El factor de crecimiento de tejido conectivo (CTGF) aparece aumentado en diferentes patologías asociadas a fibrosis, incluidas múltiples enfermedades renales. CTGF participa en procesos biológicos, como la regulación del ciclo celular, migración, adhesión y angiogénesis. Su expresión está regulada por diversos factores implicados en el daño renal, entre los que destacan el factor la angiotensina II, el factor de crecimiento transformante-beta, altas concentraciones de glucosa y situaciones de estres celular. CTGF participa en el inicio y progresión del daño renal al ser capaz de inducir una respuesta inflamatoria y promover la fibrosis, señalándole como una posible diana terapéutica en el tratamiento de patologías renales. En este trabajo revisamos las principales acciones de CTGF en la patología renal, los mecanismos intracelulares de actuación y las estrategias terapéuticas para su bloqueo.
Numerous diseases, such as proliferative disorders and fibrotic lesions, skin conditions, atherosclerosis, pulmonary fibrosis and multiple renal pathologies present high CTGF levels in tissue, mainly in fibrotic areas. 1-5 Although CTGF has traditionally been considered a profibrotic factor, it is a multi-function factor with biological activities that vary depending on the cell type. These activities include regulating cellular apoptosis/proliferation, angiogenesis, migration, adhesion and fibrosis. 2,3 In this study, we reviewed CTGF¿s role focusing on its importance in renal disease.
STRUCTURE
CTGF is a secretable cysteine-rich protein with a molecular weight of 38kDa, which was identified in cultured endothelial cells from the umbilical cord vein. 6 CTGF, also known as CCN2, belongs to the CCN family of early response genes, which is made up of five members: Cyr61 (cysteine-61 rich protein), Nov (nephroblastoma overexpressed gene), WISP-1 (Wnt-1 induced secreted protein 1), WISP-2 and WISP-3. 1,2,7,8 All members of this family are characterised by a high percentage of homology in their amino acid sequence, which ranges between 50 and 90%, and they present 38 cysteine residues that are grouped in two segments (22 in the N-terminal and 16 in the C-terminal regions). This is typical of other growth factors such as the plateletderived growth factor (PDGF), the nerve growth factor and the transforming growth factor-β (TGF-β). 3,9
Proteins in this family carry a secretory signal peptide in the NH 2-terminal end and four preserved secretion domains or modules. 9 These domains are as follows: 1) insulin-like growth factor (IGF) binding domain with the
conserved binding sequence Gly-Cys-Gly-Cys-Cys-X-XCys located within the amino-terminal region for all GFbinding proteins; 10-12 Von Willebrand type C domain, which participates in protein oligomerisation and
formation; 13 3) thrombospondin-1 domain, implicated in binding soluble macromolecules to a matrix; 14 and 4) Cterminal domain, a dimerisation domain implicated in cell-surface binding, which has mitogenic activity for
fibroblasts and is responsible for interaction with fibronectin. 15
