Calcineurin inhibitor drugs (CNI) are the mainstay of modern immunosuppression in renal transplantation. However, they contribute significantly to the chronic loss of renal grafts and the high morbidity and mortality in this population due to their deleterious effects on the renal graft, cardiovascular profile and tumour pathology. Anti-mTOR drugs, sirolimus (SRL) and everolimus (EVE) are potent immunosuppressants with antiproliferative and anti-migratory capacities. These properties mean that they have a potential protective role in graft dysfunction, in renal function optimisation and the appearance of malignant tumours. Indeed, clinical trials and observational studies have demonstrated that conversion from CNI to anti-mTOR-based maintenance therapy has beneficial effects on transplant outcomes in terms of renal function, without significant increase in acute rejection rates. This review article examines the evidence of the use of anti-mTOR in the following clinical situations following renal transplantation: 1) prevention of immune dysfunction and renal function preservation in de novo renal transplantation and after early or late CNI withdrawal; 2) chronic dysfunction of the renal graft; 3) cardiovascular effects; 4) de novo post-transplant diabetes, and 5) de novo tumour pathology.
Los fármacos inhibidores de la calcineurina (ICN) constituyen los pilares de la moderna inmunosupresión en el trasplante renal. Sin embargo, contribuyen significativamente a la pérdida crónica de los injertos renales y a la elevada morbimortalidad en esta población por sus efectos deletéreos sobre el injerto renal, el perfil cardiovascular y la patología tumoral. Los fármacos anti-mTOR, sirolimus (SRL) y everolimus (EVE), son potentes inmunosupresores con capacidad antiproliferativa y antimigratoria, propiedades que les confieren un potencial papel protector en la disfunción del injerto, en la optimización de la función renal y en la aparición de tumores. En efecto, ensayos clínicos controlados y estudios observacionales de conversión han demostrado el efecto beneficioso de estos fármacos en términos de función renal, sin incremento significativo de las tasas de rechazo agudo. En esta revisión se analizan las evidencias del empleo de los fármacos anti-mTOR en los siguientes aspectos clínicos de los pacientes con trasplante renal: 1) prevención de la disfunción inmunológica precoz y preservación de la función renal en el uso de novo y conversión precoz o tardía; 2) disfunción crónica del injerto renal; 3) efectos cardiovasculares; 4) diabetes de novo postrasplante, y 5) patología tumoral de novo.
INTRODUCTION
Calcineurin inhibitor drugs (CNI) are the mainstay of modern immunosuppression but their deleterious effects on patients and renal grafts means that long-term survival rates for renal transplantation (TX) have not improved in keeping with the good results obtained during the first year post-TX.1
In recent years, the advent of new immunosuppressants has helped for therapeutic strategies to be designed which are aimed at minimising the negative impact of CNI on chronic graft dysfunction, and cardiovascular and tumour comorbidity. Undoubtedly, this could increase survival rates, especially in long-lived individuals who receive TX from elderly donors.
