Hyponatraemia, defined as the decrease in serum sodium concentration below 135 mmol/l, is the most prevalent electrolyte disorder in the hospital environment, presenting a prevalence of 15–30 % at admission,1 being associated with an increase in hospital stay and mortality.2
SIADH constitutes the leading cause of hyponatraemia in hospitalised patients.3 Pathophysiologically it is caused by an inability to suppress vasopressin secretion, resulting in a reduction in urinary volume and concentration, leading to the decrease in serum natraemia (SNa). The introduction of vaptans, antagonists of vasopressin V2 receptors, has been the main therapeutic novelty in its management, with tolvaptan available in Europe.4 Although the lowest formally accepted dose is 15 mg/day, the risk of overcorrection sometimes leads to an initial dose of 7.5 mg/day.
As with any treatment of hyponatraemia, the main concern is the rapid overcorrection of natraemia levels, which can possibly cause life-threatening osmotic demyelination.5 To try to avoid it, a close and individualized monitoring of SNa should be performed in the first hours of initiation of treatment, marking as increase limit targets 8−12 mmol/l at 24 h and 12−18 mmol/l at 48 h.6–8
We assessed the use of tolvaptan in a tertiary hospital between March 2014 and August 2017, in order to provide knowledge regarding the use of tolvaptan. During this period 17 patients received treatment with tolvaptan. Different starting dosages were used (7.5 mg; 15 mg; 30 mg). Treatment with a dose of 7.5 mg was started on 9/17 patients. Table 1 summarises the characteristics by groups. 100 % of the patients presented chronic hyponatraemia (> 48 h). 17.6 % presented natraemia < 120 mmol/l at the start of treatment.
General characteristics of the patients, broken down by initial dose of tolvaptan.
| 7.5 mg | 15 mg | 30 mg | Total | |
|---|---|---|---|---|
| n | 9 | 7 | 1 | 17 |
| Diagnosis, n (%) | ||||
| Idiopathic SIADH | 1 (11.1) | 0 (0) | 0 (0) | 1 (5.9) |
| Pharmacological SIADH | 3 (33.3) | 0 (0) | 0 (0) | 3 (17.7) |
| Postsurgical SIADH | 0 (0) | 3 (42.8) | 0 (0) | 3 (17.7) |
| Paraneoplastic SIADH | 3 (33.3) | 1 (14.3) | 3 (17.7) | |
| SIADH secondary to neurological disease | 2 (22.2) | 2 (28.6) | 1 (100) | 4 (23.5) |
| SIADH secondary to respiratory disease | 1 (11.1) | 1 (14.3) | 0 (0) | 2 (11.8) |
| Dilutional hyponatraemia (cirrhosis) | 1 (11.1) | 0 (0) | 0 (0) | 1 (5.9) |
| Age (years), mean (IQR) | 73.0 (13.7) | 65.9 (14.9) | 59.0 | 70.9 (16.8) |
| Sex %, male/female | 66.7/33.3 | 57.1/42.9 | 100/0 | 64.7/35.3 |
| Cause for admission, n (%) | ||||
| Start of tolvaptan | 1 (11.1) | 0 (0) | 0 (0) | 1 (5.9) |
| Hyponatraemia | 6 (66.7) | 2 (28.6) | 1 (100) | 9 (52.9) |
| Other | 2 (22.2) | 5 (71.4) | 0 (0) | 7 (41.2) |
| Initial SNa (mmol/l), mean (IQR) | 125 (6) | 125 (4) | 115 | 125 (6) |
IQR: interquartile range; SIADH: syndrome of inappropriate antidiuretic hormone secretion; SNa: natraemia.
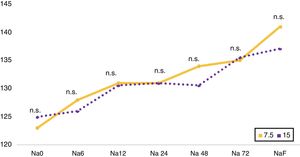
The evolutionary follow-up of SNa was uneven, with an analytical determination being made at 6 h in 23.5 % (n = 4), at 12 h in 29.4 % (n = 5), at 24 h in 76.5 % (n = 13), at 48 h in 70.6 % (n = 12) and at 72 h in 58.8 % (n = 10). A comparison (Fig. 1) was made of the evolution of SNa as a function of the starting dose of tolvaptan used (7.5 vs 15 mg), without significant differences in the different cut-off points.
Comparison between starting doses of tolvaptan 7.5 and 15 mg. Line chart of the evolution of natraemia Na 0, Na 6, Na 12, Na 24, Na 48, Na 72, NaF, which correspond to the initial natraemia, at 6, 12, 24, 48 and 72 h, and the last available natraemia, respectively.
Na: natraemia; n.s.: not significant.
Table 2 details the percentages of patients who achieved eunatraemia or presented overcorrection. No patient developed osmotic demyelination. No differences were found in the rate of overcorrection by starting dose used, sex, age or aetiological diagnosis. A trend to a higher rate of overcorrection (24 h) was observed in patients without SNa control at 6 h (0 vs 30 %; p = 0.279) and at initial SNa < 120 mmol/l (30 vs 66 %; p = 0.510), without statistical significance.
Evolution of natraemia during follow-up after the start of tolvaptan in the group of patients.
| 7.5 mg | 15 mg | Total | p | ||
|---|---|---|---|---|---|
| Eunatraemia, % | 6 h | 0 | 0 | 0 | – |
| 12 h | 0 | 0 | 0 | – | |
| 24 h | 25 | 25 | 33.33 | – | |
| 48 h | 33.33 | 0 | 20 | 0.361 | |
| 72 h | 50 | 66.67 | 57.14 | 0.513 | |
| Overcorrection, % | 6 h | 0 | 0 | 0 | – |
| 12 h | 0 | 0 | 0 | – | |
| 24 h | 50 | 25 | 38.46 | 0.501 | |
| 48 h | 0 | 0 | 0 | – |
Overcorrection is considered as increases > 5 mmol/l at 6 h; > 8 mmol at 12 h; > 10 mmol/l at 24 h; > 18 mmol/l at 48 h.
In our experience, there seems to be no differences in the evolution of natraemia in the first 72 h per starting dose of tolvaptan (7.5 vs 15 mg), and this issue should be assessed in new studies, given the inherent limitation of the small sample used. We found a high overcorrection rate at 24 h (38.5 %), higher than 5.6 % as published in the SALT-1 and SALT-2 studies9 on the drug’s safety. The explanation could come from the inclusion of patients with initial SNa < 120 mmol/l and the absence of follow-up of the evolution of SNa after the start of treatment, preventing the application of corrective measures. The variability in patient follow-up and the risks associated with an inadequate start of therapeutic management with tolvaptan show the need to follow the protocols recommended in the consensus of experts in the absence of specific studies on the subject.
AuthorshipDavid E. Barajas-Galindo and Alfonso Vidal-Casariego conceived and designed the study. David E. Barajas-Galindo analysed the data and wrote the document. All authors contributed to the acquisition of data, to the writing of the manuscript and approved the final version thereof.
Conflict of interestsDr Barajas-Galindo and Dr Gómez-Hoyos report that they have received fees from Otsuka Pharmaceutical Co. Ltd., outside of the work presented. All other authors declare that they have no conflicts of interest.
Please cite this article as: Barajas-Galindo DE, Vidal-Casariego A, Gómez-Hoyos E, Fernández-Martínez P, González MG, Hernández-Moreno A. Experiencia clínica con distintas dosis iniciales de tolvaptan en siadh. Nefrologia. 2019;39:672–674.