REGULATION
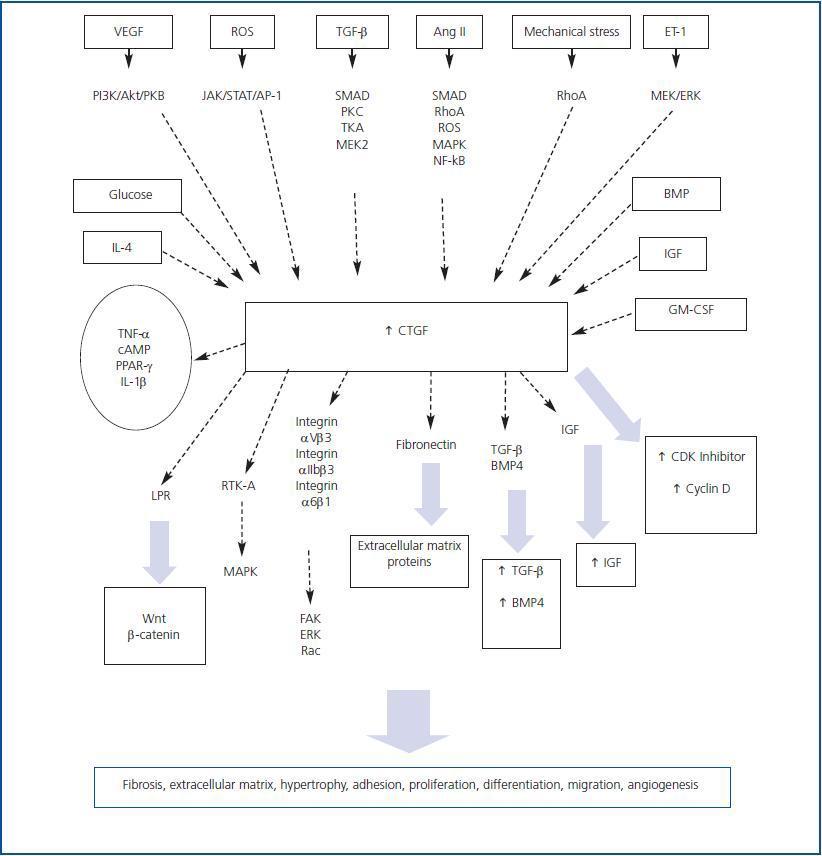
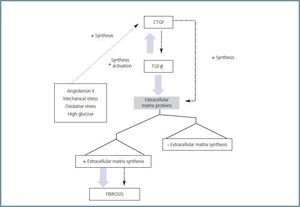
Depending on the cell type, a wide variety of factors and molecules is involved in regulating CTGF expression. G-protein-coupled receptor agonists, growth factors such as TGF-β, angiotensin II (AngII), bone morphogenetic protein (BMP), vascular endothelial growth factor (VEGF), IGF, granulocyte/macrophage colony stimulation factor (GM-CSF), interleukin-4 (IL-4), high levels of glucose, hypoxia, mechanical stress and oxidative stress cause a rapid increase in CTGF expression (figure 1). 16-24 However, other factors such as the tumour necrosis factor alpha (TNF-α), interleukin-1¿ (IL-1β), cAMP and treatment with peroxysome proliferative activated receptor-γ (PPAR-γ) inhibit CTGF expression induced by TGF-β and AngII in some cell types. 25-29 Various signal mechanisms have been related to increased CTGF. These include the SMAD protein signalling pathway, the reactive oxygen species,
the small G protein RhoA, protein kinase C, Janus kinase (JAK), phosphatydilinosotol-3 kinase (PI3K) and the mitogen-activated protein kinase (MAPK) cascades. 30-33
Most of the studies that have been carried out have focussed on investigating TGF-β-induced CTGF regulation. A SMAD-binding element has been described in the CTGF promoter, which is necessary for its induction by TGF-β. 34 In proximal tubular epithelial cells and mesangial cells, TGF-β increases CTGF production in a process that is regulated by SMAD proteins and the Ras/MEK/ERK signalling cascade. 36,37 However, this process is mediated by Rho activation 35 in renal fibroblasts. The MAPK signalling cascade also plays an important role in regulating CTGF. In hepatocytes, TGF-β induces CTGF expression and production by means of ERK1/2, 38 while in lung fibroblasts the process is mainly mediated by the kinase JNK1/2. 39 Studies performed by our group have focussed on the regulation caused by AngII. In human tubular epithelial cells, AngII induces CTGF production by activating MAPK (ERK, p38 and JNK) and Rhoassociated protein kinase, ROCK. 39 Rho involvement in CTGF regulation has been described for many cell types, including lung fibroblasts and vascular smooth muscle cells. 34,41 In the latter, CTGF increases in response to AngII through other pathways such as ROS, SMAD proteins and the kinases p38, JNK1/2, ROCK, PKC and PTK. 34,42 In fibroblasts, ERK1/2 and JNK1/2 inhibitors, but not p38 inhibitors, decrease AngII-stimulated CTGF expression 43. Nevertheless, in rat mesangial cells, we find ROS and p38 production among the pathways that are involved in AngII-induced CTGF production. 29 Recent molecular studies have revealed the presence of a highly conserved NF-κB binding sites in the proximal region of the CTGF promoter. 44 In mesangial cells, we have observed that blocking NF-κB decreases AngIIinduced CTGF production (unpublished data), which
suggests that activation of the NF-κB transcription factor is involved in CTGF regulation in the kidney.
