Despite the use of prevention strategies, cytomegalovirus (CMV) infection is the most common viral complication after renal transplant and its impact on long-term outcomes is still open to debate.
ObjectiveTo evaluate the incidence of CMV infection and disease during the use of prevention strategies in our centre and to analyse the association between CMV infection and long-term patient and graft survival and other potentially clinical events related with CMV.
MethodsWe reviewed the medical records of 377 recipients of kidney transplants performed between January 1998 and December 2008. Kaplain-Meier survival curve analysis was performed to analyse graft and patient survival by CMV infection/disease and Cox proportional hazards regression was used to identify factors associated with CMV infection/disease, graft loss and mortality.
ResultsThe incidence of CMV infection was 34.7% and CMV disease was 9.5%. Patient and graft survival was significantly lower in patients with CMV infection/disease. CMV infection/disease was associated with a higher risk of graft loss (HR 1.91, 95% CI 1.09–3.36, p=0.023), but not with a higher mortality (HR 1.29, 95% CI 0.7–2.38, p=0.4).
ConclusionCMV replication after renal transplant is a risk factor for long-term graft loss but not mortality. Prevention strategies decrease post-transplant CMV infection and disease.
A pesar del uso de estrategias de prevención y la mejora en los métodos diagnósticos, el citomegalovirus (CMV) continúa siendo la complicación viral más frecuente después del trasplante renal y su impacto en los resultados a largo plazo se sigue debatiendo.
ObjetivoConocer la incidencia de infección/enfermedad por CMV bajo estrategias de prevención y analizar su asociación con la supervivencia del paciente y del injerto y con otros eventos clínicos relacionados con el CMV.
MétodosRevisión de las historias clínicas de 377 pacientes trasplantados de riñón entre enero de 1998 y diciembre del 2008. Se analizó la supervivencia por el método de Kaplan–Meier en función de la presencia o ausencia de infección/enfermedad CMV y se usó el modelo de Cox para identificar factores asociados con infección/enfermedad por CMV y para evaluar su impacto en la mortalidad y la pérdida del injerto.
ResultadosLa incidencia de infección por CMV fue del 34,7% y de enfermedad del 9,5%. La supervivencia del paciente y del injerto fue significativamente inferior en los pacientes con infección/enfermedad CMV. La infección/enfermedad por CMV se asoció de forma significativa a mayor riesgo de pérdida del injerto (HR 1,91, IC del 95% 1,09-3,36, p=0,023) pero no con más riesgo de mortalidad (HR 1,29, IC del 95% 0,7-2,38, p=0,4).
ConclusiónLa replicación viral después del trasplante es un factor de riesgo de pérdida del injerto pero no de mortalidad a largo plazo. Las estrategias de prevención disminuyen la incidencia de infección y enfermedad por CMV postrasplante.
Cytomegalovirus (CMV) infection is a common complication in patients receiving a kidney transplant. It usually appears during first year after transplantation and; if it appears, it has direct and indirect effects on the patient and the graft, both short and long term.1 The direct effects are well known, they are associated with high rates of viral replication and they occur in the form of CMV infection/disease. However, indirect effects are more difficult to recognise and are caused by the interaction of low rates of viral replication with the immune system.2
In the period when viral prevention and monitoring strategies were not widely used, the incidence of CMV infection/disease was high (60% infection and 30% disease).3 Both donor and recipient CMV sero-pairing and the use of anti-lymphocyte antibodies were important risk factors for CMV disease.4 This served to identify the risk of infection of patients so they are classified as high, moderate or low risk of infection. This classification is still used to dictate the prevention strategy.5
Indirect effects have been associated with increased morbidity (opportunistic infections), graft loss and long-term mortality.6–13
The recent avenue of effective antiviral drugs in the control of CMV, such as ganciclovir and valganciclovir, improved diagnostic methods and the use of CMV prevention strategies (universal prophylaxis and early treatment) have represented an important milestone in improving transplant care and outcomes, reducing the risk of CMV infection/disease,6,14 the risk of acute rejection and the risk of mortality and long-term graft loss.6,7,12,15–17
This study aims not only to understand the cumulative incidence of CMV infection/disease in our setting, taking into account differences in prevention strategies over time, and to analyse whether there is a correlation between viral replication and patient and long-term graft survival, as well as other adverse events potentially related to CMV, such as cardiovascular disease, neoplasms and infections.