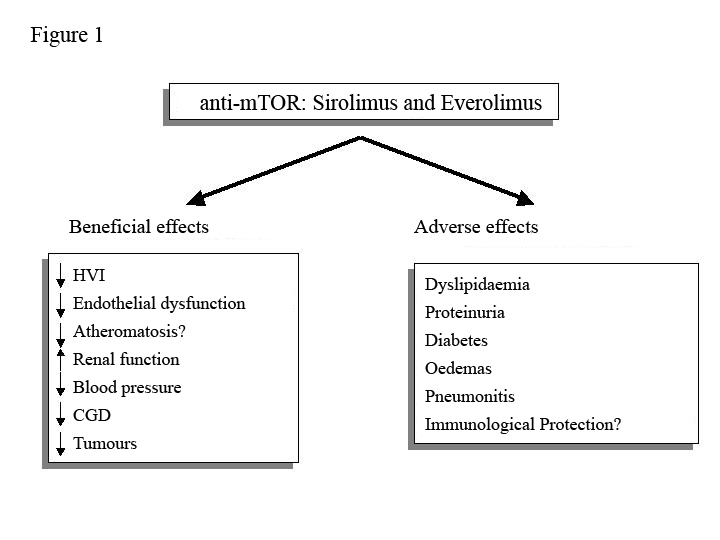
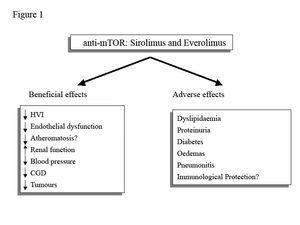
Anti-mTOR drugs, sirolimus (SRL) and everolimus (EVE) are potent immunosuppressants with antiproliferative and anti-migratory capacity that work by blocking the intracellular signalling that regulates the growth and proliferation of T2 cells. This gives them a potentially protective role in renal graft dysfunction, which could simultaneously optimise the cardiovascular profile and reduce the appearance of de novo tumours. However, their side effects may offset these benefits in the longer term (Figure 1).
In this review article, we shall analyse the evidence of anti-mTOR drug use in the field of TX, with major emphasis on the following clinical features: 1) prevention of early immune dysfunction and renal function preservation in de novo use and early or late conversion; 2) chronic dysfunction of the renal graft; 3) cardiovascular effects; 4) de novo post-transplant diabetes, and 5)de novo tumour pathology.
PREVENTION OF EARLY IMMUNE DYSFUNCTION AND PRESERVATION OF RENAL FUNCTION
De Novo Use
Initial studies showed that using SRL with or without induction and avoiding CNI was associated with better renal function compared to regimens with cyclosporine (CsA) with a similar rate of acute rejection, as long as it was associated with mycophenolate mofetil (MMF).3-11 It was also noted that, in addition to optimising renal function, using SRL instead of CsA was associated with a lower expression of genes involved in the development of chronic graft dysfunction (CGD). For example, the gene that encodes the transforming growth factor (TGF-β) protein or the monocyte chemotactic protein-1 (MCP-1).12
More recently, it was found that induction therapy with thymoglobulin in addition to SRL in de novo transplantations was associated with improved renal function compared to conventional immunosuppression therapy (prednisone, tacrolimus and MF). However, the price to pay may be a higher rate of adverse effects and loss of grafts with the use of SRL.13 The mTOR protein regulates memory CD8 T-cell differentiation.14 Furthermore, the combination of thymoglobulin and SRL is associated with a greater degree of preservation and recovery of memory T-lymphocytes compared to those receiving CsA.15 This may well explain the higher rate of immune dysfunction in SRL use in de novo transplantation compared to CNI, especially after administration of polyclonal antibodies.
Nevertheless, what happens with EVE? An excellent review article on the use of this drug showed that using 1.5 or 3 mg of EVE with full or reduced doses of CsA conferred a similar rate of acute rejections and graft survival as conventional therapy with CsA and MMF. Although there was a lower rate of severe rejections using 3mg of EVE.16 In any case, worsening renal function was the common denominator in clinical trials that used higher doses of EVE (3 mg/day) despite a reduction in CsA, which suggested the need to combine low doses of CNI and anti-mTOR.17,18 Indeed, controlled studies combining low doses of CsA and EVE have shown that preservation of renal function is associated with acceptable rates of rejection and graft survival one year after transplantation,19-21 including those patients with risk of delayed renal function (CALLISTO study).22 In terms of renal function preservation and rejection rates, something similar happens when patients are treated with EVE (1.5mg/day) and low or standard doses of tacrolimus (TAC). This could result in a higher graft survival rate.23 In any case, EVE reduces the oral bioavailability of TAC in a dose-dependent manner.24 It is therefore mandatory to adjust doses and levels of both drugs when carrying out minimisation strategies with this combination of immunosuppressants.