SIGNAL TRANSDUCTION AND BIOLOGICAL FUNCTIONS OF CTGF
There is still no known receptor that is specific to CTGF. However, it has been stated that it interacts with various
proteins, such as tyrosine-kinase and integrin, which activate multiple signalling systems. The first interaction studies revealed that there are CTGF-receptor complexes with a molecular weight of about 280kDa located in chondrocytes, osteoblasts and endothelial cells. 45 In various cell types, CTGF acts by binding different integrins, such as integrin α5β1 or αIIbβ3, to heparan sulphate proteoglycan receptors -which activates various kinases, such as focal adhesion kinase (FAK), ERK and Rac 16,47-49- and to the macroglobulin receptor for the low-density lipoprotein receptor-related protein 46 (LPR) (figure 1). It binds directly to BMP-4 and TGF-β 50 at its cysteine-rich domain, interacts with fibronectin at its carboxyl-terminal domain, and binds with IGF 52 at its amino-terminal domain. In human mesangial cells, CTGF interacts with the dual receptor tyrosine-kinase system A (RTK-A) and p75 NTR that participates in neurotrophin signal transduction in the nervous system (figure 1). Receptor tyrosine kinases bind numerous adapter proteins and activate multiple intracellular signalling pathways, which would be in keeping with CTGF¿s multi-functional properties 66 that include regulating and synthesising extracellular matrix
(ECM), 4,53,54 endothelial cell migration and angiogenesis, regulation of extracellular matrix, mesothelial cell apoptosis, 56 survival of hepatic and mesangial cells 57,58 and fibroblast and chondrocyte proliferation and differentiation. 59,60 In the kidney, CTGF actively participates in fibrosis and epithelial mesenchymal transition (EMT) and, as we described above, in the inflammatory response. We will presently give a more in-depth review of this topic.
RELATION BETWEEN CTGF AND TGF-β
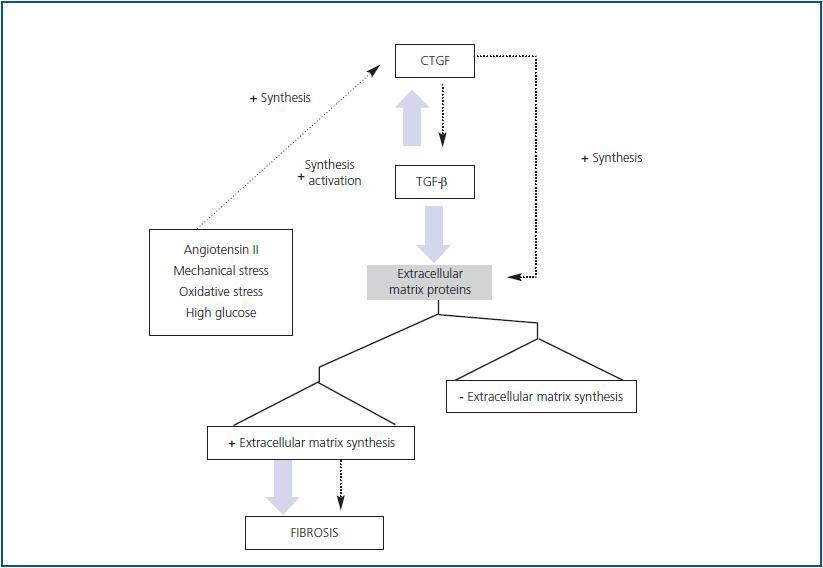
Numerous in vivo examples demonstrate that TGF-β participates in fibrotic processes. It has been stated that CTGF and TGF-β act in synergy to promote chronic fibrosis (figure 2). Subcutaneous co-injection of the two agents produces sustained and persistent fibrosis in mice. Various experimental models, such as those for unilateral ureter
obstruction, nephritis due to anti-Thy1 antibodies, diabetic glomerulosclerosis and AngII infusion 61-63 show high levels of TGF-β and CTGF during advanced stages of fibrosis, indicating that these factors may contribute to the progression of kidney damage (figure 3). As stated previously, CTGF binds directly to TGF-β and increases its responses. The mechanism is based on CTGF¿s chaperone function that increases TGF-β¿s affinity for its different receptors, creating more intense and prolonged responses. 64 This is not the only manner by which CTGF participates in TGF-β responses. Endogenous production of CTGF by TGF-β involves a transcriptional suppression of SMAD-7 through induction of the TGF-ß-inducible early gene (TIEG-1) transcription factor. By means of this mechanism, TGF-β blocks feedback regulation through SMAD-7, perpetuating the activation of TGF-β signalling. 32 This may be relevant for pathological conditions in which there is increased CTGF expression.
