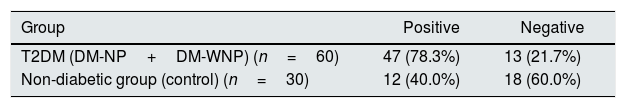Ubiquitin-52 amino acid fusion protein (UbA52) is an important factor in the pathogenesis of diabetic kidney disease (DKD) and has been suggested a potential marker in the disease. However, whether upregulation of UbA52 marks early kidney injury in T2DM mellitus (T2DM) patients remains unclear. In this study, we examine the diagnostic value of UbA52 as a biomarker in predicting early diabetic kidney disease (DKD) in T2DM patients.
MethodsWe used two-step ELISA to test UbA52 level in urine of 3 defined patient groups. Samples from T2DM patients without albuminuria or diabetic retinopathy (DM-WNP; n=30), T2DM patients with albuminuria and diabetic retinopathy, excluding other renal diseases clinically (DM-NP; n=30) and healthy controls (n=30) were analyzed. Spearman's correlation analysis and multiple linear regression model were used to analyze the correlation of urinary UbA52 level with laboratory results regarding kidney function. Receiver operating characteristic curve (ROC) was used to evaluate the diagnostic value of UbA52 in predicting T2DM and early DKD.
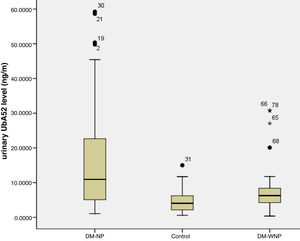
ResultsUrinary UbA52 level in DM-NP group was 1.75 times and 2.71 times higher than in DN-WNP (p=0.004) and normal control group (p<0.001), respectively. The level of urinary UbA52 correlated significantly with serum creatinine (r=0.468, p<0.001), GFR (r=−0.300, p=0.004) and proteinuria (r=0.484, p<0.001). Multiple linear regression analysis showed that proteinuria level was independently associated with urinary UbA52 level (β=0.833, p<0.001). The area under the ROC of urinary UbA52 in diagnosing T2DM and DKD was 0.751 and 0.755, respectively.
ConclusionThe level of urinary UbA52 increased significantly in T2DM patients with DKD. The level of proteinuria is independently associated with urinary UbA52 level. Urinary UbA52 could serve as an early marker in the diagnosis of DKD.
ClinicalTrials.gov Identifier: NCT02204280.
La proteína de fusión de aminoácidos ubiquitina-52 (UbA52) es un factor importante en la patogénesis de la enfermedad renal diabética (ERD), y se ha sugerido como marcador potencial en la enfermedad. Sin embargo, aún no está claro si la regulación al alza de UbA52 indica una lesión renal temprana en pacientes con diabetes mellitus de tipo 2 (DMT2). En este estudio, analizamos el valor diagnóstico de UbA52 como biomarcador para predecir la ERD temprana en pacientes con DMT2.
MétodosUtilizamos un ELISA de 2 pasos para analizar el nivel de UbA52 en la orina de 3 grupos de pacientes definidos. Se analizaron muestras de pacientes con DMT2 sin albuminuria o retinopatía diabética (DM-WNP; n=30), pacientes con DMT2 y con albuminuria y retinopatía diabética, excluyendo clínicamente otras enfermedades renales (DM-NP; n=30) y controles sanos (n=30). Se utilizó el análisis de correlación de Spearman y el modelo de regresión lineal múltiple para analizar la correlación del nivel de UbA52 en orina con los resultados de laboratorio relativos a la función renal. Se utilizó la curva de características operativas del receptor (ROC) para evaluar el valor diagnóstico de UbA52 en la predicción de la DMT2 y de la ERD temprana.
ResultadosEl nivel de UbA52 en orina en el grupo DM-NP fue 1,75 y 2,71 veces mayor que en el grupo DN-WNP (p=0,004), y en el grupo de control normal (p<0,001), respectivamente. El nivel de UbA52 en orina se correlacionó significativamente con la creatinina sérica (r=0,468; p<0,001), la TFG (r=−0,300; p=0,004) y la proteinuria (r=0,484; p<0,001). El análisis de regresión lineal múltiple mostró que el nivel de proteinuria se asociaba de forma independiente al nivel de UbA52 en orina (β=0,833; p<0,001). El área bajo las ROC de UbA52 en orina en el diagnóstico de la DMT2 y de la ERD fue de 0,751 y 0,755, respectivamente.
ConclusiónEl nivel de UbA52 en orina aumentó significativamente en pacientes con DMT2 y ERD. El nivel de proteinuria se asocia independientemente al nivel de UbA52 en orina. UbA52 en orina podría servir como marcador temprano en el diagnóstico de la ERD.
