In recent years, exposure to certain chemical substances has become a part of everyday life. Such is the case with bisphenol A (BPA), or 2,2,-bis(4-hydroxyphenyl) propane, a molecule used to synthesize polycarbonate plastics and epoxy resins. It is used extensively in the production of babies’ bottles, water and soft drinks bottles, and as the inner coating of cans and other food and drink containers. It is not surprising, then, that in 2009 around 6000000 metric tonnes of BPA were generated worldwide.1
Numerous studies have demonstrated that more than 90% of the population in the USA have detectable urinary levels of this compound and that the level of exposure of the population is above the recommended values: 50μg per kg per day.2 In a recent study conducted in Spain, Cutanda et al.3 reported that BPA was present in the urine of 97% of the population studied.
Exposure to BPA occurs mainly via the oral route, but also from dental sealants, through the skin, and by inhalation of cleaning products. Even more concerning is the fact that studies conducted in Spain,4 China, and Japan.5 have shown contamination of subterranean water and rivers with BPA.
Numerous studies1,5–8 consider that BPA interfere with to be an endocrine regulation. It has been studied the potential relationship between the oestrogenic activity (xenoestrogen) of BPA and different endocrine and metabolic abnormalities including hepatic and thyroid disorders, obesity, insulin resistance, and increased susceptibility to diabetes.9
From a renovascular perspective, the first concerns emerged in 2008 when Lang et al.10 found a significant correlation between a high urinary concentration of BPA and cardiovascular diseases in patients with type 2 diabetes.8,10 The present review will analyse first the critical role of renal function on BPA excretion, and secondly will analyse the experimental evidence that provides a solid scientific basis for translational clinical studies that implicate BPA in renovascular damage.
Accumulation of bisphenol A in patients with chronic kidney diseaseIt has been established that after ingestion, BPA is conjugated in the liver with glucuronic acid, where it loses its oestrogenic activity and is then excreted to the intestine. Both BPA and its metabolites are excreted in urine.1–3,6 Therefore, patients with chronic kidney disease (CKD) have higher serum levels of BPA than the general population11. A negative correlation has been observed between estimated glomerular filtration rate and the serum concentration of BPA.7 A recent study by Krieter et al., analysed a cohort of 152 patients with CKD and 24 controls; a significant increase in plasma concentrations of BPA was observed in CKD 3–5. The highest concentration of BPA was obtained in patients with CKD 5 (dialysis) with values of up to 6 times higher than controls without kidney disease.11,12
Currently, the BPA clearance by dialysis has not been established. This is a complex issue since the dialysis membranes themselves contain variable amounts of BPA. This has been proven by studies that demonstrate the presence of BPA in the effluents of polymethylmetacrylate, cellulose, cellulose triacetate, polyester polymer, and polysulphone membranes, particularly the latter.11–13 It has also been demonstrated that BPA levels can rise or remain unchanged after dialysis sessions. This may be due to the fact that BPA is highly protein-bound, at approximately 75%.11 Likewise, data were inconclusive on the role of residual diuresis in BPA excretion in CKD.11 Therefore, further studies are needed to clarify the potential pathophysiological implications of BPA accumulation in CKD, and to evaluate whether BPA should be added to the long list of uraemic toxins.