Blocking TGF-β activity with neutralising antibodies and/or decorin, which captures its active form, has been shown to reduce fibrosis in experimental models for kidney damage. However, TGF-β deficiency is lethal in the mouse; it
develops a lesion repair defect, with collagen deposit problems, and presents a hyperinflammatory phenotype. 65
This suggests that it would be better to find a more specific therapeutic target for fibrotic diseases. Mice that are
heterozygous for CTGF gene deletion have matrix organisation and synthesis defects during osteogenesis, which results in seriously defective development of the skeletal component of the thoracic cage; they die immediately after birth. 66 In addition, CTGF is a mediator of fibrosis caused by TGF-β and other factors that are involved in tissue damage, and for that reason, it may be a new, more useful target for antifibrotic therapy.
CTGF AS A MEDIATOR OF RENAL FIBROSIS AND EPITHELIAL-MESENCHYMAL TRANSITION
CTGF is not expressed in the healthy kidney, but its expression is induced by human renal diseases, including
glomerulonephritis, glomerulosclerosis and diabetic nephropathy; its expression levels are correlated with the
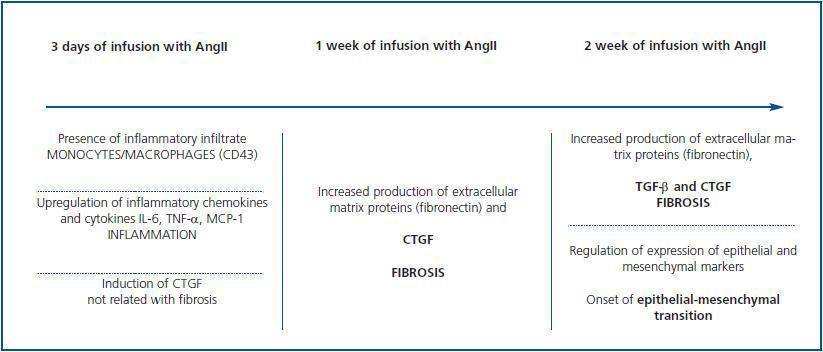
severity and progression of renal fibrosis. 5,67-69 Our group used the model of kidney damage caused by the systemic infusion of AngII in rats to study the role CTGF plays in the onset and progression of in vivo kidney damage.
The infusion of AngII rapidly induces the expression of CTGF, which appears after three days and remains high for two weeks, which was the end point of the study (figure 3). The appearance of CTGF, which was observed in tubular epithelial and glomerular cells three days after infusion, precedes the accumulation of ECM (characterised by an increase in the fibronectin deposit) which is seen after one week. This indicates that CTGF may act as a mediator of renal fibrosis caused by AngII in vivo. 70 Through in vitro studies of rat mesangial cells, we observed that blocking endogenous CTGF synthesis by using antisense oligonucleotides prevents AngII-induced fibronectin and collagen IV production. 43 These data show that CTGF is a mediator of AngII¿s fibrotic response in the kidney.
Infusion of AngII also increases expression of TGF-β in the kidney. This factor is synthesised as an inactive protein
which is anchored to the membrane before being activated. 71 In cell cultures, AngII increases mRNA expression of TGF-β, protein production and latent TGF-β activation in a process that is mediated by thrombospondin. 72 Expression levels for TGF-β, but not thrombospondin-1, appear to be higher after three days of AngII infusion.
However, active TGF-β protein levels did not increase for two weeks. 73 This suggests that CTGF is induced before
TGF-β and that it participates in the onset of fibrosis and remains high until advanced stages, which contributes to
perpetuating kidney damage (figure 3).
Much evidence suggests that tubular epithelial cells under pathological conditions may suffer epithelialmesenchymal
transition (EMT) and become fibroblasts that produce extracellular matrix and contribute to renal fibrosis and the progression of the disease. 74 Different factors participate in this process, notably CTGF, TGF-β, fibroblast growth factor (FGF), IL-1, EGF, advanced glycosylation end products (AGEs) and AngII. 39,73,75-78 CTGF promotes the human tubular epithelial cell transdifferentiation to myofibroblasts in vitro and blocking CTGF results in inhibition of
transdifferentiation caused by TGF-β, by advanced glycosylation end products and AngII. 39 In the model of AngII-induced kidney damage, the increase in CTGF expression remains present after two weeks. This coincides with the onset of EMT, which is characterised by an increase in the mesenchy marker α-SMA expression and a decrease in epithelial marker E-cadherin 73 (figure 3). Furthermore, the increase in CTGF expression in the diabetic kidney is placed over the tubular epithelium in EMT sites. 75 These data have been confirmed in other experimental models such as 5/6 nephrectomy in rats, where increased CTGF was associated with overexpression of TGF-β and PDGF in
interstitial fibroblast, increased fibrosis and increased severity of kidney damage. 80 By citing these data, we may conclude that CTGF is an inducer of fibrosis and EMT in vivo, and that it mediates the actions of profibrotic factors such as TGF-β and AngII. Furthermore, by interacting with TGF-β it contributes to the perpetuation of fibrosis.
