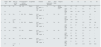
Hepatitis C virus (HCV) and chronic kidney disease (CKD) are often linked prompting a significant increase in morbimortality. New direct acting antivirals (DAA) against HCV achieve rapid response in short period of time.1 However, their use in HCV related renal disease (HCVRRD) is pretty scarce. We present 5 patients with CKD and HCV treated with them (Table 1). All subjects were males (52–57 years old) with impaired renal function and different degrees of proteinuria and microhematuria. Renal biopsy was performed in 1 patient (membranoproliferative glomerulonephritis secondary to cryoglobulinemic vasculitis) being avoided in others due to high risk of bleeding (thrombocytopenia and antiplatelet therapy). Four subjects started on sofosbuvir (SOF) and daclastavir (DAC) and one received DAC with simeprevir (SIM). While HCV viral load achieved negativization (14–30 days), a decline in renal function and a dramatic increase in proteinuria were observed in those SOF with DAC. The only biopsied patient received corticosteroids and Rituximab (375mg/m2, 4 doses) 4 weeks after starting therapy with renal recovery and proteinuria decrease. Patient receiving DAC and SIM finally died of gastrointestinal bleeding 3 months later. Three individuals were admitted during antiviral therapy: severe cellulitis in one case; 2 heart failure episodes in other; last subject required different admissions and finally started chronic hemodialysis.
Patient evolution after the start of HCV treatment.
| Gender age | HBV | HIV (yes–no/viral load) | Pre-treatment HCV viral load (IU/ml)/Genotype | Cryoglobulins | Treatment | HCV negativisation time (days) | Renal biopsy | Diagnosis | Pre | 1m | 2m | 3m | 4m | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | No | No | 990670 | Positive | DAC 60mg/24h | 24 | Yes | MPGN secondary to CV | PCr | 1.54 | 1.51 | 1.36 | 1.36 | 1.1 |
| SOF 400mg/24h | CKD-EPI | 50 | 51 | 58 | 58 | 84 | |||||||||
| 54 | 1b | P/CR ratio | 3100 | 11378a | 3244 | – | 194 | ||||||||
| 2 | M | No | Yes | 1404259 | Negative | DAC 60mg/24h | 14 | No | No | PCr | 1.12 | 1.39 | 1.4 | 1.67 | – |
| SOF 400mg/24h | CKD-EPI | 64 | 58 | – | 52 | – | |||||||||
| 52 | < 1.57 log | 1a | UP/CR ratio | 1351 | 2819 | – | 3260 | – | |||||||
| 3 | M | No | Yes | 1593228 | Negative | DAC 60mg/24h | 30 | No | No | PCr | 1.9 | 2.48 | 2.55 | 2.91 | 2.86 |
| SOF 400mg/24h | CKD-EPI | 32 | 25 | 24 | 17 | 20 | |||||||||
| 54 | <1.57log | 1b | P/CR ratio | 3532 | – | 12995 | 7572 | 10170 | |||||||
| 4 | M | No | No | 24976 | Positive | DAC 60mg/24h | 14 | No | No | PCr | 3.12 | 2.51 | 2.71 | 3.1 | – |
| SIM 150mg/24h | CKD-EPI | 15 | 28 | 25 | – | – | |||||||||
| 54 | 1b | UP/CR ratio | 148 | 423 | 219 | – | – | ||||||||
| 5 | M | No | Yes | 15240836 | Positive | DAC 60mg/24h | 15 | No | No | PCr | 2.03 | 3.03 | 2.51 | 4.1 | 5.77 |
| SOF 400mg/24h | CKD-EPI | 31 | 21 | 27 | 16 | 6 | |||||||||
| 57 | < 1.57 log | 4 | P/CR ratio | – | 4297 | 2110 | 5557 | 6142 |
HBV: Hepatitis B virus; HIV: human immunodeficiency virus; HCV: Hepatitis C virus; DAC: daclatasvir; SOF: sofosbuvir; SIM: simeprevir; MPGN: membranoproliferative glomerulonephritis; CV: cryoglobulinaemic vasculitis; PCr: plasma creatinine (mg/dl); CKD-EPI: chronic kidney disease epidemiology creatinine equation (ml/min/1.73m2); UP/CR ratio: urine protein to creatinine ratio (mg/g); pre: pre-treatment; 1m: 1 month post-treatment; 2m: 2 months post-treatment; 3m: 3 months post-treatment; 4m: 4 months post-treatment.
In our patients, CKD is related with HCV infection, confirmed at least in one case and high suspicion in the others. Treatment of HCV becomes the mainstay 1–2, not only for the infection itself but also for the kidney. In our experience, new DAA achieved a rapid decrease in viral load with an important increase of microhematuria, proteinuria and a notable worsening of renal function in 4 of these 5 patients, one of them finally requiring hemodialysis. The explanation for this disturbing phenomenon could be either an emergent acute intersticial nephritis, but the rapid increase and the amount of proteinuria and microhematuria did not support this possibility, or the exacerbation of HCVRRD, most firmly supported for the same reasons. As suggested by the favorable outcome of the only confirmed HCVRRD patient treated according to published recommendations,2 this situation could be due to an amplification of the immunological processes involved. The lack of diagnostic confirmation made us to avoid using rituximab in the others.
In our experience, the new DAA are very potent and effective drugs, but with a paradoxical worsening of the HCVRRD, mainly when sofosbuvir is used, an aspect that should be studied.
Please cite this article as: Gomis A, Díaz M, de Lorenzo A, Ruiz-Roso G, Liaño F. Precaución con el uso de los nuevos antivirales de acción directa para el tratamiento del virus de la hepatitis C en pacientes con enfermedad renal asociada. Nefrologia. 2016;36:713–715.