Patients and methodsStudy designAn observational, retrospective and single-centre study was conducted, in which CMV infection/disease after renal transplantation was the main endpoint of interest. Two cohorts of patients were created based on the existence or not of CMV infection/disease after transplantation (CMV group and non-CMV group, respectively). The results were analysed globally and according to the prevention strategy used (universal prophylaxis or preemptive therapy).
Study populationPatients receiving a kidney graft in our centre between January 1998 and December 2008. Patients who lost the kidney graft, died or were lost to follow-up less than 3 months after transplantation were excluded from the analysis (in none of these cases was the event secondary to CMV disease). Fig. 1 shows the distribution of cases in relation to prevention strategies and CMV replication.
Three hundred seventy-seven (377) patients were included, of whom 344 (91.2%) were first transplants and 33 (8.8%) were retransplants. Two hundred forty-four (64.7%) patients were male and 133 (35.3%) were female. The mean age ± standard deviation (SD) of recipients and donors at the time of transplantation was 48.6±13.4 years and 46.9±14.3 years, respectively. 93.9% (n=354) of the patients received cadaveric donor kidney grafts, 5% (n=19) from living related donors and 1.1% (n=4) from donors after cardiac death.
Patients were followed until death, lost to follow-up or the end of the study, December 2013. Patients who lost the graft (permanent return to dialysis) were followed up during the dialysis period until their death or until the end of the study in December 2013.
Data from donors and recipients were collected from the hospital's medical records. The selection of donors was consistent throughout the study period, and no histological study was performed on the kidney graft before transplantation.
The study was approved by the hospital's Independent Ethics Committee.
ImmunosuppressionThere were a 64.7% (n=244) of the patients receiving triple therapy with induction and 35.3% (n=133) triple therapy without induction.
Monoclonal antibodies were used for induction in 232 patients (95.1%) (ILR2: 181, OKT3: 51) and polyclonal antibodies in 12 patients (4.9%) (thymoglobulin in 8 and ATeGe in 4).
Triple therapy included a calcineurin inhibitor (cyclosporin in 98 patients and tacrolimus in 277), prednisone (n=377) and mycophenolate mofetil (MMF, n=358) or rapamycin (n=17) Two patients received rapamycin instead of calcineurin inhibitor and 2 patients received azathioprine instead of MMF.
Cytomegalovirus infection/disease: prevention, diagnosis and treatment strategiesPrevention strategiesUniversal prophylaxis was used in both seronegative and seropositive recipients who received induction therapy with OKT3 or with polyclonal antibodies. Before October 2003, antiviral prophylaxis was performed with ganciclovir (n=78 patients), and, after October 2003 with valganciclovir (n=43 patients). Prophylaxis lasted 3 months (mean±SD: 3.0±0.84 months).
Preemptive therapy was used in seropositive recipients that did not receive induction therapy with polyclonal antibodies or OKT3. These patients underwent virological monitoring using pp65 antigenaemia weekly during the first month, every two weeks during the 2nd and 3rd month, and monthly from the 4th to the 12th month. If viral replication was detected, treatment with ganciclovir (until October 2003, n=128) or valganciclovir (as of October 2003, n=128) was initiated.
Diagnosis: virological follow-up and diagnosis were performed by assessing pp65 antigenaemia. CMV replication was considered to be present if pp65 antigenaemia was >5 positive/2×105 polymorphonuclear cells. Patients with CMV infection had positive pp65 antigenaemia without symptoms, whereas patients with CMV disease had symptoms (viral syndrome or invasive disease) in addition to positive antigenaemia.
Treatment: in cases of CMV infection, most cases received antiviral treatment with ganciclovir (before October 2003) or valganciclovir (as of October 2003), adjusted for kidney function. In some cases, in addition to antiviral treatment, the MMF dose was reduced, while in others, only the MMF dose was reduced without administering antiviral drugs.
