Patients with chronic kidney disease have a higher risk of fractures than the general population due to the added factor of uraemia. Although the mechanisms behind uraemia-associated fractures are not fully understood, it is widely accepted that the decrease in bone mineral content and alteration in bone architecture both increase bone fragility. As chronic kidney disease progresses, the risk of fracture increases, especially once the patient requires dialysis. Among the many causes of the increased risk are advanced age, amenorrhoea, steroid exposure, decreased vitamin D, increased PTH, malnutrition and chronic inflammation. Serum phosphorus, whether high or very low, seems to correlate with the risk of fracture. Moreover, increased serum phosphate is known to directly and indirectly affect bone metabolism through the development of adaptive hormonal mechanisms aimed at preventing hyperphosphataemia, such as the increase in PTH and FGF23 and the reduction in calcitriol. These adaptive mechanisms are less intense if the intestinal absorption of phosphorus is reduced with the use of phosphorus captors, which seem to have a positive impact in reducing the risk of fractures. We describe here the possible mechanisms associating serum phosphorus levels, the adaptive mechanisms typical in kidney disease and the use of drugs to control hyperphosphataemia with the risk of fractures. We found no studies in the literature providing evidence on the influence of different treatments on the risk of fractures in patients with chronic kidney disease. We suggest that control of phosphorus should be an objective to consider.
El paciente con enfermedad renal tiene incrementado el riesgo de fracturas, y a los factores habituales de la población general se suman otros propios de la uremia. Los mecanismos que favorecen las fracturas en la uremia no son suficientemente conocidos, aunque es ampliamente aceptado que la disminución del contenido mineral óseo y la alteración en la arquitectura ósea son responsables de un aumento en la fragilidad ósea. Con la progresión de la enfermedad renal crónica (ERC), el riesgo de fractura aumenta, siendo especialmente evidente cuando el paciente requiere diálisis. Dentro de las numerosas causas implicadas en el aumento de fracturas óseas se encuentran la edad avanzada, la amenorrea, la exposición a esteroides, el descenso de la vitamina D, el aumento de la PTH y también la desnutrición y la inflamación crónica. La concentración de fósforo sérico ya sea alto o muy bajo también se ha correlacionado con el riesgo de fractura. El aumento del fosfato sérico puede afectar el metabolismo óseo directamente e indirectamente a través del desarrollo de mecanismos hormonales adaptativos que tratan de prevenir la hiperfosfatemia, como el aumento de PTH y FGF23, y la disminución del calcitriol. Estos mecanismos de adaptación son de menor intensidad si la absorción intestinal de fosforo se disminuye con el uso de captores de fósforo; los cuales parecen tener un impacto positivo en la reducción del riesgo de fracturas. En este documento se describirán los posibles mecanismos que relacionan el riesgo de fracturas con: los niveles de fósforo sérico, los mecanismos adaptativos propios de la enfermedad renal y el uso de fármacos para controlar la hiperfosfatemia. No existen estudios que proporcionen evidencia sobre la influencia de diversos tratamientos en el riesgo de fracturas en pacientes con enfermedad renal crónica. Sugerimos que el control del P debería ser un objetivo a tener en cuenta.
The relationship between chronic kidney disease (CKD) and bone abnormalities is well recognized.1 The renal patient has a fragile bone which can be explained by two main reasons: a decrease in bone mineral content (BMC) and an abnormal bone architecture. Low BMC is characteristic of osteoporosis (OP) of the general population whether it is due to an increasing age or postmenopausal. OP is defined by the World Health Organization (WHO) as a T-score lower than -2.5 standard deviations measured by «dual- energy X-ray absorptiometry »(DEXA). In Uremic patients there are alterations in bone structure due to abnormalities in bone turnover, mineralization and bone volume, which is known as renal osteodystrophy.2
In CKD patients, the risk of fracture is higher than in the general population, since the OP is the consequence of uremic factors specific to CKD plus those factors present in the general population. Uremic OP is more complex than the classical OP which occurs in the general population and of course it increases the risk of fracture.3 In the uremic patient, the coexistence of OP and the classic bone abnormalities of CKD (the so-called renal osteodystrophy) complicate the diagnosis and, of course, the treatment. The CKD patients is not usually diagnosed with OP and yet it is a very common pathology. The quantification of the risk of fracture in the general population is performed by assessing different clinical risk factors (major and minor)4 and / or using different scales, the most popular is the Fracture Risk Assessment Tool (FRAX ® ).5 The FRAX algorithm calculates the probability of a major osteoporotic fracture (vertebral, forearm, or humerus) and / or hip at 10 years (in patients without current or previous treatment). FRAX calculates risk of fracture from basic demographic and clinical data and allows recalculation of the risk by entering the DEXA value in femoral neck. FRAX has been able to predict major osteoporotic fractures in some populations of CKD patients and even mortality in dialysis patients.6
