Introducción: La insuficiencia cardíaca congestiva (ICC) es una complicación frecuente en la enfermedad renal crónica (ERC). Además de los factores de riesgo clásicos, otros relacionados más específicamente con la ERC, como la anemia, la sobrehidratación o los accesos vasculares, también podrían jugar un papel importante. Objetivos: Determinar la incidencia y las características clínicas asociadas al desarrollo de ICC en pacientes con ERC avanzada y analizar la influencia de la creación de accesos vasculares prediálisis sobre esta complicación. Pacientes y métodos: Estudio de cohorte prospectivo y de observación en el que se incluyeron 562 pacientes (edad media 65 ± 15 años, 260 mujeres) con un filtrado glomerular medio de 15,1 ± 5,0 ml/min, no en diálisis. La variable de resultado principal fue el desarrollo de al menos un episodio de ICC definida por criterios clínicos y radiológicos convencionales. Además de los datos demográficos y clínicos de interés, se incluyó también como covariable la fístula arteriovenosa (FAV). Resultados: Con una mediana de seguimiento de 461 días, la incidencia de ICC fue de 19 episodios por cada 1000 pacientes/año, presentando esta complicación un 17% del total de los pacientes. Mediante regresión logística multivariable, los mejores determinantes del desarrollo de ICC fueron, además de los factores de riesgo clásicos (mujer, añosa, obesa, diabética, con antecedentes de cardiopatía), la realización con éxito de una FAV (odds ratio: 9,541; intervalo de confianza 95%: 4,841; 18,806; p < 0,0001). Mientras que 4 de los 51 pacientes (8%) con FAV distales desarrollaron ICC, 43 de los 109 pacientes (40%) con FAV proximales desarrollaron esta complicación. No se observaron diferencias en la mortalidad de los pacientes con o sin ICC, aunque el inicio no programado (urgente) de diálisis fue mucho más frecuente entre los que desarrollaron ICC que en el resto (63 vs. 3%, p < 0,0001). Conclusiones: La incidencia de ICC es muy elevada en pacientes con ERC avanzada prediálisis. Además de los factores de riesgo clásicos, la realización de un acceso vascular incrementa significativamente la probabilidad de desarrollo de esta complicación cardiovascular.
Introduction: Congestive heart failure (CHF) is a common complication in patients with chronic kidney disease (CKD). In addition to classical risk factors (e.g. age and pre-existing cardiac diseases), other potential reversible abnormalities linked to CKD such as anaemia, volume overload, or vascular access placement may also influence the incidence and severity of acute exacerbations of CHF. Objective: This study aims to determine the incidence and main determinants of CHF in a cohort of patients with stage 4-5 pre-dialysis CKD. Patients and Method: The study group consisted of 562 patients (mean age: 65±15 years, 260 females, 31% diabetics). Native arteriovenous fistulas (AVF) were created in 160 patients who chose haemodialysis as the initial technique for renal replacement therapy. The main outcome variables were: acute decompensated CHF (defined by standard criteria), dialysis initiation (planned and unplanned), and death before dialysis initiation. In addition to demographics, comorbidities, and clinical and biochemical data, AVF creation was also included as a potential determinant of CHF in multiple logistic regression models. Results: Ninety-five patients (17%) developed at least one episode of acute decompensated CHF, and the incidence rate was 19 episodes per 1000 patient-years. In addition to classical risk factors (age, female sex, obesity, diabetes, and previous history of CHF or coronary artery disease), creation of a successful AVF significantly increased the risk of CHF (OR=9.54, 95% CI: 4.84-18.81, P<.0001). In 47 out of 95 patients who developed CHF, a functioning AVF had previously been created, 92% of which were upper arm native AVF, with a median of 51 days between the surgical procedure and CHF episode. The mortality of patients with CHF was similar to that of the rest of the study patients, although unplanned dialysis initiation was significantly more frequent in those who developed CHF. Conclusions: Acute decompensated CHF episodes are common in pre-dialysis CKD patients. In addition to classical risk factors, pre-emptive AVF placement was strongly associated with the development of CHF.
INTRODUCTION
Cardiovascular diseases are very prevalent in patients with chronic kidney disease (CKD), and congestive heart failure (CHF) is one of the most common manifestations of this condition.1-5
Factors such as age, sex, arterial hypertension, volume overload, ischaemic heart disease, anaemia, and hypoalbuminaemia have all been shown to influence the development of CHF in CKD patients,1-3 which is accompanied by morphological and functional heart disorders, especially in the left ventricle, such as hypertrophy and/or ventricular dilation, along with systolic/diastolic dysfunction.6
Closely linked to the advanced CKD processes, the creation of an arteriovenous fistula (AVF) can also become a risk factor for developing CHF.7-10
Several studies have demonstrated that starting chronic haemodialysis treatment with an AVF has substantial advantages for the survival of these patients as opposed to those with venous catheters as their first vascular access point.11-13 As a result, using an AVF for planned haemodialysis is currently one of the most highly recommended measures in pre-dialysis care.14,15
This study was motivated by the observation of a high incidence of episodes of CHF in our patients during follow-up visits for advanced CKD. The aims of our study were to determine the incidence and clinical characteristics associated with the development of CHF, and to analyse the possible role of creating pre-dialysis vascular accesses in this condition, as well as the consequences of this cardiovascular issue in patient mortality and unplanned start of haemodialysis.
