Introduction: Decreased levels of 25 hydroxyvitamin D (25[OH]D) have been reported in patients with chronic kidney disease (CKD). The pleiotropic effects of vitamin D are known to go beyond mineral metabolism. Objetives: The aims of this study were to: 1) Determine the 25(OH)D levels in predialysis outpatients. 2) Find out the clinical and biochemical characteristics of patients with 25(OH)D deficiency, and predictive factors for the deficiency. Patients and methods: An observational study in 79 predialysis outpatients was performed. Clinical and biochemical parameters were analysed in terms of nutrition, inflammation and mineral metabolism in relation to serum levels of 25(OH)D. Levels of 25(OH)D lower than 15ng/ml were considered to be deficient. Results: Serum levels of 25(OH)D were deficient in 41 patients (52%). The comparative study regarding levels of vitamin 25(OH)D showed the group of patients with a deficiency, i.e. those with less than 15ng/ml, were older (70 ± 11.97 vs. 61 ± 14.5; p = 0.005), had a greater body mass index, BMI, (30±4.06 vs. 27.1 ± 5.08; p = 0.003) and increased proteinuria (1.42g/24h (0.53-2.96) vs. 0.51 (0.20-1.48), p = 0.009). This group included a greater number of diabetic patients: 20 (76.9%) vs. 6 (23%), p = 0.002. They had a higher level of parathyroid hormone (PTH): 359 (239-658) vs. 233 (129-323), p = 0.000; and more patients were under treatment with Calcitriol: 28 (62.2%) vs. 17 (37.8%), p = 0.024. In the multivariate analysis, high levels of PTH (OR 13.38; CI 95% [2.94-60.89]; p=0.001), increased proteinuria (OR 4.41; CI 95% [1.12-17.25]; p = 0.033); and being diabetic (OR 5.713; CI 95% [1.43-22.77]; p = 0.014) were independent predictor factors for patients with 25(OH)D deficiency. Conclusions: In our study, we observed a high prevalence of 25(OH)D deficiency among patients with CKD. The increased levels of PTH, the increase of proteinuria and the presence of diabetes were independent predictors for 25(OH)D deficiency.
Introducción: Se ha descrito una disminución de los niveles de 25 hidroxivitamina D (25[OH]D) en los pacientes con enfermedad renal crónica (ERC). Conocemos que el efecto pleiotrópico de la vitamina D va más allá del metabolismo mineral. Objetivos: Los objetivos del estudio fueron: 1) determinar los niveles de 25(OH) D en pacientes con ERC seguidos en consulta de prediálisis, y 2) analizar características clínicas y bioquímicas de los pacientes con respecto a los niveles de 25(OH)D y los posibles factores predictivos de la deficiencia en 25(OH)D. Pacientes y métodos: Realizamos un estudio observacional en 79 pacientes con ERC. Analizamos datos clínicos y parámetros bioquímicos en cuanto a nutrición, inflamación y metabolismo mineral en relación con los niveles de 25(OH)D. Se consideró deficiencia de 25(OH)D si los niveles eran inferiores a 15 ng/ml. Resultados: Cuarenta y un pacientes (52%) presentaron deficiencia de 25(OH)D. Comparando los datos analizados según los niveles de vitamina 25(OH)D mayores o menores de 15 ng/ml, el grupo de pacientes con deficiencia en 25(OH)D tenían: más edad (70 ± 11,97 frente a 61 ± 14,5; p = 0,005), mayor índice de masa corporal (IMC) (30 ± 4,06 frente a 27,1 ± 5,08; p = 0,017), y mayor proteinuria (1,42 g/24 h [0,53-2,96] frente a 0,51 [0,20-1,48]; p = 0,009). La hormona paratiroidea (PTH) se encontraba más elevada (359 [239-658] frente a 233 [129-323]; p = 0,000), y más pacientes recibieron tratamiento con calcitriol oral (28 [62%] frente a 17 [37,8%]; p = 0,024). El porcentaje de diabéticos fue mayor en este grupo (20 [76,9%] frente a 6 [23%]; p = 0,002). En el análisis multivariante, la presencia de diabetes (OR: 5,713; IC95% [1,43-22,77]; p = 0,014), el aumento de PTH (OR: 13,38; IC95% [2,94-60,89]; p = 0,001), y la proteinuria más elevada (OR: 4,41; IC95% [1,12-17,25]; p = 0,033) fueron factores predictivos independientes de la deficiencia en 25(OH)D. Conclusiones: Los pacientes con ERC tienen alta prevalencia de deficiencia de 25(OH)D. El aumento de PTH, la elevación de la proteinuria, y la presencia de diabetes fueron factores predictivos independientes de la deficiencia en 25(OH)D.