Severe cases of CMV disease were treated with IV ganciclovir (5mg/kg every 12hrs, 2–3 weeks), while the MMF dose was decreased, followed with oral ganciclovir or valganciclovir for another 2–3 weeks.
The antiviral treatment was usually discontinued after 2 consecutive negative pp65 antigenaemia tests.
Definition of other endpointsDelayed graft function: defined as the need for dialysis in the first week after transplantation.
Surgical complications in the first 6 months after transplantation: defined as bleeding requiring reintervention, vascular thrombosis, urinary fistula or lymphocele requiring surgical intervention, obstruction of the urinary tract by distal ureteral stenosis, etc.
Acute rejection: biopsy-proven rejections that occurred at any time after transplantation were considered for the analysis.
Clinical events after transplantation: the following clinical events were recorded: bacterial infections requiring hospitalisation after transplantation; neoplasms after transplantation; diabetes mellitus (defined as taking oral antidiabetics or insulin) after transplantation; and cardiovascular disease, ischaemic heart disease (acute myocardial infarction or angina), cerebral ischaemia (transient ischaemic or cerebrovascular accident) or peripheral artery disease (limb amputation or artery stent placement) after transplantation.
Statistical analysisDescriptions of quantitative endpoints were expressed as the mean±SD or as the median and interquartile range (IQR). The chi-squared test was used to study the correlation between qualitative endpoints, and the Student's t-test was used to compare means of quantitative endpoints.
Analysis of survivalAn analysis of patient and graft survival was performed using the Kaplan–Meier method. Survival curves were compared using the log-rank test. In the analysis of patient survival, the patient's death from any cause was considered an event, while “lost to follow-up” cases and cases in which the event did not occur at the end of the study were considered censored observations (31 December 2013). Patients who lost the graft were followed up until their death or the end of the study.
In the analysis of graft survival, graft loss from any cause—defined as the definitive return to dialysis—was considered an event, while “lost to follow-up” cases, cases in which the event did not occur at the end of the study (31 December 2013) and deaths with functioning graft were considered censored observations.
To investigate factors that could be potentially associated with the risk of CMV infection/disease, mortality and graft loss, a univariate and multivariate analysis was performed with proportional hazard regression models or Cox model. Endpoints with clinical effects and a value of p<0.2 in the univariate analysis were included in the multivariate model. It was considered significant when p<0.05. These analyses were performed in duplicate: first, considering CMV infection/disease as an aggregated endpoint (model 1) and, second, considering CMV infection/disease separately (model 2).
ResultsCytomegalovirus infection/diseaseA total of 377 patients were included (Fig. 1). The median follow-up time was 8 years (IQR 3.7 months-15.9 years).
The cumulative incidence of CMV infection in the first year was 34.7% (n=131) and of CMV disease (viral syndromes) 9.5% (n=36). The remaining patients (55.7%, n=210) did not have CMV replication after transplantation.
Table 1 shows the baseline demographic characteristics of recipients and donors based on the presence of CMV infection/disease.
Demographic characteristics of recipients and donors, and transplant data according to CMV replication after transplantation.