Even so, a major clinical trial (the SYMPHONY study) showed that the SRL use in de novo transplantation without CNI was associated with a high rate of acute immune dysfunction and worse renal function compared with other guidelines that incorporated full or reduced doses of CNI.25 In any case, low doses and levels of SRL were used, which may result in a bias in the efficacy. If we add to this the fact that early or late use of an anti-mTOR (SRL or EVE) is associated with a high rate of surgical wound complications and that these drugs are a risk factor even without these complications,26,27 its use in de novo transplantations may be questionable. The KDIGO (Kidney Disease Improving Global Outcomes) clinical practice guidelines discourage the use of these drugs as initial immunosuppressants in renal transplantations.28 Therefore, in accordance with extensive evidence: 1) the use of an anti-mTOR (low doses) in de novo transplantation without CNI is associated with a higher rate of immune dysfunction and surgical wound complications, and is therefore not recommended as an initial immunosuppressant and 2) the combined use of low-dose CNI with an anti-mTOR provides immune protection, at least in the short-term.
Early and late conversion
In light of these results, it is possible that the main indication of anti-mTOR drugs (SRL or EVE) lies in replacing CNI in the first months post-TX, once the initial danger of the immune system attacking the new graft has subsided. Nevertheless, what evidence is there for this therapeutic approach? In line with this reasoning, initial studies have shown that the reduction or elimination of CsA in therapy with SRL during the first months post-TX could offer improved renal function with acceptable graft survival rates despite a slight increase in the rate of acute rejection.10 This was endorsed in a systematic review with its corresponding meta-analysis that included six controlled clinical trials in which the withdrawal of CNI with SRL therapy resulted in better renal function despite a slight increase in the rate of immune dysfunction.29 More recently, randomised conversion of CsA to SRL between the second and third weeks post-TX was associated with a significant improvement of renal function compared to patients who continued with CsA (SMART study).30 Similar findings have been observed with TAC. The withdrawal of this drug in the first months post-TX, while under SRL therapy, preserved renal function compared to potent immunosuppression (TAC with SRL) two year after transplantation, without a significant increase in proteinuria.31 It is possible that this early beneficial effect is at least partly due to the decrease in intrarenal vascular resistance parameters, as described earlier.32
On the other hand, in therapeutic regimens without this initial drug combination (TAC with SRL), the substitution of CsA/TAC for SRL starting from the sixth month post-TX improved renal function significantly, especially in those patients who started with a better glomerular filtration rate (>40ml/min). Furthermore, there was a lower incidence of tumours (CONVERT study).33 Similarly, the conversion of CsA for SRL at the third month post-TX with withdrawal of steroids at the eighth month was associated with significant improvement in renal function (CONCEPT study).34 However, this improvement in glomerular filtration rate was not accompanied by less interstitial fibrosis in protocol biopsies performed one year after transplantation.35