The incidence of T2DM as well as life expectancy of T2DM patients has been increasing worldwide, resulting in the soaring of patients with diabetic nephropathy.1,2 In developed countries, diabetes has become the leading cause of kidney disease. It is estimated about 1 in 4 adults with diabetes has kidney disease in the United States.3 In developing countries, T2DM is rapidly replacing infectious diseases to be the leading cause of kidney failure.4 In China, DKD is the second leading cause of end-stage renal disease (ESRD) and accounts for approximately 16.4% of all cases of ESRD.5
Early identification of patients with DKD who are at risk for progressive renal dysfunction enables early intervention and may lead to better clinical outcome. Glomerular filtration rate (GFR) marker serum creatinine is the best marker of renal excretory function. However, an increased in serum creatinine level is only detectable when GFR declined significantly and thus is insensitive for early DKD diagnosis. Currently, moderately increased albuminuria, which indicates widespread microvascular damage in kidney, is the earliest and the most commonly used laboratory marker in evaluating DKD. A constant increase in albuminuria can predict DKD progression in T2DM patients.6 However, many factors may cause the fluctuation of albuminuria level, including serum glucose, blood pressure, smoking, pregnancy, and urinary tract infection. Evidence also shows that the incidence of normoalbumin diabetic kidney disease (NA-DKD) is increasing in T2DM patients.7–9 Thus, a more reliable marker is needed to identify T2DM patients with high risk for or has developed early DKD.
Urine proteome analysis in diabetic patients has provided insights into identification of novel diagnostic markers for DKD.10,11 Dihazi et al.,12 using SELDI-TOF mass spectrometry and SAX2 protein arrays, found a processed form of ubiquitin with m/z 14766 called ubiquitin-52 amino acid fusion protein (UbA52) that was missing in the urine of patients with DM, but existed in DKD patients. Based on the finding, they concluded UbA52 could be used as a diagnostic marker for DKD. However, the identification process for UbA52 based on mass spectrometry was complicated and expensive, which may not be suitable for clinical use. Thus, other methods are needed to identify diabetic patients at high risk for DKD. With early diagnosis of DKD, there are opportunities for early medical intervention and preventing disease progression. The objective of this study was to test urinary UbA52 using enzymatic ELISA approach in T2DM patients and healthy people, and analyze its correlation with laboratory and pathological results.
Materials and methodsStudy designWe recruited participants from the third hospital of Hebei Medical University (Hebei, China). Patients with T2DM were eligible if they were <80 years old and >18years, and diagnosed with T2DM. T2DM was defined according to the following criteria: (i) HbAlc≥6.5%; (ii) fasting blood glucose≥7.0mmol/L; (iii) 2-h plasma blood glucose≥11.1mmol/L during an oral glucose tolerance test (OGTT); (iv) with classic symptoms of hyperglycemic crisis, a random blood glucose≥11.1mmol/L and had not received renal replacement therapy. Patients with T2DM were excluded if they had acute complications of diabetes, such as ketoacidosis or hyperosmolar coma; malignancy, particularly tumors affecting hepatic and renal function, and history of radiotherapy and/or chemotherapy; heart failure, particularly New York Heart Association grade>III; incomplete information that could affect the experimental results; out-patients; pregnant or breastfeeding; severe hepatic insufficiency, with aminotransferase level 2 times higher than normal; history of respiratory infections or other serious illnesses; or unwilling to cooperate in the study. Another 30 healthy subjects were recruited from the Center of Health Examination of the Third Affiliated Hospital, Hebei Medical University (Hebei, China).
Three patient groups were defined on the basis of clinical course, examination of the optic fundus, moderately increased albuminuria, urinary albumin and urine creatinine ratio, and urinary albumin excretion rate. The groups included patients with DM with moderately increased albuminuria(defined as the urinary albumin/creatinine ratio (UACR) >30mg/g, or persistent microalbuminuria >300mg/24h, or >1.0g/24h for more than 3 months), diabetic retinopathy, excluding other renal diseases clinically were defined as diabetic kidney disease (DM-NP; n=30), with DM without albuminuria or diabetic retinopathy, some have chronic kidney disease due to hypertension, gout, nephrotoxic drugs and other reasons, excluding diabetic kidney disease clinically (DM-WNP; n=30), and healthy controls (n=30). Three DM-NP patients had biopsy-proven DN.
The protocol was approved by the appropriate institutional review boards, and written informed consent was obtained from all participants. Clinical sample procurement and analysis as well as data management were approved by the local institutional ethical review committee of the third hospital of Hebei Medical University (Hebei, China).
Urine samplesFirst morning midstream urine samples, 10ml, were taken before breakfast. Urine samples were centrifuged for 15min at 3000g and stored at −80°C. The supernatant was divided into 1.5-mL aliquots and immediately stored at −80°C. All samples were stored for 4–5 months and did not undergo any freeze-thaw cycles.