INTRODUCTION
The skin and certain foods are sources of vitamin D. The action of UV rays on the skin produces provitamin D (cholecalciferol D3). This process involves factors such as age and skin pigmentation.1 Dietary vitamin D depends on the consumption of dairy products, fish and fortified cereals, producing ergocalciferol D2.2
Subsequently, vitamin D binds to proteins and is transported in the blood to the liver where hydroxylation of carbon 25 occurs, forming calcidiol (25[OH]D). This process is not regulated by hormones, as levels depend only on the availability of the substrate. Calcidiol (25[OH]D) is an indicator of vitamin D deficiency
There is then a second hydroxylation in the kidney via the enzyme 1-alpha-hydroxylase, resulting in the formation of calcitriol (1,25-[OH]2D), the active form of vitamin D.3 Both calcidiol and calcitriol can activate the vitamin D receptors which are widely spread throughout the body. These receptors’ pro-differentiation, antiproliferative and immunomodulatory activities have many physiological effects.4 This uses 80% of vitamin D, although the best known action of vitamin D is the regulation of mineral metabolism.
Knowledge of vitamin D, the substrate 25(OH)D and the active form 1,25-(OH)2D is of most interest because of their pleiotropic actions. The involvement of vitamin D in the health of the general population is becoming increasingly understood.5,6
A deficiency in 25(OH)D has been linked to cardiovascular disease, diabetes, the progression of chronic kidney disease (CKD) and immune system disorders.7
CKD patients have a deficiency of the substrate 25(OH)D and the active form 1,25-(OH)2D. A deficiency in the substrate 25(OH)D, both for those with CKD 8-11 and the general population,12 has been associated with age, loss of appetite and factors affecting cutaneous synthesis, such as low sun exposure13 and skin pigmentation.14
A deficiency in the active form 1,25-(OH)2D would be produced by a reduction in the substrate 25(OH)D and decreased activity of the enzyme 1-alphahydroxylase, due to reduced renal mass.15 Currently, the discovery of fibroblast growth factor (FGF)-23 has opened up other avenues for understanding mineral metabolism disorders from the early stages of CKD. FGF-23 is a protein produced in the bone, which acts on the kidneys producing phosphaturia and suppressing the expression of the enzyme 1-alphahydroxylase, which decreases production of 1,25-(OH)2D.16,17 FGF-23 increases in parallel with a decrease in renal function: the increase of phosphorus in the diet would stimulate FGF-23 resulting in a decrease of 1,25-(OH)2D from the early stages of CKD.
Moreover, activity of the enzyme 1-alphahydroxylase in other tissues leads us to believe in the importance of maintaining optimal levels of 25(OH)D.
The study objectives were as follows:
1. Determine the 25(OH)D levels in predialysis outpatients.
2. Compare clinical and laboratory data regarding nutrition, inflammation and mineral metabolism with levels of 25(OH)D.
3. Analyse potential predictors for a deficiency in 25(OH)D.
MATERIAL AND METHOD
Study population
An observational study was conducted on 79 stable CKD predialysis outpatients between March and September 2008.
Patients with an infection or a vascular disease during that period were excluded. The 25(OH)D level was measured between February and March 2008, due to its seasonal nature.
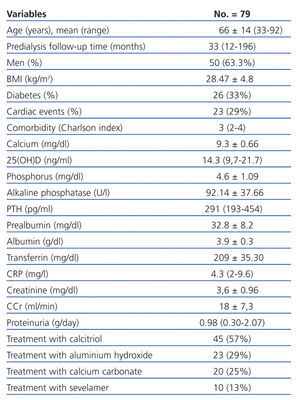
The mean follow-up period for predialysis consultation was 33 months, with a range of 12-196 months. Twenty-six patients (33%) were diabetic, 76 (97.4%) were hypertensive, 32 (40.5%) had a cardiovascular event, and 23 (29%) had cardiac events only. Included in the latter were: angina (10 patients), acute myocardial infarction (3), episodes of heart failure (4), tachyarrhythmias (5) and pericarditis (1).