Nevertheless, what happens with EVE? In early abrupt conversions from CsA to EVE (seven weeks post-TX), there is a significant increase in renal function with an acceptable rate of acute rejection six months after transplantation.36 Furthermore, observational studies have shown that the conversion from a CNI to EVE preserved renal function six months after transplantation. This is a relevant fact if we take into account that many conversions were performed due to the chronic deterioration of graft function.37-39 A recent clinical trial in patients with no immunological risk, who received conventional immunosuppression for 6 months, showed that patients converted from CsA to EVE had lower rejection rates and improved renal function than those who remained on treatment with MMF or CsA.40 Preliminary data from a controlled, multicentre Spanish study (ERIC study) shows that the conversion from TAC to EVE after three months post TX is associated with a tendency to improve the GFR, with a slight increase in the rate of acute rejections.41 Therefore, in light of available evidence (a moderate level of evidence), we can claim that the conversion from a CNI to an anti-mTOR is associated with stable renal function, which can help prevent the progression of CGD. As indicated by the KDIGO guidelines, this therapeutic approach should be considered especially in low immunological risk individuals.28
CHRONIC RENAL GRAFT DYSFUNCTION
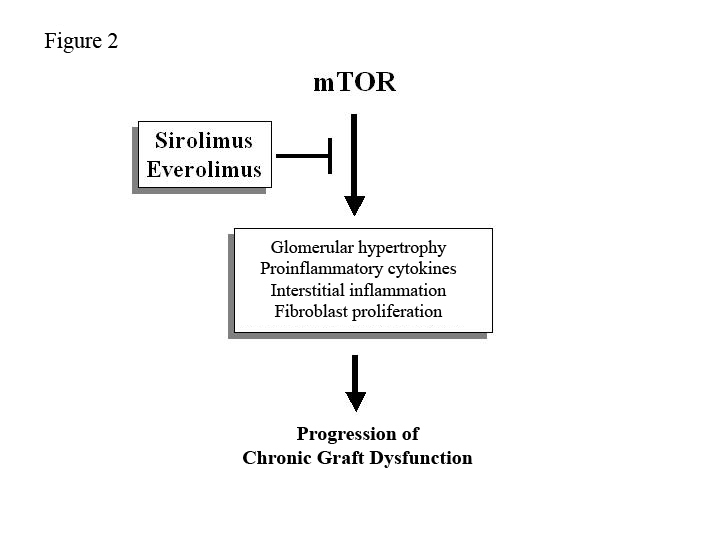
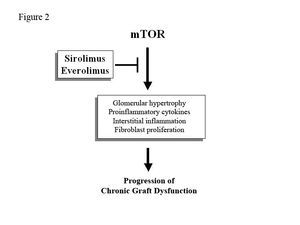
The mTOR protein intervenes in the pathogenic mechanisms of the progression of chronic renal disease. Therefore, SRL and EVE can play an important role in the prevention of CGD due to their antiproliferative actions (Figure 2). Nevertheless, what evidence is there?
In the chronic rejection animal model, the administration of EVE minimises the histological lesions inherent in CGD through antiproliferative mechanisms or by stimulating the apoptosis of cells that are involved in tissue remodelling.42 In sensitised rats receiving a renal graft, the administration of EVE dramatically reduced cellular infiltration of the allogeneic response and minimised interstitial fibrosis/tubular atrophy. This can help slow the progression of CGD.43
In the clinical field, the withdrawal of CsA in SRL therapy three months post-TX can prevent the occurrence of CGD lesions and optimise renal function three years after transplantation.44 A randomised study on patients with CGD showed that the administration of SRL markedly reduced interstitial and vascular expression of profibrogenic molecules compared to those who received reduced doses of CsA with MMF. This was accompanied by an improvement in graft survival two years after transplantation.45 Observational studies of late conversion from a CNI to an anti-mTOR (SRL or EVE), mainly due to CGD, have consistently shown improvement or stabilisation of renal graft function.37-39,46 However controlled studies are needed to confirm these findings. In light of the limited evidence available, the KDIGO guidelines only “suggest” (but do not categorically recommend) this therapeutic change in patients with CGD.28 Moreover, anti-mTORs preserve the production of interferon-gamma; a molecule with an important antiviral action,47 which can also prevent the occurrence of chronic polyomavirus BK nephropathy.48
Even so, anti-mTOR drugs can lead to proteinuria, especially in patients with previous renal damage.49 This proteinuria is a predictor of endothelial dysfunction and mortality in patients with TX. This may worsen the overall TX results in the longer term.50 In light of this information, SRL and EVE may represent therapeutic alternatives in patients with CGD and minimal or no proteinuria, as long as they are not associated with a CNI (low level of evidence). The exact moment of conversion to optimise TX results remains uncertain.