UbA52 antibody testingIn total, 10mg horseradish peroxidase (HRP)was added to 0.2ml of 1.25% glutaraldehyde solution and kept at room temperature overnight. The reaction mixture was collected in a dialysis bag surrounded by normal saline. The dialysis bag was stored for 24h at 4°C. At the same time the normal saline was exchanged twice to remove unconjugated glutaraldehyde solution. A few drops of normal saline were placed in the reaction mixture to ensure a total volume of 1ml. An amount of 5mg goat anti-UbA52 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, US) per 1ml normal saline was added to the solution. An amount of 0.1ml of 1M carbonate buffer solution, pH 9.5, was added; the reaction mixture was vortex-mixed and kept for 24h at 4°C effectively to form a crosslinked structure. An amount of 0.2ml of 0.2M lysine was added to the reaction mixture with constant shaking. The preparation was stored at room temperature for 2h. Then the same volume of saturated ammonium sulfate solution was added with constant stirring and incubated for 1h at 4°C. The mixture was centrifuged at 2862g for 15min. The supernatant was discarded and the deposit was washed twice with half-saturated ammonium sulfate solution. The final precipitation was suspended in 0.15M PBS, pH 7.4. Then the mixture was collected in a dialysis bag surrounded by 0.15M PBS, pH 7.4, to remove ammonium ion. The mixture was centrifuged at 17886g for 30min. The supernatant was the purified solution of HRP-conjugated goat anti-human UbA52 polyclonal antibody, then repackaged into smaller packages to store at 4°C or −80°C.
UbA52 measurement by double-antibody sandwich two-step ELISAAn amount of 10μL goat anti-UbA52 polyclonal antibody was dissolved in coating buffer on 96- and 48-well ELISA plates. 0.15ml of the mixture was putted in every well and allowed to incubate overnight at 4°C. The concentration of the coating antibody was 2μg/ml. The plate surface was then gently washed with washing buffer 4 times and dried with filter paper. Eight wells were for standards. Sample diluent solution was added to the standard solution and the concentrations of standard solution were 200, 100, 50, 25, 12.5, 6.25, 3.12, and 0ng/ml. An amount of 100μL sample was added to 96- and 48-well ELISA plates and incubated for 90min at 37°C. The plate surface was then gently washed with washing buffer 5 times and dried with filter paper. The HRP-conjugated antibody was diluted 5000 times with PBS. Then 100μl was added to 96- and 48-well ELISA plates with constant shaking and incubated for 90min at 37°C. Each well was washed 5 times in washing buffer and dried with filter paper. After 100μL of TMB (3,3′,5,5′-tetramethylbenzidine hydrochloride) solution was added, the wells were incubated for 10min at 37°C in the dark. The reaction was stopped with 2M of sulfuric acid. The levels of each sample were extrapolated by referring to a standard curve (4-parameter logistic curve fitting) of optical density (OD) 450nm.
Statistical analysisThe data were analyzed by using SPSS 20 (SPSS Inc., Chicago, IL). For continuous variables, data are presented as mean±SD or median (range), and means were compared by one-way ANOVA. Spearman's correlation analysis and multiple linear regression model were used to analyze the correlation between UbA52 and patients’ laboratory indexes. Receiver operating characteristic curve (ROC) was used to evaluate the value of UbA52 in T2DM mellitus and diabetic kidney disease diagnosis. p<0.05 was considered statistically significant.
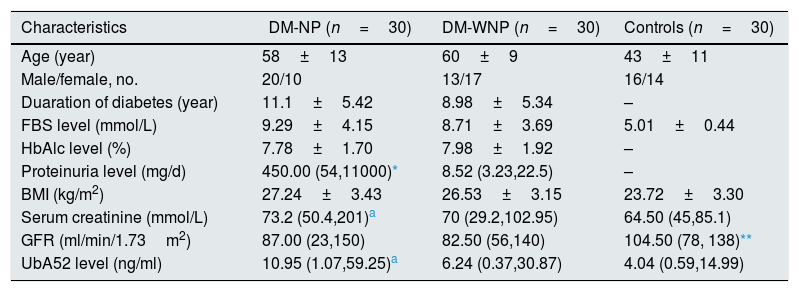
ResultsPatient characteristicsOf the 90 patients enrolled in the study, 49 were male (54.4%). The mean age, duration of diabetes, gender distribution, fasting blood glucose level, body mass index and hemoglubulinAlc level did not differ between DM-NP and DM-WNP groups (p>0.05) (Table 1). The 24-h total proteinuria level and serum creatinine level were higher for DM-NP than DM-WNP and controls (p<0.05) and did not differ between DM-WNP patients and controls (p>0.05).