CTGF PARTICIPATES IN THE RENAL INFLAMMATORY RESPONSE
By using the AngII infusion model, we observed that a clear inflammatory response occurs in the kidney after three days, 81 that this response is characterised by the presence of infiltrating inflammatory cells (T cells, macrophages and granulocytes) in tubulo-interstitial and glomerular areas (figure 3). In this animal study, we also observed an increase in production of classic inflammatory mediators such as IL-6, TNF-β and MCP-1, and in CTGF production in mesangial, podocytes and tubuloepithelial cells, 70 which suggests that CTGF could be implicated in inflammatory response regulation in kidney damage situations. Numerous in vitro studies in glomerular mesangial, tubular epithelial, pancreatic and hepatic cells have demonstrated that CTGF regulates inflammatory mediators. 58,82,83
Our group recently showed that systemic CTGF administration in mice caused an inflammatory response after 24 hours, which was characterised by inflammatory cell recruitment (macrophages and T cells) in the interstitium, production of chemotactic factors (MCP-1 and RANTES), proinflammatory cytokines (INF-γ, IL-6 and IL-4), and activation of the NF-κB transcription factor. 84 Treatment with parthenolide, an NF-κB inhibitor (24 hours before the CTGF injection) reduced the renal inflammatory response, which shows that this factor is crucial for CTGF actions in the kidney. 84
THERAPEUTIC STRATEGIES AGAINST THE PROGRESSION OF KIDNEY DAMAGE
For patients with diabetic nephropathy, it has been shown that high CTGF plasma levels can be considered early markers of progressing renal dysfunction in the diabetic kidney 85, and they also predict the evolution of renal disease. 86 Studies of patients with IgA nephropathy have observed that high urine levels of CTGF and TGF-β are
correlated with the degree of tubulo-interstitial damage. 87 On the other hand, in patients with chronic cardiac damage, plasma CTGF levels provide information on the appearance of myocardial fibrosis, and can be considered a new marker for cardiac dysfunction. 88 These data suggest that CTGF may be a marker for fibrosis and damage progression in different diseases.
Out of current clinical treatments for stopping kidney damage progression, blocking AngII is one of the most widespread pharmacological options, and has proven protective effects on organs. 89 Treatment with AT 1 receptor antagonists and angiotensin conversing enzyme inhibitors 89,90 decreases renal CTGF expression and fibrosis in various experimental models for kidney damage. 70 However, these drugs only slow the advance of the disease, and a new therapeutic option is necessary if we are to reverse renal fibrosis and halt the EMT process.
CTGF blockage is one of the most promising options amongst the new therapeutic approaches. At present, CTGF inhibition studies are directed toward the development of antisense oligonucleotides, interference RNA or neutralising antibodies that block endogenic CTGF. Experimental studies have shown that blocking CTGF with antisense oligonucleotides reduces the ccumulation of ECM in transgenic TGF-β 1 mice with a nephrectomy 91 and in mice with diabetic nephropathy. 92 In one hepatic fibrosis model, treatment with interference RNA for CTGF via the intraportal vein attenuated hepatic fibrosis. 93 However, CTGF blocking effects in the renal inflammatory response have not been studied yet, and a more in-depth examination of this field is necessary. All of these data suggest that
blocking endogenous CTGF could be a good alternative when treating renal pathologies associated with inflammation and fibrosis.
FINAL CONCLUSION
This review reveals the immense complexity of the intracellular signalling pathways that regulate CTGF, which vary according to the cell type and the induction factor. It also refers to the presence of the activating and negative regulatory factors determining CTGF synthesis and the development of CTGF responses. With regard to renal pathology, CTGF is involved in all stages of kidney damage: it participates in the inflammatory response (by activating the NF-κB factor that regulates chemokines and cytokines) and promotes fibrosis and EMT, thus showing itself as a good therapeutic target for the treatment of renal diseases.
Figure 1.
Figure 3.
Figure 2.