5±7.6 vs. 12.9±10.1kg; p=0.061), although the pre-established statistical significance criterion was not met. We observed no relevant changes in the QMS in group C (11.9±7.5 vs. 10.3±5.6kg; p=0.442). As for the functional tests, we observed a significant increase in the distance covered (40.3m) in the 6MWT (14.6%, 234.4±117.7 vs. 274.7±144.9m; p=0.004), as well as a shorter STS10 time (29.9±10.6 vs. 25±7.8s; p=0.004) in group E at the end of the study. We observed no relevant changes in any of these functional tests in group C. In the monthly assessment of the patients used to adapt the overall exercise intensity, we observed improvements in the average number of repetitions with the dominant arm, complete knee extensions, mean cycloergometer use times, revolutions per minute (rpm) and the number of revolutions (group E). No patients chose to leave the study, nor were there any observed exercise-related adverse effects at the conclusion of the study.
Assessment of muscle strength, functional capacity and exercise intensity (monthly).
| Group E (n=11) | Group C (n=11) | |||||
|---|---|---|---|---|---|---|
| Baseline | Final | *p | Baseline | Final | *p | |
| Muscle strength | ||||||
| HG (kg) | 16.6±8.7 | 18.2±8.9 | 0.019* | 19.9±9.4 | 18.3±10.6 | 0.011* |
| QMS (kg) | 10.5±7.6 | 12.9±10.1 | 0.061 | 11.9±7.5 | 10.3±5.6 | 0.442 |
| Functional capacity test | ||||||
| 6MWT (m) | 234.4±117.7 | 274.7±144.9 | 0.004* | 213.9±104.4 | 210.8±126.5 | 0.801 |
| STS10 (s) | 29.9±10.6 | 25±7.8 | 0.004* | 44±14.2 | 45.9±13.8 | 0.265 |
| Monthly assessment: exercise intensity | ||||||
| Rep Flex-Ext arm (n) | 34.7±9.2 | 35.5±14.8 | 0.878 | – | – | |
| Rep knee extension (n) | 24.4±6.8 | 34.1±15.7 | 0.045* | – | – | |
| Cycle time (min) | 8.2±5.3 | 14.6±9.1 | 0.011* | – | – | |
| RPM (n) | 29±11.1 | 46±24.5 | 0.022* | – | – | |
| Completed revolutions (n) | 252.8±195.6 | 920.9±426.2 | 0.001* | – | – | |
Group E: n=11 and C: n=11; baseline vs. end of study.
6MWT: 6-min walk test; C: control; E: exercise; Ext: extension; QMS: quadriceps muscle strength; Flex: flexion; HG: hand grip of the dominant arm; m: metres; min: minutes; n: number; Rep: repetitions; RPM: revolutions per minute; s: seconds; STS10: sit-to-stand-to-sit 10.
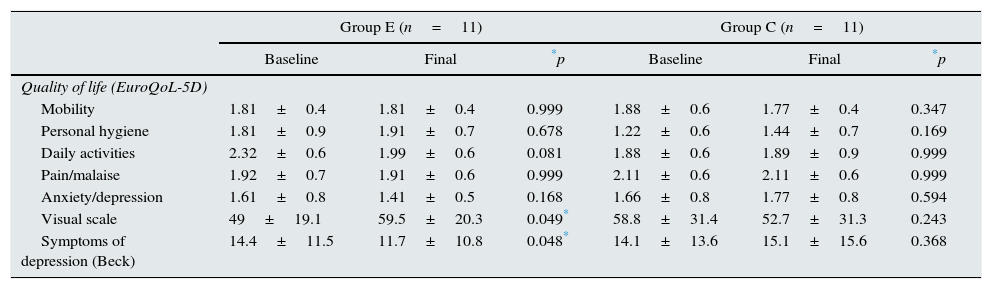
Table 4 shows the results for symptoms of depression and quality of life. Regarding depression, the patients from group E reported a significant improvement in their mood (14.4±11.5 vs. 11.7±10.8; p=0.048) at the end of the study, while the patients of group C reported no changes. No modifications were made in the prescribed antidepressant medication for any of our patients over the course of the study (30 vs. 22% anxiolytics, 30 vs. 24% antidepressants and 7 vs. 14% hypnotics in groups E and C, respectively, throughout the study). As for quality of life, no relevant changes were observed in the different dimensions of the EQ-5D in any of the study groups at the conclusion of the adapted exercise programme. In group E, however, we observed an improvement in the daily activity dimension, although this did not reach statistical significance. Likewise, a significant improvement was observed in the EQ-5D assessment of overall health status using a visual scale (49±19.1 vs. 59.5±20.3; p=0.049) in group E at the end of the study. These latter results were not observed in group C.