Characteristics of patients with diabetes and healthy controls.
| Characteristics | DM-NP (n=30) | DM-WNP (n=30) | Controls (n=30) |
|---|---|---|---|
| Age (year) | 58±13 | 60±9 | 43±11 |
| Male/female, no. | 20/10 | 13/17 | 16/14 |
| Duaration of diabetes (year) | 11.1±5.42 | 8.98±5.34 | – |
| FBS level (mmol/L) | 9.29±4.15 | 8.71±3.69 | 5.01±0.44 |
| HbAlc level (%) | 7.78±1.70 | 7.98±1.92 | – |
| Proteinuria level (mg/d) | 450.00 (54,11000)* | 8.52 (3.23,22.5) | – |
| BMI (kg/m2) | 27.24±3.43 | 26.53±3.15 | 23.72±3.30 |
| Serum creatinine (mmol/L) | 73.2 (50.4,201)a | 70 (29.2,102.95) | 64.50 (45,85.1) |
| GFR (ml/min/1.73m2) | 87.00 (23,150) | 82.50 (56,140) | 104.50 (78, 138)** |
| UbA52 level (ng/ml) | 10.95 (1.07,59.25)a | 6.24 (0.37,30.87) | 4.04 (0.59,14.99) |
FBS, fasting blood glucose; HbA1c, hemoglobulinA1c; BMI, body mass index; DM-WNP, T2DM without nephropathy and without moderately increased albuminuria; DM-NP, DM with moderately or severely increased albuminuria and diabetic retinopathy; Data are mean±SD unless indicated.
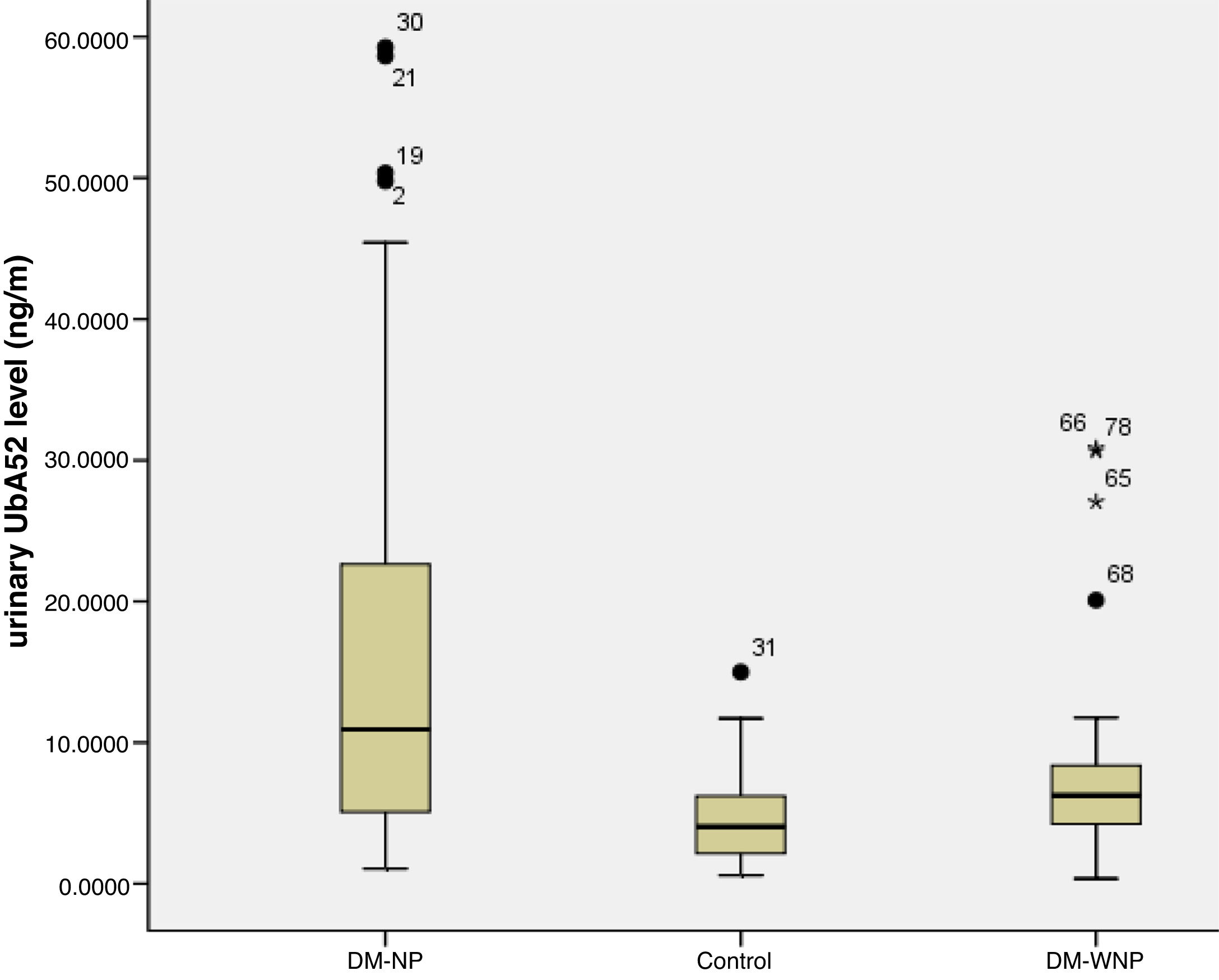
Three DM-NP patients had a diagnosis of diabetic kidney disease based on renal biopsy. On ELISA, the titers of UbA52 for these patients were 50.4, 58.6, 59.3ng/ml. The other patients in this group showed high moderately increased albuminuria without renal biopsy. The urinary UbA52 content was higher for DM-NP than DN-WNP patients and controls (p<0.05) and did not differ between DM-WNP patients and controls (p>0.05). The levels of urinary UbA52in DM-NP group was 1.75 times and 2.71 times of that in DN-WNP (p=0.004) and normal control group (p<0.001) respectively (Table 1 and Fig. 1).