| Recipients | CMV (n=167) | Non-CMV (n=210) | p |
|---|---|---|---|
| Age, years (SD) | 50.5 (13.2) | 47 (13.2) | 0.006 |
| Men, n (%) | 107 (64.1) | 137 (65.2) | NS |
| CKD aetiology, n (%) | NS | ||
| Diabetes mellitus | 11 (6.8) | 8 (3.8) | |
| No diabetes mellitus | 156 (93.2) | 201 (96.2) | |
| Type of dialysis before Tx, n (%) | NS | ||
| HD | 97 (58.1) | 112 (53.3) | |
| PD | 68 (40.7) | 90 (42.9) | |
| No dialysis before Tx | 2 (1.2) | 8 (3.8) | |
| BMI, kg/m2(SD) | 24.9 (3.72) | 24.7 (4.50) | NS |
| Time on dialysis, months (SD) | 31 (30.4) | 36 (52.6) | NS |
| HTN before Tx, n (%) | 121 (73.8) | 154 (77.8) | NS |
| DM before Tx, n (%) | 17 (10.2) | 14 (7.1) | NS |
| Dyslipidaemia before Tx, n (%) | 59 (37.3) | 73 (38) | NS |
| Donors | CMV (n=167) | Non-CMV (n=210) | p |
|---|---|---|---|
| Age, years (SD) | 49.4 (13.3) | 45 (14.7) | 0.003 |
| Men, n (%) | 100 (60.2) | 131 (63.9) | NS |
| Transplant data | CMV (n=167) | Non-CMV (n=210) | p |
|---|---|---|---|
| HLA-DR incompatibilities, n (%) | NS | ||
| 0 incompatibilities | 48 (28.9) | 58 (28.3) | |
| 1 incompatibility | 88 (53) | 116 (56.6) | |
| 2 incompatibilities | 30 (18) | 31 (15.1) | |
| Cold ischaemia time, hr (SD) | 15.3 (6.3) | 15 (6.6) | NS |
| Warm ischaemia time, min (SD) | 52.5 (18.7) | 52.4 (16.3) | NS |
| Induction IS, n (%) | NS | ||
| Monoclonal antibodies (anti-CD25) | 80 (47.9) | 101 (48.1) | |
| Polyclonal antibodies (OKT3, thymoglobulin, ATeGe) | 22 (13.2) | 41 (19.6) | |
| No induction | 65 (38.9) | 68 (32.4) | |
| Maintenance IS, n (%) | NS | ||
| Tacrolimus | 114 (68.3) | 163 (77.6) | |
| Cyclosporin A | 51 (30.5) | 47 (22.4) | |
| Rapamycin | 2 (1.2) | – | |
| DGF, n (%) | 32 (20.8) | 27 (12.9) | NS |
| Acute rejection by biopsy, n (%) | 35 (21) | 33 (15.7) | NS |
| Surgical complications, n (%) | 33 (19.9) | 35 (16.7) | NS |
SD: standard deviation; DM: diabetes mellitus; PD: peritoneal dialysis; CKD: chronic kidney disease; HD: haemodialysis; HTN: hypertension; BMI: body mass index; IS: immunosuppression; DGF: delayed graft function (need for dialysis in the first week); Tx: transplantation.
Table 2 shows the cumulative incidence of CMV infection/disease and the incidence of viral non-replication, taking into account the prevention strategy and antiviral drug used. CMV infection (n=131) occurred on average at 2.4±1.9 months after transplantation (IQR 0.5–12 months) and CMV disease (n=36) at 2 months (IQR 1–36 months) after transplantation.
Cumulative incidence of CMV infection/disease in the first year.
| Prophylaxis with ganciclovir (n=78) | Prophylaxis with valganciclovir (n=43) | Preemptive therapy with ganciclovir (n=128) | Preemptive therapy with valganciclovir (n=128) | |
|---|---|---|---|---|
| CMV infection, n (%) | 23 (29.5) | 14 (32.5) | 44 (34.4) | 54 (42.2) |
| CMV disease, n (%) | 12 (15.4) | 3 (7.0) | 14 (10.9) | 7 (5.5) |
| No viral replication, n (%) | 43 (55.1) | 26 (60.5) | 70 (54.7) | 67 (52.3) |
The predictive factors of CMV infection/disease are shown in Table 3.
