INTRODUCTION
Biobank is an establishment containing biological samples that are associated with clinical information. These samples are collected, processed, stored and managed according to quality criteria in order to make them available to serve society in a non-profit way with the aim of promoting biomedical investigation.
As we infer from that definition, biobanks are not the active agent for a single centre’s research; rather, they are complementary tools to facilitate the research of others. Biobanks have come to be in the last five years; their origin lies in the enormous growth of scientific production in biomedicine, the need to coordinate basic and clinical studies by fostering cooperative models, and the possibility of increasing the efficiency of informed consent so that the same biological material may serve for different studies. Naturally, all of this must be done according to criteria ensuring the quality and suitability of the stored samples, and the ethical and legal requirements that guarantee citizens’ rights. Although these factors were already being taken into account be researchers, they were not precisely regulated. The combination of all of the above aspects led to the development of biobanks being considered a top-priority strategic need by both the EEC and, at the Spanish national level, the Carlos III Health Institute.
Biobanks are intended to produce wealth through creating a mass of human biological capital intended to foster biomedical research and the promotion of health. Samples must have an overall quality that is not only associated with their collection and storage conditions, but also to their traceability and the precision and reliability of the clinical data associated with those samples.
Present and future of biobanks
At this time, few biobanks are present in Spain. Due to strategic initiatives by health authorities in this country, an important development is foreseen in the near future. The development of a biobank passes through the following phases: defining its purpose, predicting necessities, obtaining funding, installing infrastructure and preparing techniques, preparing documentation, implementing a management system, and beginning operations. In the medium term, biobanks will begin to function as a network, to maximise their potential on both the national and European levels.
Biobanks could be intended to manage the biological material that would be necessary to develop a large project, which would become increasingly associated with a high degree of specialisation (National DNA Bank). They could also attend to the needs of an institution - a hospital, for example - or they could study a disease, as is the case of the Spanish HIV BioBank. These purposes would not be exclusive, although in the future we would expect a tendency towards a certain degree of specialisation.
Bases for guaranteeing the effectiveness of biobanks
As mentioned previously, biobanks are a very effective tool at the service of the scientific community. When storing samples on a large scale, complex technical procedures must necessarily be used for sample collection, transport, identification, traceability maintenance, storage at the proper temperature, and for computerised data processing, etc. The bases that will guarantee the effectiveness of biobanks will comprise:
- Standardisation of procedures: the use of homogeneous, well-defined protocols for sample collection, processing, conservation and storage will guarantee sample quality. Well-defined standard notation of associated data will also create quality clinical information. This will reflect the sample’s importance as biological capital, since a sample with no associated information lacks value. On the other hand, the fact that the collection and storage of samples and associated clinical data will not be scattered among different groups, centres, collections, etc. will mean that all samples will share the same conditions and will be able to be compared with each other in different studies.
- Guaranteed sample traceability, by drawing on proper methodology for sample coding and identification, which will guarantee its traceability at all times.
- A dynamic and effective management by means of computer applications that allow us to list all of the processes to which samples and associated data have been submitted.
- The implementation of a quality management system to make the organisation function effectively, improving biobank customer satisfaction.
- Employment of qualified personnel who will be exclusively dedicated to the operation of the biobank.
Bases for managing biobanks in Spain
The entire process that takes place from collecting the samples up to their use by researchers must be in accordance with applicable legislation, which for biobanks is principally composed of:
- Spanish Law 14/2007 of 3 July regarding biomedical research,1 which contemplates its origins and regulates its organisation, functioning and records. At present, there is no developmental legislation to clarify it, meaning that we must wait to know what conditions will be necessary for authorisation and for havinga national registry. By law, biobanks must guarantee the quality, safety and traceability of the biological data and samples that they house. Likewise, they must guarantee protection of the rights of subjects who have donated biological material and who may be affected by the research.