CARDIOVASCULAR EFFECTS
In the heart, SRL inhibits the mTOR protein, which regulates intracellular protein synthesis involved in the development of cardiac ventricular hypertrophy as a result of pressure stimulus. The administration of SRL in mice subjected to cardiac pressure overload decreased the growth of myocardial cells by 50% compared to the control group. This was accompanied by reduced expression of proteins involved in mechanisms of cardiac hypertrophy such as the ribosomal protein S6.51,52 Furthermore, in the chronic renal disease animal model, administration of SRL reduces cardiomyocyte growth and reverses intermyocardiocytic fibrosis,53 which could improve cardiac remodelling. Observational studies with low numbers of patients show that the clinical conversion of a CNI to SRL significantly reduces left ventricular mass (approximately 30%), regardless of blood pressure.54 In any case, longitudinal studies are needed with a greater numbers of patients to confirm these interesting findings.
Anti-mTOR drugs produce dose-dependent dyslipidaemia that can contribute to the development of post-TX cardiovascular disease.55 However, in the atheromatosis animal model (apolipoprotein E knockout mice), the administration of increasing doses of SRL was able to reduce aortic atheromatous lesions in more than 50% of cases. This was accompanied by decreased expression of interleukins involved in the atheromatous process (IL-10) compared to the control group.56 This way, SRL preserves endothelial function when compared to CsA, maintaining acetylcholine-dependent vasodilation and lowering endothelin-1 levels.57,58 These actions may explain the prevention of graft vasculopathy and renal function improvement in therapeutic regimens that use anti-mTOR drugs. A clinical symptom may be a lower arterial stiffness, as was observed in a controlled study in which the conversion from CsA to EVE was associated with a slower pulse wave compared to those who continued with CsA.59 Lastly, administration of anti-mTOR decreases the incidence of CMV infection,60,61 an effect that could result in a lower occurrence of indirect effects potentially caused by this opportunistic infection such as ischaemic heart disease or peripheral vascular disease.62,63 Future prospective studies will clarify these interesting issues.
POST-TRANSPLANT DE NOVO DIABETES
The mTOR protein regulates beta cell proliferation and is involved in the intracellular signalling pathway of insulin. In stable patients with CGD, the conversion from CsA to SRL produces insulin resistance shown by an increased expression of cellular insulin receptor substrates (IRS-1 and IRS-2).64,65 An observational study of the American registry with thousands of patients showed that the risk of post-TX de novo diabetes was significantly higher in all drug combinations that included SRL compared to other therapeutic associations without this drug.66 In fact, the administration of rapamycin in animal models decreases insulin sensitivity and reduces the pancreatic beta cell mass by 50% through increased apoptosis of these cells, especially when there is a predisposition to developing diabetes mellitus, as happens in obese rats.67
Therefore, albeit with a low level of evidence, anti-mTORs can slow left ventricular growth and progression of atheromatosis. Although, they may predispose individuals towards development of post-TX diabetes, especially in predisposed patients.