Analysis of risk factors for CMV infection/disease.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Endpoint | HR | 95% CI | p | HR | 95% CI | p |
| Recipient's age (years) | 1.01 | 1.01–1.03 | 0.00 | 1.02 | 1.008–1.03 | 0.00 |
| CMV D+/R− | 2.27 | 1.19–4.32 | 0.01 | 2.46 | 1.28–4.73 | 0.00 |
| CMV D+/R+ | 2.04 | 1.17–3.55 | 0.01 | 1.89 | 1.08–3.29 | 0.02 |
| Diabetes mellitus | 1.31 | 0.78–2.21 | 0.29 | |||
| Recipient's BMI (kg/m2) | 1.01 | 0.97–1.05 | 0.46 | |||
| Donor type (living vs. cadaveric) | 0.75 | 0.35–1.61 | 0.46 | |||
| Treatment IS (Tacro vs. CyA) | 0.64 | 0.46–0.89 | 0.00 | 1.51 | 1.08–2.09 | 0.01 |
| Time on dialysis before Tx (months) | 0.99 | 0.99–1.00 | 0.34 | |||
| Acute rejection (by biopsy) | 11.34 | 0.95–1.90 | 0.09 | |||
Patients with CMV infection/disease had a greater tendency to develop cardiovascular disease after transplantation (CMV group: n=33 [19.8%] vs. non-CMV group: n=24 [11.6%], p=0.06) and diabetes mellitus after transplantation (CMV group: n=47 [28.3%] vs. non-CMV group: n=41 [19.8%], p=0.05). However, no statistically significant differences were found with bacterial infections and neoplasms after transplantation.
Twenty-one per cent (21%; n=35) of patients with CMV infection/disease had acute rejection, confirmed by renal biopsy, vs. a 15.7% (n=33) in patients without CMV infection/disease (p=0.18).
Patient survival and predictors of overall mortalitySixty patients (15.9%) died during the follow-up period: 44 patients died with a functioning graft and 16 patients after graft loss while being on dialysis.
Thirty-three patients (8.7%) had CMV infection and twenty-seven (7.2%) had no viral replication. The causes of death were similar in both groups: 6 patients in the CMV group and 7 in the non-CMV group died of cardiovascular causes; 6 patients in the CMV group and 2 in the non-CMV group died of infection; and 6 patients in the CMV group and 9 in the non-CMV group died of neoplastic disease. Five (5) patients in the CMV group and 3 in the non-CMV group died of other causes.
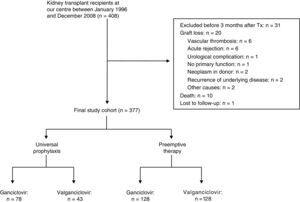
Long-term patient survival is shown in Fig. 2 which was significantly superior in patients without CMV infection/disease (CMV group: 64.2% vs. non-CMV group: 76.9% at 15.8 years, p=0.019). Considering prevention strategies, survival was significantly lower only in patients who received universal prophylaxis and who had CMV infection/disease (57.2% CMV group+universal prophylaxis vs. 81% non-CMV group+universal prophylaxis at 14 years, p=0.03) (Fig. 2b). There were no statistically significant differences in survival in patients who received preemptive therapy whether they had CMV infection/disease or not (67.7% CMV group+early treatment vs. 72.6% non-CMV group+early treatment at 15.3 years, p=0.178).
Patient survival according to the presence or absence of CMV infection/disease. Patient survival in the overall study cohort was higher in patients without CMV infection/disease (a) and in those receiving universal prophylaxis (b). Patients with preemptive therapy had similar patient survival in both groups (c).
The analysis of factors associated with the risk of mortality is shown in Table 4.
Analysis of risk factors for long-term mortality.