- Organic Law 15/1999 of 13 December regarding the Protection of Personal Data.2 This law must be applied to samples from identifiable subjects and to samples for which identification could be re-associated or decoded, which are considered to be personal health-related data. It requires guaranteeing confidentiality and the proper useof samples in the exclusive field of the approved research study. The law does not apply to anonymous or irreversibly dissociated samples.
Biobanks may be owned publicly or privately, but for all cases they must be non-profit organisations. Their organisational scheme must contain a scientific director and a file manager, and in addition, they must be attached to two external committees: a scientific committee and an ethics committee.
Legal considerations for using biological samples are different for biobanks and sample collections. In both cases, however, the informed consent of the source subject is necessary in order to obtain the samples. Given biobanks’ mission of public service, consent is granted in more wide-ranging terms. This means it is not necessary to give details about the specific project in which the sample is to be used, as would be the case for collections; also, there must be a clause regarding ceding the sample to third parties, which is also impossible for normal data collecting without obtaining further consent.
In this way, samples can be kept indefinitely in a biobank and be at the scientific community’s disposal for future investigations that are not planned atthe moment the sample is taken. The nature of the sample is respected in any case.
Samples can be provided at no cost for use in research projects that have been approved by both the scientific and ethics sides.
Only the minimum necessary quantity will be ceded. Samples and associated data that are transferred will be kept anonymous or dissociated. In the cession agreement, the researcher will commit to not use the sample for any purpose other than that for which the sample was requested. Likewise, he/she may not cede the sample to third parties.
Who benefits from biobank development?
We expect that the benefits provided by biobanks will affect various segments of the population, including the following:
- Researchers:
- Sample quality and organisation criteria allow them to carry out epidemiological studies under proper conditions.
- Biobanks provide them with legal and ethical assessment and regulate the exchange of biological samples for investigation using standard procedures and transparent workings.
- Biobanks act as an instrument for cooperative investigation that is multi-centre, interdisciplinary and multi-sectorial.
- Biobanks contribute to gaining new financing and patents for the Health System.
- Patients:
- By using biological samples, researchers can develop biomarkers and target treatments that may lead to advances in prediction, early diagnosis and disease treatment methods.
- Society in general:
- Biobanks may serve to develop diagnostic tools for preventing diseases.
- They may identify risk factors if studies are carried out relating lifestyles or environmental factors with diseases.
- The creation of biological capital will foster the creation of social wealth by acting as a factor promoting the development of the pharmaceutical industry.
Other considerations related to biobanks
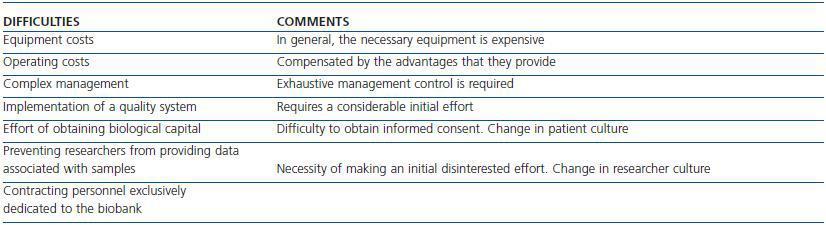
Despite the positive aspects that biobanks can contribute, summarised above, their start-up is not free from difficulties, some of which are shown in table 1.
Biobanks and Nephrology
Our speciality is not unaware of the need to have these tools at our disposal, and in the future they will be completely necessary if we wish to deepen our study of the diseases that concern us. In addition, the possibility of real-time access to samples stored in biobanks will further increase nephrologists’ capacity for research. Given that the SEN (Spanish Society of Nephrology) has been a participative scientific society throughout its history, we are certain that the development of biobanks will lead to better cooperation among us, increasing our research potential, and therefore our contribution to improving care for kidney disease patients. Although we are still in a develop ment phase,3 we are placing the Ramon y Cajal UH-CIFRA biobank at the disposal of Spanish nephrologists wishing to use it for their projects.
Table 1. Difficulties involved in starting up biobanks