Quality of life (EuroQoL-5D) and symptoms of depression (Beck questionnaire).
| Group E (n=11) | Group C (n=11) | |||||
|---|---|---|---|---|---|---|
| Baseline | Final | *p | Baseline | Final | *p | |
| Quality of life (EuroQoL-5D) | ||||||
| Mobility | 1.81±0.4 | 1.81±0.4 | 0.999 | 1.88±0.6 | 1.77±0.4 | 0.347 |
| Personal hygiene | 1.81±0.9 | 1.91±0.7 | 0.678 | 1.22±0.6 | 1.44±0.7 | 0.169 |
| Daily activities | 2.32±0.6 | 1.99±0.6 | 0.081 | 1.88±0.6 | 1.89±0.9 | 0.999 |
| Pain/malaise | 1.92±0.7 | 1.91±0.6 | 0.999 | 2.11±0.6 | 2.11±0.6 | 0.999 |
| Anxiety/depression | 1.61±0.8 | 1.41±0.5 | 0.168 | 1.66±0.8 | 1.77±0.8 | 0.594 |
| Visual scale | 49±19.1 | 59.5±20.3 | 0.049* | 58.8±31.4 | 52.7±31.3 | 0.243 |
| Symptoms of depression (Beck) | 14.4±11.5 | 11.7±10.8 | 0.048* | 14.1±13.6 | 15.1±15.6 | 0.368 |
Group E: n=11 and C: n=11; baseline vs. end of study.
EuroQol-5D analysis by dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) and overall assessment using a visual scale for health.
C: control; E: exercise.
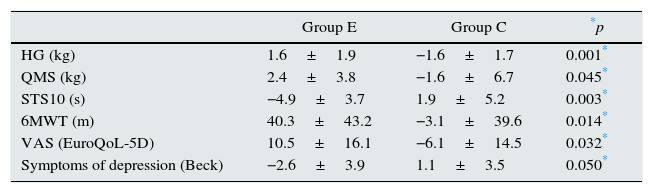
Table 5 shows the results of the comparative analysis for the main variables between the study groups using the difference of means. Significant differences were detected for HG (1.6±1.9 vs. −1.6±1.7kg; p=0.001), QMS (2.4±3.8 vs. −1.6±6.7kg; p=0.045), STS10 (−4.9±3.7 vs. 1.9±5.2s; p=0.003), 6MWT (40.3±43.2 vs. −3.1±39.6m; p=0.014), symptoms of depression (−2.6±3.9 vs. 1.1±3.5; p=0.050) and visual health scale of the EQ-5D (10.5±16.1 vs. −6.1±14.5; p=0.032) for groups E and C, respectively.
Analysis by study groups; analysis of the difference of the means of the main variables between the study groups using the non-parametric Mann–Whitney U test.
| Group E | Group C | *p | |
|---|---|---|---|
| HG (kg) | 1.6±1.9 | −1.6±1.7 | 0.001* |
| QMS (kg) | 2.4±3.8 | −1.6±6.7 | 0.045* |
| STS10 (s) | −4.9±3.7 | 1.9±5.2 | 0.003* |
| 6MWT (m) | 40.3±43.2 | −3.1±39.6 | 0.014* |
| VAS (EuroQoL-5D) | 10.5±16.1 | −6.1±14.5 | 0.032* |
| Symptoms of depression (Beck) | −2.6±3.9 | 1.1±3.5 | 0.050* |
Group E: n=11 and C: n=11.