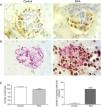
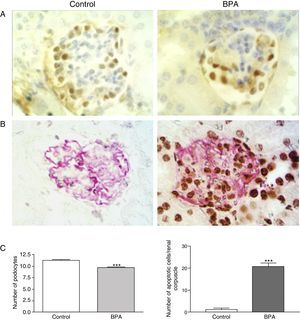
Bisphenol A induces podocyte damage and proteinuriaA recent study described a new type of podocytopathy induced by BPA.14 Olea-Herrero et al. observed that BPA could induce hypertrophy and apoptosis in cultured mouse podocytes. These effects were accompanied by an increase in the synthesis of molecules classically involved in the pathogenesis of glomerulosclerosis, such as the cyclin-dependent kinase inhibitor p27kip1, the TGF-β system, and collagen IV. Furthermore, in these cells, BPA reduced the synthesis of nephrin and podocin, proteins of the filtration slits involved in the mechanisms of both proteinuria and podocyte survival. As would be expected from these in vitro results, the kidneys of animals treated with BPA developed hypertrophy, hyperfiltration, and proteinuria. Along with the increased renal expression of p27kip1, TGF-β, and collagen IV, mesangial expansion and a decrease in the number of podocytes due to apoptosis (Fig. 1) were also seen. Electron microscopy showed hypertrophy of podocytes and pedicles. It should be noted that even when animals treated with BPA did not develop hyperglycaemia, their kidneys showed structural and functional changes similar to those that occur in the initial stages of diabetic nephropathy (DN). Although there are limitations to the use of animal models in the development of renal failure or long-term histomorphological renal changes,15 these findings may have pathophysiological implications, given that proteinuria is a good predictor of progression of kidney disease.16
BPA produces podocytopoenia in mice. (A) Immunohistochemistry for WT-1. In mice treated with BPA the number of podocytes (brown nuclei) was lower than in controls. 300×. (B) TUNEL assay (black nuclei) combined with immunohistochemistry for podocin (grey expansions). The renal corpuscles of those mice treated with BPA showed a higher number of apoptotic podocytes than controls. 300×. (C) Left, graph representing the statistical analysis of the number of podocytes. Right, histogram representing the number of apoptotic cells in mice treated with BPA and in controls. ***P<.001 using ANOVA for analysis of variance.
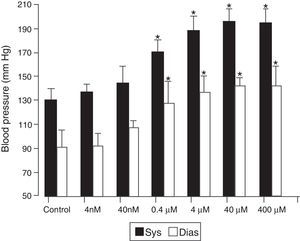
Subsequent studies17 demonstrated that animals treated with BPA developed arterial hypertension and endothelial dysfunction, in a dose-dependent manner. This effect could be observed at doses lower that half of those considered safe (Fig. 2). Microarray analysis of genetic expression in murine endothelial cells treated with BPA demonstrated the activation of genes involved in vascular regulation such as angiotensin II and calcium-calmodulin kinase II (CaMKII). This was subsequently observed in vivo as well. This activation is responsible for the endothelial dysfunction and hypertension induced by BPA, given that CaMKII activation promotes the enzymatic uncoupling of endothelial nitric oxide synthase. This leads to the production of oxygen free radicals instead of nitric oxide, a primary vasodilator and endothelial protector. This increased production of oxygen free radicals indicates that BPA, as well as inducing hypertension, could participate in vascular damage mechanisms and in the progression of atherosclerotic lesions.
BPA induces hypertension in mice. The mice were treated with BPA at the doses indicated or with nothing (controls). Blood pressure was assessed 30 days after the administration of BPA. *P<.05 compared with corresponding control (n=10).
Recent studies have emphasised the interest of experimental rodent models for the study of BPA toxicity. It is important to recognise that BPA has equal potency in human and animal cells.18 Experimental data implicating BPA in renovascular damage have gained particular relevance. Results obtained experimentally are supported by epidemiological studies conducted in the populations of New York,19 Shanghai,20 and Seoul,21 which describe an association between human exposure to BPA and an increased in proteinuria and hypertension.
From a nephrological perspective, two large population studies should be mentioned. One of them was a large adult Chinese population (N=3077)20 and the other involved 710 children in the United States.19 Both studies demonstrated a significant association between urinary excretion of BPA concentration and albuminuria. This association persisted independently of sex, diabetes, smoking status, hypertension, or CKD. The authors of both studies speculated about the possible role of oxidative stress and endothelial dysfunction to explain the findings. However, current experimental data show that even a low-grade albuminuria associated with BPA exposure could promote podocytopathy of uncertain (or at least unexplored) prognosis. This indicates the need to conduct further studies and to re-evaluate the necessity of preventing or limiting BPA exposure.