DE NOVO TUMOUR PATHOLOGY
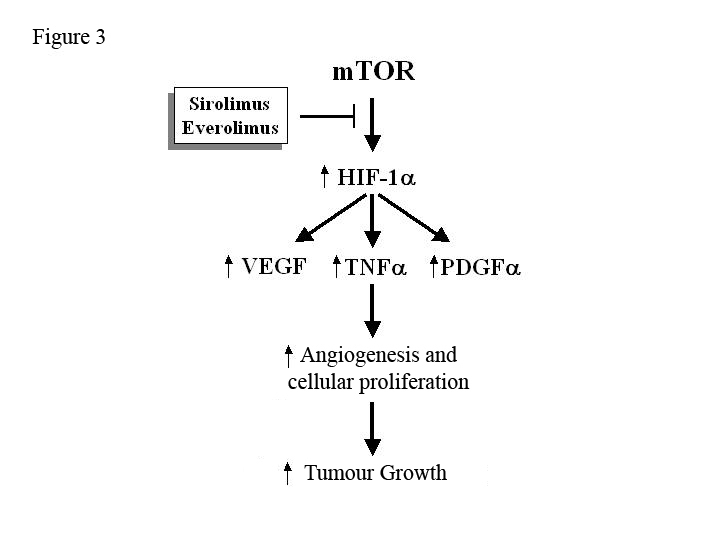
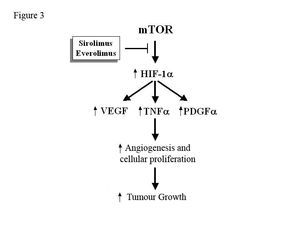
In general, there is an increase in the incidence of tumours after TX and CNI plays a crucial role in their development.68 The mTOR protein stimulates the production of growth factors (VEGF, PDGF-α, TNF-α) that increase angiogenesis, cellular proliferation and tumour growth (Figure 3). Hence, SRL and EVE can potentially slow the development and growth of neoplastic cells.69 An observational study of the American registry of more than 30,000 patients showed that immunosuppressive treatment that included SRL or EVE reduced the risk of cutaneous and non-cutaneous cancer by 60% as compared to treatment with CNI.70 An intention-to-treat analysis of a randomised clinical trial showed that the incidence of cutaneous and non-cutaneous carcinomas was lower in patients who received SRL compared to those who received CsA with SRL. However, the control group received more potent immunosuppressive drug, which constitutes an important bias for evaluating the anti-tumour effect of anti-mTOR drugs.71 As noted previously, a lower incidence of tumours was observed in the CONVERT study in patients who were converted from a CNI to an SRL.33 Lastly, a more recent controlled trial in patients with non-melanocytic skin tumours showed that treatment with SRL can slow the development of new neoplasms and cause pre-existing lesions to regress compared to those patients who received CsA (moderate level of evidence).72
At the same time, some viruses such as herpes or the Epstein-Barr virus may be responsible for the increase in neoplasms after TX. Anti-mTOR drugs can optimise the specific antiviral lymphocyte response (T lymphocytes) through an increase in CD4 and CD8. This is a mechanism that could explain the lower incidence of viral infections with these drugs.73 Obviously, this could result in a lower occurrence of neoplasms of viral origin. In fact, in a surprising study of patients with TX and Kaposi's sarcoma, the substitution of CsA for SRL led to a disappearance of histologic tumours six months after transplantation. This seemed to be due to decreased angiogenesis and reduced vascular endothelial growth factor (VEGF).74 In view of this, the KDIGO guidelines suggest the use of anti-mTOR drugs in patients undergoing TX who have this pathology.28 In either case, controlled studies are needed to confirm the benefit of anti-mTOR drugs in the treatment of tumours.
KEY CONCEPTS
1. The use anti-mTOR in de novo transplantation without a CNI leads to a greater risk of rejection and surgical wound complications after TX.
2. The initial combination of low doses of an anti-mTOR and a CNI provides acceptable short-term immune protection.
3. The early conversion from a CNI to SRL/EVE may, at least, offer stable renal function. Whether this therapeutic manoeuvre prevents chronic graft dysfunction is still unknown.
4. With a low level of evidence, the anti-mTOR drugs reduce the left ventricular mass and may potentially slow atheromatosis after TX.
5. Anti-mTOR drugs can increase the risk of post-TX diabetes, especially in predisposed individuals.
6. SRL and EVE reduce the incidence of post-TX de novo neoplasms.
ACKNOWLEDGEMENTS
This study was financed in part by the Ministerio de Ciencia e Innovación de España (Ministry of Science and Innovation of Spain [SAF2007-60314]).
Figure 1. Potential clinical benefits and adverse effects of anti-mTOR drugs
Figure 2. Mechanisms of action of anti-mTOR drugs to slow the progression of chronic graft dysfunction.
Figure 3. Potential mechanism of action of anti-mTOR drugs for inhibiting tumour growth