6MWT: 6-min walk test; C: control; E: exercise; VAS: visual analogue scale for health; QMS: quadriceps muscle strength; HG: handgrip of the dominant arm; s: seconds; STS10: sit-to-stand-to-sit 10 test.
In recent years, we have been witnessing a progressive increase in the number of elderly patients in renal replacement therapy programmes, mainly those involving HD.3,13 The latest advances made in the treatment of renal disease and the development of new HD techniques have been able to improve the uraemic symptoms and life expectancy of these patients.6,11 Thus, in coming years, it will not be uncommon to find more and more elderly patients in HD units, characterised by increased comorbidities and greater complexity, more dependence to perform daily activities due to deteriorating physical condition associated with the sedentarism of replacement therapy itself and poor quality of life.12,20
In recent decades, several studies have been published about improvements in the functional capacity and health-related quality of life of renal patients after regular physical exercise. Most of these studies report the beneficial effects of physical exercise observed in functional capacity, psychological status and health-related quality of life.21,22 Recently, several studies have been published about low-intensity exercise programmes adapted to individual patient characteristics23–26 as some exercises could not be done safely and satisfactorily by all participants because they caused muscle injuries, adverse cardiovascular events and a high number of drop-outs. Mercer et al.23 observed improvements in functional capacity and the ability to perform activities of daily living after a combined low-intensity physical exercise programme (aerobic and strength training) that lasted 12 weeks and included 22 HD patients. Identical results were observed by van Vilsteren et al.24 in 96 patients after a 12-week exercise programme that was predominantly aerobic, as well as by Chen et al.25 after a 48-week low-intensity strength/resistance programme in both lower extremities in a study that included 50 HD patients. In the only national study published to date, Segura et al.26 evaluated 27 patients in an HD programme who were randomised to 2 comparative groups of either a strength/resistance programme or a low-intensity, predominantly aerobic programme, both of which lasted 24 weeks; the authors found evidence of improved functional capacity and health-related quality of life in both groups.
In our study, the implementation of an adapted low-intensity physical exercise programme in elderly HD patients (>80 years) improved muscle strength, functional capacity, symptoms of depression and health-related quality of life. Using similar functional tests and studies, the overall results obtained by our study are identical to those previously reported in the literature. However, the main differences of our study were the fact that we exclusively evaluated a group of senior patients over the age of 80, with their elevated associated comorbidities, and that we adapted the exercise type and intensity, both aerobic and anaerobic, according to patient characteristics.
As for muscle strength, we observed improved muscle strength in the upper extremities estimated by HG, which is a reliable indicator and prognostic in the assessment of overall strength in geriatric patients.28,34,35 This increased strength could correlate with morphologic and functional changes of the muscle fibres that would entail greater activation and recruitment of the muscle groups involved and, consequently, greater strength.16,17,36 In the lower extremities, there was an evident tendency towards improvement in group E, but this result did not reach statistical significance. These results could be attributed to the advanced muscle atrophy of elderly patients with multiple comorbidities who had not trained previously, to the difficulty involved in correctly performing the test (QMS) in these patients, as well as the limited sample size. Nonetheless, the increase in the number of repetitions in the lower extremities measured monthly as well as the increasing intensity in cycloergometer use over the course of the study indirectly suggest an increase in muscle strength and functionality in the lower extremities. As for the function tests, it should be stated that both the 6-min walk test and the STS10 are widely used tests to assess functional capacity.30,31 Results of more than 3.4kg para HG, shorter times for the STS10 (8.4s) or an increase in the distance covered in the 6MWT of 66.3m represent changes with important associated clinical value and indicate improved strength and functional capacity of the extremities involved.37 The higher activation and recruitment of the previously mentioned muscle fibres could explain the improvement observed in the function tests that were only observed after the adapted physical exercise programme. While our results are slightly inferior to previous publications, this is probably due to the characteristics of our elderly population.