Fractures in CKDThere is evidence that the CKD patient has a higher incidence of OP and an increase frequency of fractures, particularly in advanced stages of CKD.7 The renal patient with fractures may require immobilization, with the complications that this entails, such as infections, ulcers and vascular complications.8,9 OP is a cause of fractures in the general population, however, in patients with CKD the disorders in mineral metabolism increase the risk of fractures further.1 In a recent study, a group from Korea with almost 90,000 patients followed for four years found that the risk of hip fracture in dialysis patients is 66% higher than that of CKD patients on predialysis (OR 1, 66; 95% CI 1.54–1.82; p < 0.001) after adjusting for other risk factors.10
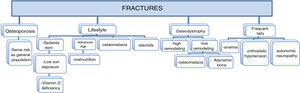
Cause of fracturesMost studies find a higher risk of fractures in CKD patients than in the general population.11 The bones of the renal patient are fragile for several reasons (Fig. 1). They are usually patients with advanced age, the women are usually postmenopausal or amenorrheic (especially if they are on dialysis), exposure to steroids is not uncommon and, both the decrease in vitamin D (inadequate levels of calcidiol due to a diet poor in vitamin D or insufficient sun exposure, and/or low levels of calcitriol due to decreased renal hydroxylation of 1- 〈D3) as well as the increase in parathyroid hormone (PTH) are factors that alter bone quality.
There are no studies aiming to know to what degree the alterations in the mineral metabolism in the uremic patient are responsible for the occurrence of fractures.12 In fact, there is only indirect evidence that treatments aiming at controlling the alterations in the mineral metabolism actually reduce the incidence of fractures.13 Moreover, the role of PTH is controversial and the evidence for the relationship between plasma levels of calcium and phosphorus (P) with the risk of fracture are poor.14 Therefore, fractures of CKD patients should take into account other factors common to the general population and not only alterations in mineral metabolism.15 Other elements such as anemia, orthostatic hypotension after dialysis or autonomic neuropathy are common in CKD and may favor dizziness with falls and therefore the risk of fracture.16 The importance of fractures lies mainly in the fact that they are associated with increased mortality. Hip fractures in patients with CKD increase mortality 2.7 times as compared those without fracture.17
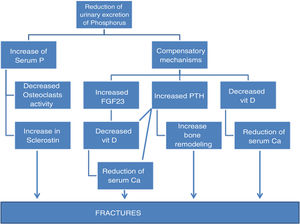
Phosphorus and boneWhen the estimated glomerular filtration rate (GFR) is less than 75 mL / min / 1.73 m2, the renal filtration of phosphate is reduced and adaptive mechanisms develop to prevent body accumulation of phosphorus. Only when glomerular filtration rate drops below 15 mL / min / 1.73 m2 the serum P increases and this usually coincides with the development of hypocalcemia. The overload of P that occurs in the early stages of CKD stimulates the development of compensatory mechanisms to maintain phosphaturia as the increase in fibroblast growth factor 23 (FGF23) and the elevation of serum PTH levels. FGF23 reduces vitamin D in any of its forms because it decreases the activity of 1 〈€-hydroxylase (CYP27B1) and increases that of 24-hydroxylase (CYP24A1).18 The decrease in vitamin D causes a reduction in serum calcium concentration. The decrease in calcium, the difficulty in eliminating P and the resistance to the action of PTH19 maintain elevated the production of PTH even when the GFR is 60 mL / min / 1.73 m2.20
Phosphorus together with calcium are the main ions that form bone, where it is found in the form of hydroxyapatite.21 The bioavailability of P is crucial for a normal mineralization.22 The processes that cause osteomalacia and rickets can be separated into those secondary to vitamin D alterations and the hypophosphatemia non-related to vitamin D, hereditary or acquired. The classification of the various forms of hipophosphatemic rickets is more rational after the discovery of the central role of FGF23 in the pathogenesis of hereditary or acquired forms of the disease.23 Tumoral calcinosis is a rare disease characterized by hyperphosphatemia due to hypophosphaturia and ectopic calcifications. Primary tumor calcinosis can be of the hyperphosphatemic or normophosphatemic variety. The primary hyperphosphatemic variety is an autosomal recessive disorder.24 Secondary tumor calcinosis is associated with conditions such as hyperparathyroidism, chronic kidney disease, vitamin D toxicity, milk-alkali syndrome, and osteolysis.