A number of patients were treated with phosphorus binders: 23 (29%) with aluminium hydroxide, 10 (13%) with sevelamer and 20 (25%) with calcium carbonate. While 45 patients (57%) received oral calcitriol as the only form of vitamin D.
Parameters analysed
The following were measured: Mineral metabolism analysis 25(OH)D, including D2 and D3, calcium (Ca), phosphorus (P), alkaline phosphatase (ALP) and parathyroid hormone (PTH).
Renal function parameters
Serum creatinine (CRP), creatinine clearance (CCr) and proteinuria at 24 hours were measured.
Nutritional parameters
The weight and height of all patients were measured to calculate their body mass index (BMI). The levels of albumin, prealbumin and transferrin (TF) were also determined.
The inflammatory state was determined by C-reactive protein (CRP) by immunoturbidimetry. The reference values were 0-10mg/l.
Comorbidity was analysed using the Charlson comorbidity index.
A fasting blood test was performed on all patients after collecting their urine 24 hours before. Biochemical data were analysed in the hospital’s central laboratory.
PTH was measured by chemiluminescence with reference values of 16-87pg/ml.
Vitamin 25(OH)D levels were measured by chemiluminescent radioimmunoassay (CLIA), Dia-Sorin LIAISON. Its concentration was nanomolar, only 3% was free, and it mostly circulates in the serum bound to lipophilic proteins. The reference values were 8-60ng/ml. A deficiency in 25(OH)D levels was considered as below 15ng/ml, insufficiency if the levels were between 15 and 30ng/ml, and normal values were above 30ng/ml, according to the KDOQI guidelines.18
Statistical analysis
The normality of the variables’ distribution was determined using the Kolmogorov-Smirnov test. Those variables with a normal distribution were expressed as mean±SD, while those that did not have a normal distribution were expressed as median and 25-75 percentiles. Categorical variables were expressed as number and percentage.
The comparison between continuous variables was performed using the Mann-Whitney U test. For more than two groups, the Kruskal-Wallis test was used. Categorical variables were compared using the chi square test.
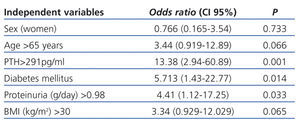
The Spearman’s bivariate correlation was used to determine the association of the analysed data with 25(OH)D levels. Statistically significant variables were selected as potential independent 25(OH)D deficiency predictors for the multivariate binary logistic regression analysis, with the dependent variable being a deficiency or not of 25(OH)D. The independent variables introduced with the following cutoff points were: PTH median of 291pg/ml, proteinuria median of 0.98g/24h, age over 65 years, BMI greater than 30kg/m2, women and the presence of diabetes.
The statistical analysis was performed using the statistical program SPSS version 15 (SPSS). Values of P<.05 were considered significant.
RESULTS
The CKD stage distribution of the 79 patients was as follows: stage 3: 6 patients (8%); stage 4: 50 patients (63%); and stage 5: 23 patients (29%).
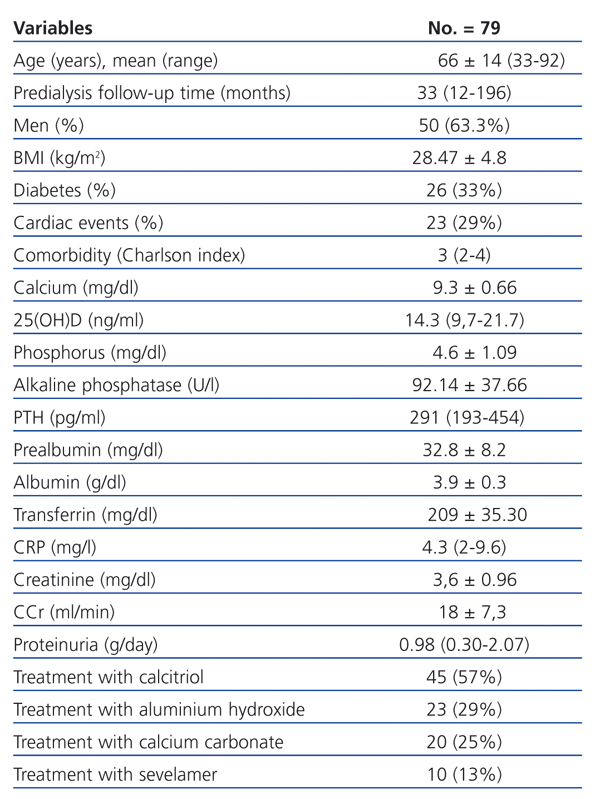
The clinical and biochemical data of the 79 patients are shown in Table 1.