| Model 1 | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Endpoint | HR | 95% CI | p | HR | 95% CI | p |
| CMV infection+disease | 1.74 | 1.04–2.89 | 0.03 | 1.29 | 0.70–2.38 | 0.40 |
| Recipient's age (years) | 1.07 | 1.05–1.10 | 0.00 | 1.09 | 1.05–1.13 | 0.00 |
| Diabetes mellitus | 3.99 | 2.09–7.58 | 0.00 | 1.38 | 0.53–3.58 | 0.50 |
| Recipient's BMI | 1.14 | 1.07–1.21 | 0.00 | 1.08 | 1.01–1.17 | 0.02 |
| Donor's age (years) | 1.03 | 1.01–1.04 | 0.00 | 0.98 | 0.96–1.01 | 0.30 |
| Cold ischaemia time (hr) | 1.04 | 1.00–1.08 | 0.04 | 1.07 | 1.02–1.11 | 0.00 |
| Initial graft function | 0.58 | 0.34–0.97 | 0.04 | 1.03 | 0.50–2.10 | 0.93 |
| Cardiovascular disease | 1.69 | 0.95–3.01 | 0.07 | 0.93 | 0.46–1.89 | 0.84 |
| Graft loss | 2.85 | 1.60–5.07 | 0.00 | 2.78 | 1.30–5.92 | 0.00 |
| Model 2 | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Endpoint | HR | 95% CI | p | HR | 95% CI | p |
| CMV disease | 2.53 | 1.25–5.11 | 0.00 | 1.26 | 0.52–3.04 | 0.59 |
| CMV infection | 1.60 | 0.91–2.82 | 0.10 | 1.26 | 0.64–2.49 | 0.49 |
| Recipient's age (years) | 1.07 | 1.05–1.10 | 0.00 | 1.09 | 1.05–1.13 | 0.00 |
| Diabetes mellitus | 3.99 | 2.09–7.58 | 0.00 | |||
| Recipient's BMI | 1.14 | 1.07–1.21 | 0.00 | 1.08 | 1.01–1.17 | 0.03 |
| Donor's age (years) | 1.03 | 1.01–1.04 | 0.00 | |||
| Cold ischaemia time (hr) | 1.04 | 1.00–1.08 | 0.04 | 1.01 | 0.95–1.07 | 1.01 |
| Initial graft function | 0.58 | 0.34–0.97 | 0.04 | 1.05 | 0.52–2.15 | 0.87 |
| Cardiovascular disease | 1.69 | 0.95–3.01 | 0.07 | 1.08 | 0.52–2.22 | 0.83 |
| Graft loss | 2.85 | 1.60–5.07 | 0.00 | 3.00 | 1.42–6.35 | 0.00 |
The lost of kidney graft was more frequent among patients with CMV infection/disease than patients without CMV: 30 (18%) vs. 26 (12.4%), respectively. The causes of graft loss were similar in both groups. In the CMV group, graft loss was due to acute rejection in 4 cases, chronic rejection in 21 cases, recurrence of underlying disease in 1 case and other causes in 4 cases. In the non-CMV group, the causes were acute rejection in 2 cases, chronic rejection in 18 cases, recurrence of underlying disease in 2 cases and other causes in 4 cases.
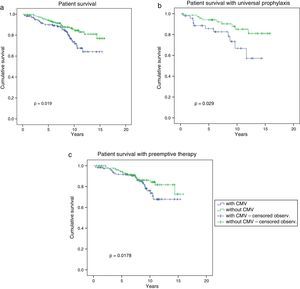
Long-term graft survival is shown in Fig. 3, which was significantly better in patients without CMV infection/disease (CMV group: 68% vs. non-CMV group: 74.1% at 15.8 years, p=0.034). Considering the prevention strategy used (Fig. 3b), we found that graft survival was significantly lower only in patients with CMV infection/disease who received universal prophylaxis (36.6% CMV group+universal prophylaxis vs. 77.2% non-CMV group+universal prophylaxis at 14.3 years, p=0.001). There was no statistically significant difference in graft survival between patients with and without CMV infection/disease receiving preemptive therapy (82.6% CMV group+early treatment vs. 55.2% non-CMV group+early treatment, p=0.84).
Graft survival according to the presence or absence of CMV infection/disease. Graft survival in the overall study cohort was higher in patients without CMV infection/disease (a) and in those receiving universal prophylaxis (b). Patients with preemptive therapy had similar graft survival in both groups (c).
The analysis of possible factors associated with the risk of graft loss is shown in Table 5.