From a cardiovascular perspective, numerous clinical and epidemiological studies have demonstrated that exposure to BPA is associated with hypertension and vascular damage.22–25 In a sample representative of the adult USA population, Shankar and Teppala22 found that high urinary BPA excretion was associated with arterial hypertension independently of other classic risk factors. Similar results were found in the adult population of Seoul, where 1511 analyses were performed on 521 individuals.24 Likewise, Bae and Hong21 studied the acute effect of oral exposure to BPA in 60 people following the ingestion of 2 servings of the same substance (soya milk) packaged in either a can or a bottle: with canned drinks, high urinary BPA was associated with increased blood pressure 2h after consumption.
There are various studies associating BPA exposure to vascular damage. In Norfolk (United Kingdom), Melzer et al.24 evaluated 758 cases of coronary artery disease and 861 control subjects over a follow-up period of 10.8 years. They demonstrated a significant association between high urinary BPA concentration and incident coronary artery disease. Similar results have been published in studies from the National Health and Nutritional Survey (NHANES) of the USA25 in which the authors, after analysing 745 subjects, found a significant association between urinary BPA level and peripheral arterial disease, independently of classic risk factors.
The implications of these findings are obvious due to both the high incidence in the population and the morbidity and mortality of renovascular disease. It is well established that cardiovascular disease is the most common cause of death in developed countries, with approximately one quarter of the population having some form of this disease.17,21–25 The same may occur in CKD, in which BPA accumulates and could be deemed a uraemic toxin.
It is difficult to definitively characterise the potentially harmful concentration of BPA. However, it is worth mentioning the findings of 2 independent studies in which the consumption of a daily dose of canned beverage produced, after 321 to 526 days, an increase in urinary BPA concentration of over 1000% (over 20ng/mL). Li et al.20 described an association between BPA exposure and albuminuria in adults, with some individuals having a mean urinary BPF concentration of 1ng/mL. Thus, the BPA exposure demonstrated in repeated epidemiological studies, conducted mainly in developed countries,27 is within the range associated with proteinuria and hypertension.
In addition, since the end of the last century, a worldwide epidemic of type 2 diabetes – the most common cause of CKD in developed countries – has been detected. Data from the Registry of the Spanish Society of Nephrology estimate that in 21% of patients, CKD is caused by Diabetic Nephropathy (DN).26 Although classically DN has been considered a metabolic disease, studies by Navarro et al.28 demonstrated the presence of an inflammatory component of DN. Given that only 20–40% of patients with diabetes develop nephropathy, it is suggested that there are other (genetic) diabetes-inducing factors involved that are yet to be discovered.29 Current investigations allow us to hypothesise that the environmental factor BPA may induce or potentiate changes in the kidney that occur in diabetes mellitus. It is worth mentioning, finally, that in USA the FDA has proposed to sponsor research on BPA.30
ConclusionsThe available scientific data allow the identification of BPA as a new environmental factor implicated in renovascular damage. This is characterised by podocytopathy with proteinuria, arterial hypertension, and vascular dysfunction. These data also support the need for translational studies in an attempt to clarify the potential role of BPA in hypertension and in the progression of kidney disease, particularly in patients with diabetes.
RJB received funding from the Spanish Ministry for Science and Innovation (SAF2009-12009-C02-01) and from the Instituto de Salud Carlos III-FEDER (PI12/02825 and PI15/02139). MS received funding from the Spanish Ministry for Economy and Competitiveness (SAF 2012-35141) and a grant from the Spanish Society of Nephrology Foundation (Ayudas Fundación Senefro) 2012. CZ received funding from the Spanish Ministry for Economy and Competitiveness (SAF 2008-04629 and SAF 2011-28375), and the Instituto de Salud Carlos III-FEDER (PI14/02022). N. Olea has a research contract with the Autonomous Community of Madrid-Programme of Activities in R+D in Biosciences 2010 (S2010/BMD-2378). P. Reventún has a research contract with the Ministry of Economy and Competitiveness.
Please cite this article as: Bosch RJ, Quiroga B, Muñoz-Moreno C, Olea-Herrero N, Arenas MI, González-Santander M, et al. El bisfenol A: un factor ambiental implicado en el daño nefrovascular. Respuesta. Nefrología. 2016;36:5–9.