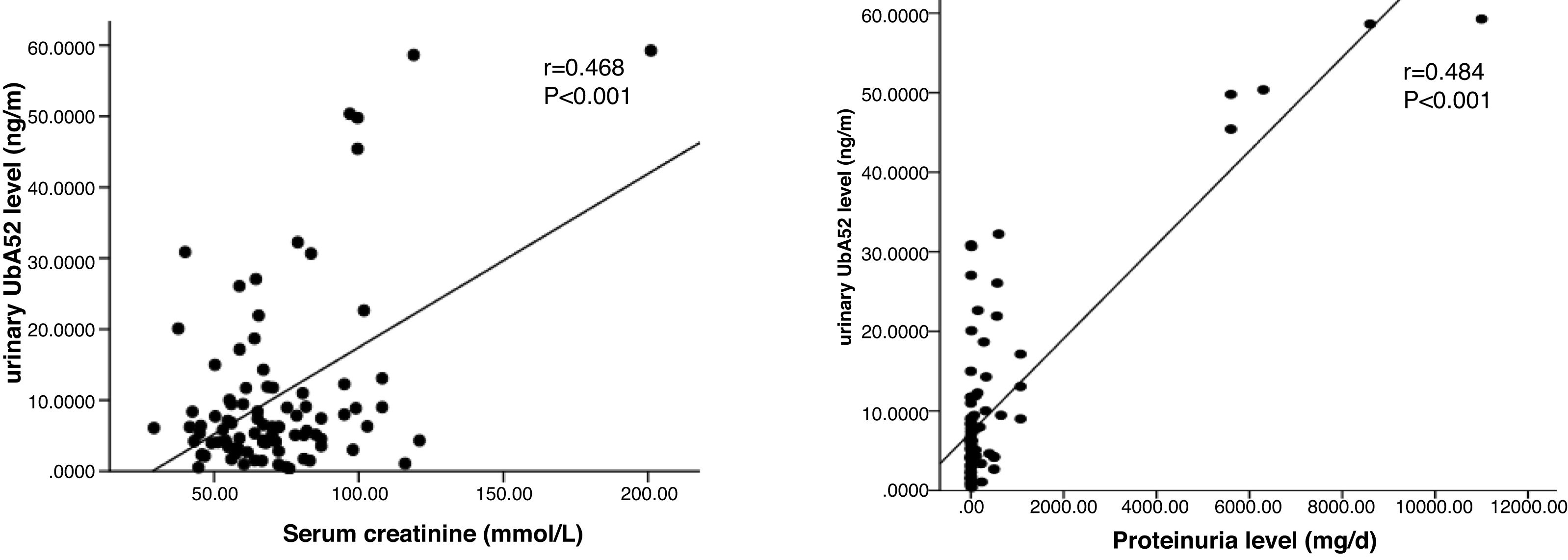
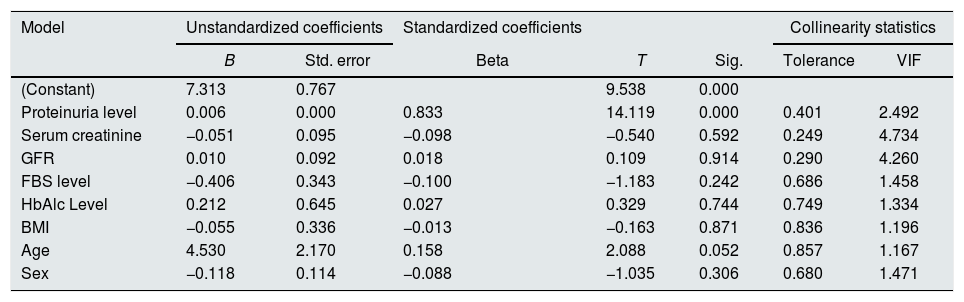
Correlation analysis of urinary UbA52 level with clinical indicatorsThe level of urinary UbA52 was significantly correlated with serum creatinine (r=0.468, p<0.001), GFR (r=−0.300, p=0.004) and proteinuria level (r=0.484, p<0.001) (Fig. 2). Multiple linear regression analysis (Enter; variables: proteinuria level, serum creatinine, GFR, FBS level, HbAlc level, BMI, age, and sex) showed that only proteinuria level was independently associated with urinary UbA52 level (β=0.833, p<0.001) (Table 2). No evidence of influential collinearity was observed.