Bone is an organ that through the production of hormones25 can regulate the stores of P reserves and its own mineralization. The bone produces FGF23,26 which stimulates urinary excretion of P and decreases the production of vitamin D which in turn reduces intestinal absorption of P and also, by other mechanisms, decreases bone reabsorption, all of this prevents the increase in serum P level.27 In the general population the influence of P on bone mineral content has not been sufficiently studied; however, in this regard, there is more information on renal patients.28
Phosphorus as a cause of fracture. Direct and indirect mechanismsThere is no evidence showing a direct relationship between the serum P levels and bone loss and the incidence of fractures; however, some studies suggest that there may be some association. The potential mechanisms are reflected in Fig. 2. It is necessary to differentiate between high levels of P in serum, which can act directly on osteoclastic activity, and the ingestion of high amounts of P that induces hormonal changes18 which affect bone metabolism.29
Phosphorus intake and fracturesA high intake of P has been associated with loss of bone mineral content in a healthy population,30 however such association has not been substantiated in CKD. In experimental animals, it has been seen that a high P intake increases the bone synthesis of sclerostin, a molecule related to abnormalities in bone remodeling,31 which inhibits bone formation. Osteopontin (OPN), a bone matrix protein, is another factor that mediates an increased in bone resorption in response to a high phosphorus diet. The increase in bone resorption associated to a high phosphorous diet appears to be mediated in part by OPN and PTH.32 Diets containing high amounts of inorganic phosphate as additives may negatively affect bone and mineral metabolism and these effects are more pronounced in people with advanced age.
Serum phosphorus levels and fracturesExtreme levels of serum phosphate (P), both high and low, have been associated with mineralization defects and increased risk of fracture.
There are several mechanisms that may link serum P levels and bone fractures:
- •
Direct effect of P on bone metabolism,33,34 by affecting the maturation of the growth plate; differentiation, proliferation and apoptosis of osteoblasts, inducing bone resorption or mineralization, and thus, being directly responsible for the loss of bone mineral content.35 Serum P has been shown to be inversely associated with lumbar spine bone mineral density (BMD) in men (36)·
- •
Indirect effect through the stimulation of other molecules such as PTH, FGF23 and a secondary decrease in vitamin D, all of them with direct effects on bone metabolism, including osteoprotegerin.33
- •
P may be just a marker of bone turnover.
Experimental studies have shown that high concentration of inorganic phosphate in the culture media directly inhibits the generation of new osteoclasts and also inhibits bone resorption by mature osteoclasts. This effects are mediated by several mechanisms: overstimulating osteoprotegerin mRNA expression, without affecting the mRNA of the receptor ligand for the activation of nuclear factor kappa B (RANKL), by direct action on the precursor cells of osteoclasts and by the direct induction of osteoclast apoptosis.33 The direct implication of P on bone health has been demonstrated in experimental animals, showing its implication in bone loss and vascular calcification simultaneously.34,35
A Dutch study in a normal population36 shows that an increase in serum P levels, even within the normal range, is directly associated with the risk of fracture in both sexes (HR 1.47; 95% CI 1.31−1, 65 for each mg / dL of P), regardless of BMD and phosphate intake after adjustments for possible confounding factors (PTH and FGF23). Serum P level was inversely related to bone mineral density in the lumbar spine (predominantly trabecular bone) in men, but not with BMD in the femoral neck (predominantly cortical bone) in either sex. Especially interesting in this study, as in previous studies37 is that the risk of fracture associated with serum P was independent of the adjustments for FGF-23, so that P per se (and not secondary hormonal alterations) could be responsible of the increased risk of fracxtures in this cohort.
In patients with CKD, it has also been observed that for every 1 mg / dL increase in serum P concentration, the risk of hospitalization for fracture increases by 12%.38
FGF23 and fracturesThe difficulty in eliminating P through the kidney in CKD patients stimulates an increase in FGF23.18 There is a borderline correlation between FGF23 and BMD and disappears when adjusted for other causes of osteopenia.39 There are also no consistent data regarding a potential relationship between elevated levels of FGF23 and risk of fractures; the data obtained is not uniform, some in favor40 and others against41 a relationship between FGF23 and risk of fractures.
Rupp et al. have shown that in patients with osteoporosis, higher levels of FGF23 are associated with poor trabecular bone microarchitecture,42 but they have not been able to prove that serum FGF23 concentration is a marker of fracture risk in these patients.