Regarding depressive symptoms, certain psychological problems, such as depression and anxiety, are rather common in HD patients. Factors that lead to its appearance include the chronic nature of renal replacement therapy, physical symptoms like fatigue, nighttime insomnia or thirstiness and life expectancy conditioned by renal transplantation.38,39 Given the repercussions in the quality of life of these patients, prevention and early treatment of symptoms are important. In this regard, the results obtained by our study highlight the benefits of physical exercise, as previously published, from a psychological standpoint. The reason behind it, first of all, is based on certain theories such as the release of neurotransmitters (e.g. endorphins) into the circulation that cause a sensation of well-being. Furthermore, exercise affects different emotional and behavioural aspects, such as the substitution of negative thoughts and low self-esteem, while reducing anxiety and notably improving one's attitude. Last of all, group exercise promotes socialisation by participating in a fun, organised activity during HD sessions.21,22,40–42
As we have mentioned previously, in the medical literature there are reports that physical exercise improves the health-related quality of life of renal patients in HD.18–22 Our patients were elderly and had elevated comorbidity, prolonged HD permanence and limited life expectancy because the option for kidney transplantation had been excluded in the majority. Nonetheless, the improved muscle strength, functional capacity and symptoms of depression were accompanied by a significant improvement in the quality of life score estimated by the visual scale for state of health in group E. Curiously, the only striking change was obtained in the daily activity dimension for this group. This finding is of great clinical interest as it suggests that a small improvement in the physical activity level of these persons could delay the deterioration from a state of independence to a state of disability. The result would avoid the decline in quality of life and dependence of HD patients, with all the unfavourable clinical consequences and use of healthcare resources that this entails.
We should highlight the effectiveness and safety observed in our adapted low-intensity exercise programme, with no drop-outs or unfavourable effects over the course of the study. These results show that, in spite of the potential risks of regular physical exercise at low or moderate intensity in elderly HD patients, the benefits obtained with these adapted low-intensity exercise regimens are clearly greater.
Among the numerous limitations of our study, we would like to comment on the lack of randomisation of the study groups, with the possible biases that this could raise. Patient allocation was conditioned by the lack of external funding or any additional resources, so it therefore seemed reasonable to assign patients in accordance with the nurses’ workload. We should also mention that the limited sample size required the use of non-parametric tests and the follow-up time was very short, although it is similar to most previously published studies. Furthermore, in our study we observed no changes in muscle tone or in central biochemical data. Perhaps the use of specific analytical methods for body composition, which were not used in our study, could have shown some changes in these aspects. Nonetheless, the characteristics of elderly patients themselves, the low intensity and the short intervention time, in our opinion, make unlikely any significant favourable changes in body composition or nutritional biochemical parameters. In this regard, more extensive and better designed studies would be necessary in order to establish the potential benefits of physical exercise in this particular group of patients.
In conclusion, a low-intensity physical exercise programme adapted to elderly patients (>80 years) improved muscle strength, functional capacity, symptoms of depression and health-related quality of life in our HD patients. Until future studies are completed, the results obtained in our study support the benefits of physical exercise, even in elderly patients in an HD programme. Exercise should be considered an essential part of the integral management of HD patients in order to avoid the progressive deterioration in physical condition and functional capacity.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank all the patients and nursing staff for their valuable collaboration in this study.
This research project was completed within the framework of the Doctorate in Medicine programme at the Autonomous University of Barcelona.
Please cite this article as: Simo VE, Jiménez AJ, Guzmán FM, Oliveira JC, Nicolas MF, Potau MP, et al. Beneficios del ejercicio físico de baja intensidad durante la sesión de hemodiálisis en el paciente anciano. Nefrologia. 2015;35:385–394.