Results of multiple linear regression analysis.
| Model | Unstandardized coefficients | Standardized coefficients | Collinearity statistics | ||||
|---|---|---|---|---|---|---|---|
| B | Std. error | Beta | T | Sig. | Tolerance | VIF | |
| (Constant) | 7.313 | 0.767 | 9.538 | 0.000 | |||
| Proteinuria level | 0.006 | 0.000 | 0.833 | 14.119 | 0.000 | 0.401 | 2.492 |
| Serum creatinine | −0.051 | 0.095 | −0.098 | −0.540 | 0.592 | 0.249 | 4.734 |
| GFR | 0.010 | 0.092 | 0.018 | 0.109 | 0.914 | 0.290 | 4.260 |
| FBS level | −0.406 | 0.343 | −0.100 | −1.183 | 0.242 | 0.686 | 1.458 |
| HbAlc Level | 0.212 | 0.645 | 0.027 | 0.329 | 0.744 | 0.749 | 1.334 |
| BMI | −0.055 | 0.336 | −0.013 | −0.163 | 0.871 | 0.836 | 1.196 |
| Age | 4.530 | 2.170 | 0.158 | 2.088 | 0.052 | 0.857 | 1.167 |
| Sex | −0.118 | 0.114 | −0.088 | −1.035 | 0.306 | 0.680 | 1.471 |
Dependent variable: urinary UbA52 content.
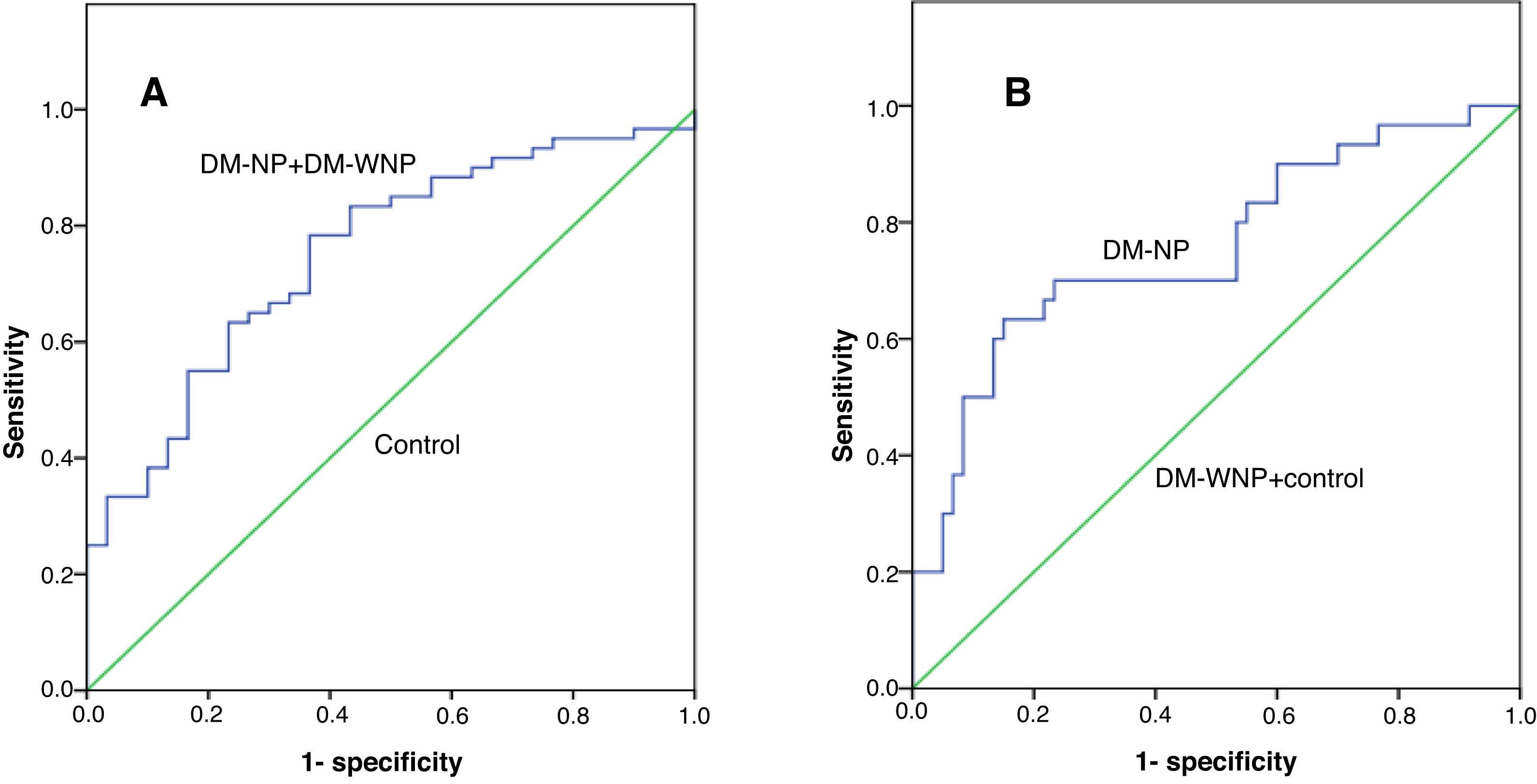
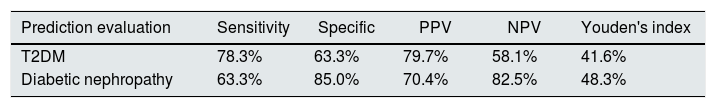
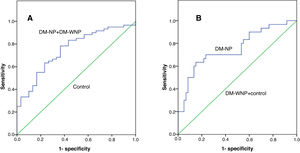
The area under the receiver operating characteristic curve (AUC) of UbA52 content in diagnosing T2DM mellitus was 0.751(95%CI 0.684–0.855, p<0.001). The sensitivity and specificity were 78.3% and 63.3%, respectively, and the cut off value was 4.32.
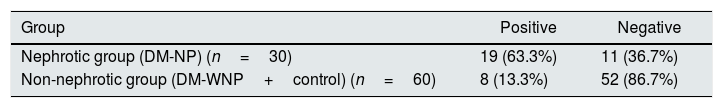
The AUC of UbA52 content in diagnosing diabetic nephropathy was 0.755(95% CI 0.644–0.866, p<0.001).The sensitivity and specificity were 63.3% and 85.0%, respectively, and the cut off value was 8.97 (Fig. 3 and Tables 3–5).
The ROC curve for Uba52 content as a diagnostic marker of T2DM and diabetic nephropathy. (A) ROC curve of Uba52 content in diagnosis of T2DM mellitus; Patients were divided into normal control group (control) and T2DM group (DM-NP+DM-NP). (B) ROC curve of Uba52 content in diagnosis of diabetic nephropathy; Patients were divided into groups proteinuric group (DM-NP) and non-proteinuric group (DM-WNP+control).