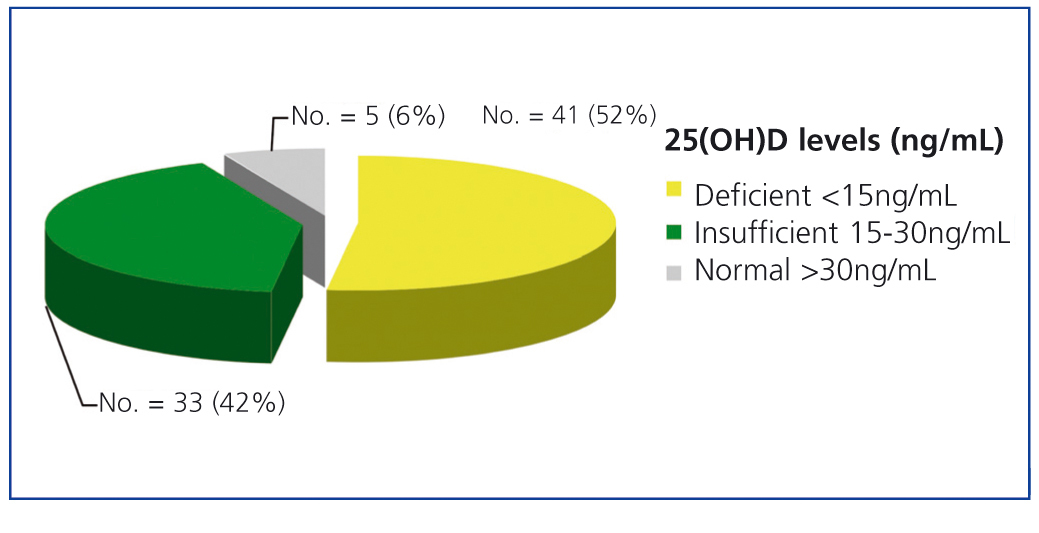
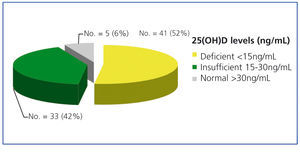
Levels of 25(OH)D in the 79 patients were distributed as follows: 41 (52%) were deficient (less than 15ng/ml), 33 (42%) were insufficient (between 15 and 30ng/ml), and only 5 patients (6%) had normal levels (greater than 30ng/ml), see Figure 1.
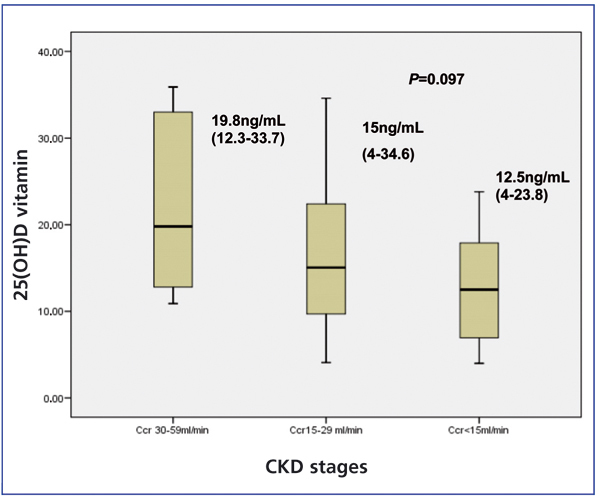
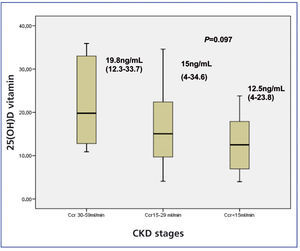
Levels of 25(OH)D in CKD stages 3, 4 and 5 expressed as median and interquartile range were: stage 3: 19.8ng/ml (12.3-33.7); stage 4: 15ng/ml (4-34.6); and stage 5: 12.5ng/ml (4-23.8). Decreased levels were observed in all CKD stages, with a tendency to decrease as the CKD stage increased (P=.097), see Figure 2.