MATERIAL AND METHOD
Patients
Our study included a 562 (mean age: 65±15 years, 260 female, 302 male) incident and prevalent patients in the advanced chronic kidney disease outpatient unit at the Hospital Infanta Cristina of Badajoz from 1 January 2004 to 1 July 2010.
The inclusion criteria were: non dialysis patients over 18 years old with stage 4-5 chronic renal failure not secondary to a failed kidney transplant.
The most common aetiology of CKD was unknown (223 patients), followed by diabetic nephropathy (109 patients) and primary glomerulopathies (91 patients).
Comorbidities were very prevalent. A total of 177 patients were diabetic, 97 had a history of ischaemic heart disease, 74 patients had cerebral ischaemia, and 52 had peripheral ischaemia. At least one episode of congestive heart failure was recorded in 84 patients, 56 had chronic atrial fibrillation, and 48 patients had been diagnosed with chronic obstructive pulmonary disease.
The most commonly prescribed drugs were: anti-hypertensive agents (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and beta blockers), diuretics, statins, anti-platelets, phosphorous binders, and erythropoietin.
Clinical and laboratory analysis values
In addition to the demographic data, we included measurements of systolic and diastolic blood pressure (using an automated Omron M3 device) and body mass index. The level of comorbidity was determined using the method developed by Davies et al.16
During the study period, patients were monitored and treated using standard criteria. The creation of a permanent vascular access (native arteriovenous fistula) was scheduled for patients with severely deteriorated glomerular filtration rates (GFR<12ml/min/1.73m2) and who needed haemodialysis.
All vascular accesses were autologous (no prostheses were used) and were created in the arms. The venous territory (proximal or distal) was decided upon by the vascular surgeon following a clinical examination.
The causes for not achieving a functioning vascular access during the study period were: too short follow-up period of the patients on ACKD visits, patient preference for peritoneal dialysis, patient refusal for vascular access, failed attempts, or scant possibility of success based on the criteria of the vascular surgeon.
Upon admission to the study, we extracted blood for a haematological (haemogram) and biochemical analysis, including the following parameters: urea, creatinine, albumin (bromocresol purple method), sodium, potassium, chlorine, calcium, phosphorous, and 24-hour urine proteinuria (expressed as milligrams per gram of creatinine). We performed all biochemical analyses using an auto-analyser (Advia® Chemistry, Siemens Healthcare Diagnostics). We also measured bicarbonate levels in venous blood (ABL 800 FLEX gas analyser, Radiometer Ibérica).
GFR was estimated using the MDRD-4 formula.17
Study design and statistical method
Ours was a prospective, observational cohort study, in which the primary study variable was the development of at least one episode of decompensated CHF as defined by conventional clinical and radiological parameters.18 The study was carried out at our hospital during the established study period. Other events that we analysed included death for any cause during the pre-dialysis follow-up period, and start of dialysis, whether planned or unplanned.
The median follow-up time was 461 days (interquartile range: 215-897 days).
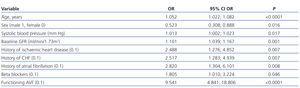
In order to establish the determining factors for the development of heart failure, we used a multivariate logistic regression model, into which we introduced the following covariates: age, sex, body mass index, systolic and diastolic blood pressure, haemoglobin, GFR, baseline serum levels of uric acid, total calcium, phosphorous, alkaline phosphatase, bicarbonate, potassium, albumin, parathyroid hormone, history of heart failure, atrial fibrillation, ischaemic heart disease, prescription of diuretics, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, beta blockers, and functioning arteriovenous fistula.
We chose the covariates for the model automatically using stepwise backwards elimination.
In order to establish whether there was an independent association between the type of vascular access performed and the development of CHF, we used multivariate Cox proportional hazards models, and determined hazard ratios and 95% confidence intervals (CI).
For the comparison of two independent continuous variables, we used un-paired Student’s t-tests, or non-parametric Mann-Whitney U-tests, according to the distribution of the variables. We used chi-square tests to compare discreet variables.
We have presented our study data as a mean and standard deviation (± SD), or as a median and interquartile range or minimum-maximum values. A P-value <.05 was considered to be statistically significant. We performed all statistical analyses and created our figures using SPSS software version 15.0 (SPSS, Chicago, USA).
RESULTS
The clinical and laboratory variables for the study patients are summarised in Table 1.
A total of 95 patients suffered at least one episode of CHF (17%). The global incidence of this phenomenon was 19 episodes/1000 patient-years.
Table 1 shows the differences between patients that developed CHF and those that did not. The clinical profile of patients that developed pre-dialysis CHF can be summarised as: female sex, elderly, obese, diabetic, with a history of ischaemic heart disease or previous episodes of CHF or atrial fibrillation, with higher systolic BP, but no important differences in laboratory variables except for a higher alkaline phosphatase level, greater use of diuretics and beta blockers, and more successful vascular access, preferentially proximal (humero-cephalic or humero-basilic).