DKD refers to kidney disease that is specific to diabetes. In most cases, careful screening instead of kidney biopsy is able to identify DKD patients. The diagnosis of DKD is made based in part on the finding of elevated urinary albumin excretion and the presence of diabetic retinopathy.13 Diabetic nephropathy (DN) refers to specific pathological changes in kidney biopsy and functional changes in DM patients that result from detrimental effects of DM.6,7 Given that the diagnosis of DKD is based on clinical manifestations and laboratory findings, however, about 30% DKD diagnosed by clinical criteria are biopsy-proven nondiabetic glomerular diseases combined with diabetes, suggesting a urgent need for a sensitive yet specific marker for DKD. SELDI-TOF/MS found UbA52 can be considered as a reliable biomarker to identify patients with diabetic nephropathy among diabetic patients. And more importantly, to distinguish biopsy-proven diabetic nephropathy from nondiabetic-CKD in diabetic patients.
In current study, we examined the value of urinary UbA52 as a diagnostic biomarker for DKD in patients with diabetes by two-step ELISA assay. Multiple linear regression analysis showed that proteinuria level was independently associated with urinary UbA52 level (β=0.833, p<0.001). The area under the receiver operating characteristic curve (ROC) of urinary UbA52 in diagnosing T2DM mellitus and diabetic nephropathy was 0.751 and 0.755, respectively. Based on our results, urinary UbA52 measured by ELISA is expected to be a potential biomarker for the diagnosis of diabetic nephropathy.