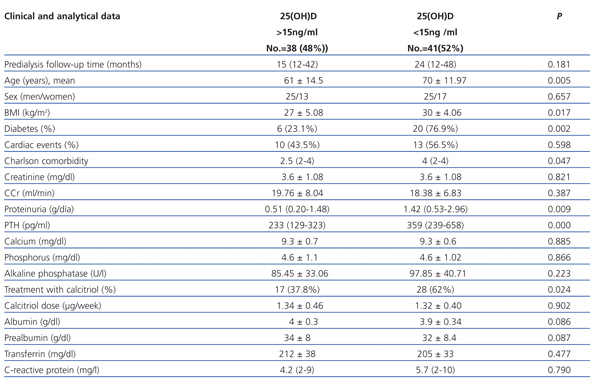
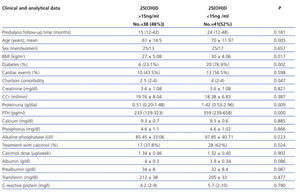
Table 2 compares the data analysed with respect to 25(OH)D levels higher or lower than the cut-off point of 15ng/ml. The 25(OH)D deficient group was older (70±11.97 vs 61±14.5 years, P=.005) had a higher BMI (30±4.06 vs 27±5.08, P=.017), and had more diabetic patients (20 [76.9%] vs 6 [23%] P=.002). No differences were found in the number of cardiac events: 10 (43.5%) vs 13 (56.5%), P=.598.
There were no differences in renal function, as measured by serum creatinine and creatinine clearance. Proteinuria in g/24h was higher in this group, 1.42 (0.53-2.96) vs 0.51 (0.20-1.48), P=.009.
Regarding mineral metabolism, PTH figures were higher, 359 (239-658) vs 234 (129-323), P=.000. There were no differences in the levels of calcium, phosphorus or alkaline phosphatase. Most patients were treated with calcitriol, 28 (62.2%) vs 17 (37.8%), P=.024, with no difference in the dose received.
For nutritional parameters: albumin (3.9±0.34 vs 4±0.3), P=.086; prealbumin (32±8.4 vs 34±8), P=.087; and transferrin (205±33 vs 212±38), P=.477 showed no differences with respect to 25(OH)D levels. The inflammatory state as measured by CRP also showed no differences with respect to the 25(OH)D levels: 4 (2-9) vs 5.75 (2-10), P=.790.
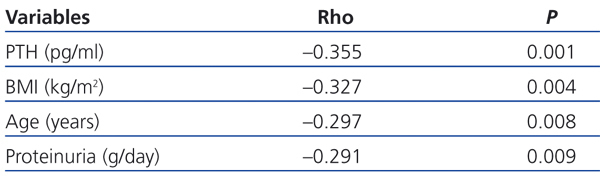
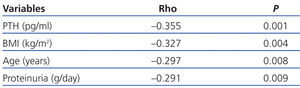
In the univariate analysis (Table 3), the 25(OH)D levels correlated inversely with PTH (r=-0.355, P=.0001), age (r=-0.297, P=.008), BMI (r=-0.327, P=.004) and proteinuria (r=-0.291, P=.009).
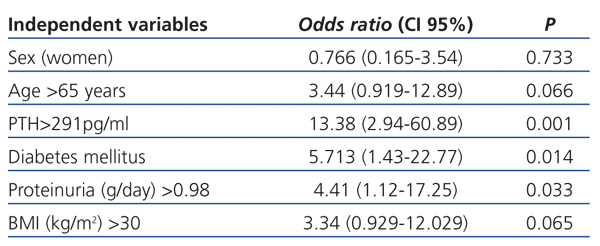
In the multivariate analysis (Table 4), the presence of diabetes (OR: 5.713, 95% CI [1.43-22.77], P=.014); increased PTH (OR: 13.38, 95% CI [2.94-60.89], P=.001); and higher proteinuria (OR: 4.41, 95% CI [1.12-17.25], P=.033) were independent predictors for 25(OH)D deficiency.