The multivariate logistic regression revealed the determining factors for developing an episode of CHF (Table 2), with a permanent vascular access being the most important one (OR: 9.451; 95% CI: 4.841-18.806; P<.0001).
During the study period, a total of 160 vascular accesses were successfully created (51 radio-cephalic and 109 humero-cephalic AVF). Whereas 4 of the 51 patients (8%) with distal AVF developed CHF, 43 of the 109 patients (40%) with proximal AVF developed this complication.
The median time elapsed between the creation of the AVF and the first episode of CHF was 51 days (interquartile range: 26-138 days).
Figure shows that the risk of developing CHF during the period following creation of the AVF was significantly higher in patients with a proximal AVF than in those with a distal AVF (log-rank: 12.13; P<.0001).
A total of 97 patients died during the follow-up period (17%), with an annual mortality rate of 8%-12%.
We observed no significant differences in mortality between patients that developed CHF and those that did not (16.5% vs 17%, respectively).
A total of 304 patients (54%) needed dialysis. Those that had developed CHF required dialysis more frequently than those that did not (67% vs 51%; P=.005). Additionally, the start of dialysis was unplanned (emergency) much more frequently in patients that developed CHF than in those that did not (63% vs 3%; P<.0001).
DISCUSSION
The results of our study show that the incidence of CHF is very high in pre-dialysis patients with advanced CKD. The combination of characteristics that defined high-risk patients for developing this complication (female, elderly, diabetic, obese, with a history of heart disease, etc.) is the typical one, and can be easily recognised by any experienced nephrologist. However, the most notable finding of this study was a strong association between creating an AVF and developing CHF.
The short time span between creating the AVF and the development of CHF, along with the higher frequency of this complication in patients with proximal AVF support a connection between these phenomena. Although the association between AVF and CHF in dialysis patients has previously been considered incidental,7-10 creating an AVF causes several haemodynamic changes that can favour the development of CHF: increased cardiac venous return, increased heart rate and contractility, and increased filling pressures, which leads to a greater cardiac output, increased plasma volume, and decreased peripheral resistance.10,19-21
In some patients, especially those with a previous history of cardiac disease, this hyper-dynamic circulatory state cannot be compensated, triggering CHF. Furthermore, if this condition persists chronically, it can produce structural cardiac changes —hypertrophy or left ventricular dilation—, as suggested by the fact that this alteration reverses when AVF are ligated22; at the same time, this condition can predispose the patient to myocardial ischaemia due to the imbalance between the subendocardial oxygen provided and the increased demand resulting from the greater cardiac output.23
The increase in cardiac output is proportional to arteriovenous flow,24,25 and this can aid in explaining the higher probability of developing CHF in patients with a proximal AVF.
The results from our study pose two questions: why does CHF develop more frequently in pre-dialysis patients with an AVF than in those already on dialysis? and how can this complication be prevented?
The first question could be explained by the limited capacity for compensation of many of these patients in cases of increased cardiac output and since, in contrast to patients already on haemodialysis, an exhaustive control (three times per week) and effective treatment (ultrafiltration) of occurring CHF is not possible in patients that are only treated in ACKD visits.
The simplest option for preventing this cardiovascular complication could be not creating an AVF prior to dialysis, although this option does not seem very recommendable, since the cost/benefit ratio of a pre-dialysis AVF is probably much lower than with temporary catheters. As our study showed, CHF is not associated with a higher pre-dialysis mortality rate, and the most notable adverse effect was an emergency (unplanned) start of haemodialysis.
The results of our study also recommend vascular surgeons to make all possible effort to achieve distal AVF in pre-dialysis patients, particularly in those with risk factors for developing CHF, although it is the very clinical parameters and the availability of the proper vessels which poses the greatest difficulty for creating radial AVF in these patients.
Finally, we would recommend a strict follow-up of cardiovascular tolerance and response to the creation of the AVF, which would allow for an early diagnosis of CHF, facilitating control of this condition and, when necessary, overhydration, especially in high-risk patients.
This study had its limitations. We did not measure haemodynamic parameters (AVF flow, cardiac output, etc.) nor did we take sequential echocardiograms, and so our conclusion of a pathogenic connection between the appearance of CHF and the AVF was based solely on clinical observations and epidemiology data. We also did not collect information on the incidence and clinical details of CHF in patients already on haemodialysis, and so the assertion of a less significant association between episodes of CHF related to vascular accesses in these patients is based on the experience that others reported in the medical literature.
In conclusion, the incidence of CHF is very high in pre-dialysis patients with advanced CKD. In addition to classical risk factors, the creation of a vascular access significantly increases the probability of developing this cardiovascular condition. This must be taken into account when dealing with high-risk patients for whom a vascular access intervention is planned.
Conflicts of interest
The authors have no potential conflicts of interest to declare.
Table 1. Clinical and laboratory parameters for all patients and each sub-group according to the presence or absence of congestive heart failure
Table 2. Influential factors in the development of decompensated heart failure in our patients, as determined by multiple logistic regression
Figure 1. Risk of developing congestive heart failure following creation of an arteriovenous fistula