DKD plays an important role in the development of ESRD. Kidney biopsy, the gold standard for diagnosis of glomerular disease, represents the current method for a definite diagnosis of diabetic nephropathy, but is only performed in diabetic patients with atypical presentations. In 2010, the Research Committee of the Renal Pathology Society first divide diabetic nephropathy into four hierarchical glomerular lesions with a separate evaluation for degrees of interstitial and vascular involvement.14 For the most part, the diagnosis of DKD is based on the course of clinical manifestations and laboratory findings such as moderately increased albuminuria, which is considered a prognostic predictor of the progression of DKD. However, moderately increased albuminuria has a limited predictive value because of its poor correlation with reduced glomerular filtration rate.15,16 Some patients with diabetic nephropathy proven by renal biopsy have normal serum creatinine level without moderately increased albuminuria,17,18 whereas others, before moderately increased albuminuria is diagnosed, may show progressive loss of kidney function.19,20 Recent studies suggested that the moderately increased albuminuria in several DKD patients can even return to normal values.16,21 While this phenomenon is more common in type 2 diabetes mellitus, this situation is termed as normoalbuminuric diabetic kidney disease (NADKD) and is characterized by more obvious tubular interstitial lesions.22,23
Therefore, developing more sensitive and specific markers is urgently needed to identify patients with diabetes who are at high risk of DKD. At the molecular level, several proteins and their functions in DKD have been characterized, whereas others remain to be discovered. UbA52 is a ubiquitin fusion protein. Sun et al.24 found that the expression of UbA52 mRNA in the kidney was proportional to blood glucose levels. In situ hybridization and immunohistochemistry revealed that UbA52 exclusively localized to renal tubules, and its expression was markedly increased in mice with DN.18 UbA52 can be detected in serum, but at low concentrations which rule out its effect on urine concentration.25
Our study shows the level of urinary UbA52 was significantly correlated with serum creatinine. The expression of UbA52 is regulated by a variety of injuries, such as oxidative and carbonyl stress, which are important in the pathophysiology of DKD and apoptosis.24,26 High glucose in the cells of diabetic patients can lead to the formation of superoxide compounds and increased production of reactive oxygen species (ROS). Oxidative stress can increase the activity of ubiquitin activase (E1) and ubiquitin binding enzyme (E2) and then increase the expression of UbA52.27,28 Stimulation of cells with hydrogen peroxide, which mimics oxidative stress, can increase the expression of UbA52.29 The selective expression of UbA52 in renal tubules suggest that the ubiquitin proteasome proteolytic system is indeed operating in this compartment of the kidney.24
We combined HRP-labeled antibody with ELISA to determine whether urine could be used for identifying a marker specific for DKD and evaluated the relationship between urinary UbA52 content and DKD. Urinary UbA52 content was greater in DM-NP patients than in controls or DM-WNP patients. Our results confirm the altered urinary UbA52 content in patients with DN. UbA52 may be an important molecule relevant to the pathobiology of DKD. With urinary UbA52 secretion, we can estimate the degree of microangiopathy in the renal system of patients with diabetes.
The prevalence of nondiabetic renal disease in patients with T2DM mellitus (T2DM) varies from 27% to 79%.30 So a patient with T2DM with a high proteinuria could have a diagnosis of DKD or of T2DM with nondiabetic renal disease. Because the proteinuria level was independently associated with urinary UbA52 level, UbA52 may have a role in the pathogenesis of renal disease with proteinuria. UBA52 was significantly increased in patients with focal segmental glomerular sclerosis (FSGS) and minimal change nephropathy (MCD),31 which was much higher than that in patients with diabetic nephropathy in our study. UBA52 may not be a specific marker, but it may be helpful to identify these diseases with different cut off values. Further studies with larger patient samples are needed to explore the function of UbA52 in DKD and nondiabetic renal diseases.
This study has several limitations. First, we had only 3 cases of diabetic nephropathy with a diagnosis by renal biopsy. The other cases of DM-NP were defined by moderately increased albuminuria and diabetic retinopathy, which are not accurate indictors and related to many factors. Second, we did not follow up patients to evaluate the prognosis, which was whether relevant to the concentration of UbA52 in urine. Third, since we did not enroll patients with normoalbuminuric diabetic kidney disease (NADKD), the significance of UBA52 in NADKD needs further study.
In summary, we found altered urinary UbA52 content in DM-NP patients. The ubiquitin fusion protein UbA52 may be another important molecule relevant to the pathogenesis of DKD. It is expected to facilitate identification of diabetic nephropathy among diabetic patients. This observation gives an impetus to research on the function of UbA52, which could further enhance our understanding of the pathogenesis of DKD. The identification of this protease and longitudinal studies in larger patient groups will determine its usefulness as a marker for predicting the clinical course and the potential role of the protease in the pathophysiology of diabetic renal involvement.
FundingThis study was funded by the Science and Technology Program of Guangdong Province Grant 2016A090922005.
Conflicts of interestThe authors declare to have no conflicts of interest.
We would like to express our gratitude to all the patients who have participated, as well as to all the staff of the Division of Nephrology of the Third Affiliated Hospital of Hebei Medical University for their collaboration and enthusiasm in this study.