DISCUSSION
A high prevalence of 25(OH)D insufficiency was confirmed in CKD patients,7,10,11 with only 6% of the 79 patients having levels considered normal.
In CKD patients, there is an association of 25(OH)D deficiency with age, as there is in the general population.12 This could be explained by a combination of factors, such as poor nutrition, gastrointestinal disorders or a lack of skin synthesis due to low sun exposure.1 In CKD patients, dietary restriction and loss of appetite due to uraemia may be stronger determining factors for 25(OH)D deficiency.
The nutritional parameters analysed did not differ with respect to 25(OH)D levels, so they cannot be considered as a global malnutrition marker.
The BMI was higher in patients with decreased 25(OH)D levels, and obesity has been associated with decreased 25(OH)D,19 although the cause is not well known. Vitamin D is fat soluble and could be metabolically active within stores of fat (adipocytes). Therefore, an increased BMI could be accompanied by lower vitamin 25(OH)D plasma levels.
However, a study in obese and overweight patients20 found an inverse relationship between vitamin D3 levels and weight and BMI, but not with body fat measured by bioimpedance.
The number of diabetic patients in the 25(OH)D deficient group was higher, and diabetes, among others, was a predictor of 25(OH)D deficiency.
Vitamin D receptors have been found in the endocrine pancreatic beta cells; therefore, vitamin D deficiency has been associated with cellular changes in the endocrine pancreas, which would lead to obesity and insulin resistance, thereby favouring a diabetic state.21-23
Proteinuria was higher in the 25(OH)D deficient patient group and was also a predictor for vitamin D deficiency. We know that vitamin D circulates mostly bound to proteins; hence, the higher the proteinuria, the lower the 25(OH)D level.24
A deficiency in 25(OH)D has been documented in other protein loss states, such as nephrotic syndrome25 and treatment with peritoneal dialysis.26,27
For diabetic patients with diabetic nephropathy, proteinuria should be a factor to be considered in the 25(OH)D deficiency found.
Over the last 10 years, cardiovascular disease has been associated with 25(OH)D deficiency.28 The inhibitory effect of vitamin D receptors on the expression of the reninangiotensin-aldosterone system protects against cardiac hypertrophy, increasing left ventricular contractility.29,30 No difference was found in the number of cardiac events according to the level of 25(OH)D in this study. The study design features and the number of patients may condition these results.
A 25(OH)D deficiency had no influence on the inflammatory status of patients measured by the CRP.
Regarding mineral metabolism, calcium and phosphorus figures were controlled. In the 25(OH)D deficient group, the PTH was higher and more patients had been treated with oral calcitriol. An increased level of PTH was another independent predictor of deficiency.
It has been shown that 25(OH)D deficiency leads to changes in bone mineralisation, independent of PTH levels and the active form 1,25-(OH)2D.31 Additionally, 25(OH)D levels directly regulate the PTH, regardless of the active form, 1,25-(OH)2D.32
From the results, it can be concluded that there is a high prevalence of 25(OH)D deficiency and insufficiency in CKD patients, and that increased PTH and proteinuria and the presence of diabetes are independent predictors for 25(OH)D deficiency.
A deficiency in 25(OH)D should be corrected at different stages of CKD,33,34 with special attention paid to patients with diabetes and increased proteinuria. If the 25(OH)D deficiency continues, the pleiotropic activity is being neglected and the development of secondary hyperparathyroidism is enhanced.
Long-term studies are needed to evaluate the impact of maintaining adequate 25(OH)D levels at different stages of CKD.
Table 1. Clinical and biochemical characteristics of patients
Table 2. Comparison of clinical and biochemical data regarding 25(OH)D levels
Table 3. Bivariate correlation (Spearman) between 25(OH)D levels and variables studied
Table 4. Logistic regression multivariate analysis: potential predictors of vitamin 25(OH)D deficiency
Figure 1. Distribution of patients according to 25(OH)D levels
Figure 2. 25(OH)D levels in CKD, stages 3,4 and 5