Activation of the Wnt pathway is essential for normal bone mineralization.43 The increase in serum P, PTH and FGF23 is associated with elevations of sclerostin and Dickkopf 1 (Dkk1),44 two potent inhibitors of the Wnt/®-catenin pathway, which would be responsible for a deficit in mineralization. Experimental studies carried out in uremic rats showed that a high phosphorus content produced mineralization defects secondary to the inhibition of Wnt. In this study, FGF23 directly inhibited the Wnt osteoblastic pathway through a process mediated by soluble Klotho / MAPK that required induction of Dkk1, demonstrating that induction of Dkk1 by soluble FGF23 / Klotho in osteoblasts inactivates Wnt / ®-catenin signaling and may have an important impact on the development of loss of bone mineral content in renal patients.45
In conclusion, the direct effect of FGF23 on bone mineral content and risk of fractures yields contradictory data in the different clinical studies analyzed, however, experimental studies have demonstrated the direct role of FGF23 in the inhibition of the osteoblastic Wnt pathway, which would condition a loss of bone mineralization, through which FGF23 could participate in the increase in fractures observed in kidney patients. More studies are needed to support this findings.
PTH and fracturesThe difficulty to eliminate P through the kidney with CKD produces an increase in FGF23, and this in turn reduces vitamin D which increases PTH.18 These alterations occur at very early stages of CKD, with filtrations of 60 mL/ min / 1.73 m2.46 PTH (together with alkaline phosphatase) is the molecule that best characterizes bone histomorphometry. Both low and high PTH values alter bone turnover and increase the risk of fracture.47–49 High PTH values correlate with loss of cortical bone, increasing the risk of fracture, especially in women patients in hemodialysis (HD).50
High PTH, in the presence of high or normal serum P, has a catabolic effect on bone,51 it is associated with loss of cortical rather than trabecular bone, and produces altered bone microarchitecture. It also contributes to the onset and progression of vascular calcification in uremic rats.34,52 In the presence of a high P intake, a low PTH, attenuates the deterioration of the cortical bone.53
Vitamin D and fracturesVitamin D is a hormone with a decisive role in bone formation and in maintaining BMC. The 25-OH vitamin D or serum calcidiol is the molecule that reflects the deposits of vitamin D, although it is only one of the different molecules that are substrate of the hormone activation. It is not well known what are the serum calcidiol levels necessary to reduce fractures.54 There is information about the BMC in CKD patients with different levels of vitamin D. The optimal level of 25-OHD for an appropriate bone turnover seems to be between 20 and 40 ng / mL. Levels below 20 ng / mL produce a defect of mineralization and bone formation, and levels above 40 ng / mL trigger a reduction in bone turnover,55 although their involvement in fractures was not evaluate. In fact, few studies have analyzed the relationship between low levels of calcidiol and fractures, and those that have found that low levels increase this risk.56 In a meta-analysis, no benefit was found in supplementing with vitamin D to reduce fractures in patients with CKD,57 although this is also a highly debated topic for the general population.
A recent study shows an increase in stress fractures associated with low concentrations of vitamin D in serum in a normal population.58
Phosphorus binders and fracture reductionFor years it has been known that in animal models calcium-free P binders improve bone remodeling stimulating bone formation.59 Lanthanum carbonate and sevelamer improve bone mineral content in dialysis patients with adynamic bone, probably due to the decrease in calcium intake and the consequent increase in PTH levels. It is likely that other binders (eg iron-based) may have the same effect, as long as there is no unwanted systemic accumulation of iron. Patients with moderate CKD treated with any type of binder improve bone mineral content as compared to untreated patients.60 However, there is still uncertainty regarding the real impact of phosphorus binders in relation to fractures due to the scarcity of controlled studies of phosphorus binders with placebo that include this item since the objective of the research has focused mainly on demonstrating the reduction of vascular calcification and cardiovascular mortality.61–63 However, recent studies have shown that in CKD patients and advanced CKD patients on dialysis the risk of fracture was 20% higher in those not taking P binders as compared to patients taking P binders.64
ConclusionsThe patient with kidney disease has an increased risk of fractures. The mechanisms responsible are the ones usually identified in the general population plus those associated with uremia, although the diagnosis of bone lesions responsible for the fractures continues to be a pending issue. Serum phosphorus seems to correlate with the risk of fracture, and it is known how adaptive mechanisms to control phosphate and increased P absorption are closely related to fractures. The increase in FGF23 and PTH and the decrease in vitamin D are responsible for bone fragility and increased fractures; sclerostin secreted by the bone also contributes to bone fragility. In the absence of studies that adequately analyze the influence of various treatments on the increased risk of fractures in patients with CKD, we suggest that phosphorus control should be an objective to take into consideration.
Conflict of interestsNone of the authors has a conflict of interest in this article.
Please cite this article as: González-Parra E, Bover J, Herrero J, Sánchez E, Molina P, Martin-Malo A et al. Control del fósforo y prevención de fracturas en el paciente renal. Nefrologia. 2021;41:7–14.