Analysis of risk factors for long-term graft loss.
| Model 1 | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Endpoint | HR | 95% CI | p | HR | 95% CI | p |
| CMV infection+disease | 1.80 | 1.06–3.06 | 0.03 | 1.92 | 1.03–3.36 | 0.02 |
| Recipient's age (years) | 0.96 | 0.94–0.98 | 0.00 | 0.95 | 0.93–0.98 | 0.00 |
| Diabetes mellitus | 1.68 | 0.66–4.27 | 0.27 | |||
| Recipient's BMI | 1.03 | 0.96–1.11 | 0.36 | |||
| Donor's age (years) | 1.01 | 0.99–1.03 | 0.10 | |||
| Cold ischaemia time (hr) | 1.04 | 1.00–1.09 | 0.03 | 1.05 | 1.00–1.09 | 0.05 |
| Delayed graft function | 2.03 | 1.20–3.44 | 0.00 | 1.54 | 0.83–2.84 | 0.16 |
| Acute rejection (by biopsy) | 1.80 | 1.03–3.13 | 0.04 | 2.44 | 1.33–4.47 | 0.00 |
| Universal prophylaxis | 2.80 | 1.64–4.76 | 0.00 | 1.85 | 1.03–3.34 | 0.04 |
| Surgical complications | 1.80 | 1.06–3.06 | 0.03 | 2.17 | 1.21–3.91 | 0.01 |
| Model 2 | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Endpoint | HR | 95% CI | p | HR | 95% CI | p |
| CMV disease | 2.70 | 1.33–5.48 | 0.00 | 2.60 | 1.21–5.85 | 0.01 |
| CMV infection | 1.46 | 0.80–2.65 | 0.21 | 1.73 | 0.91–3.29 | 0.09 |
| Recipient's age (years) | 0.96 | 0.94–0.98 | 0.00 | 0.96 | 0.93–0.98 | 0.00 |
| Cold ischaemia time | 1.04 | 1.00–1.09 | 0.03 | 1.05 | 1.01–1.10 | 0.01 |
| Acute rejection (by biopsy) | 1.80 | 1.03–3.13 | 0.04 | 2.57 | 1.38–4.77 | 0.00 |
| Universal prophylaxis | 2.80 | 1.64–4.76 | 0.00 | 1.77 | 0.98–3.20 | 0.05 |
| Surgical complications | 1.80 | 1.06–3.06 | 0.03 | 2.17 | 1.19–3.94 | 0.01 |
The cumulative incidence of CMV infection in the first year after transplantation was 34.7% and of CMV disease 9.5%. Our data are consistent with the observations of other groups that use prevention strategies6,14 and reflect a significant decrease in the incidence of CMV infection/disease with the use of such strategies. There are however differences between the prevention strategies used in our study and in those by other authors.18–21 High-risk patients who received universal prophylaxis had a higher rate of CMV disease than patients with preemptive therapy. This was facilitated by the absence of CMV-specific cellular immunity, which adequately protects patients against viral reactivation. By contrast, patients with early treatment had higher rates of CMV infection than patients with universal prophylaxis, a fact that is inherent in the strategy itself.
The change from ganciclovir to valganciclovir decreased the incidence of disease, which demonstrates the efficacy of valganciclovir in preventing CMV disease. This efficacy was already reported by Paya et al.22 and is attributed to the increased degree of exposure to ganciclovir achieved with valganciclovir (approximately 1.7 times greater). However, a stratified Cox analysis by period of time showed that the effect of the drug used at each time period was not different in terms of overall patient or graft survival (data not shown).
The main result of our study is the significant correlation between the presence of CMV infection/disease in the first months after transplantation and long-term graft loss, with a median follow-up of 8 years.
The multivariate analysis showed that patients with CMV infection were 1.7 times more likely to lose the graft than patients without CMV infection, and that patients with CMV disease were 2.6 times more likely to lose the graft than those who did not have CMV disease. Other authors have also shown this negative effect of viral replication on graft survival.17,23–26
However, the greater risk of graft loss may change according to the prevention strategy used. A greater risk of graft loss is associated with viral replication after transplantation, if previous universal prophylaxis is not effective, resulting in CMV infection/disease. This shows that the deleterious effect of long-term CMV on the graft is maximum in high-risk patients.
Other studies, including meta-analyses, have found no correlation between CMV replication and graft survival,21,27–29 possibly because the follow-up period of these studies was much shorter than ours—the importance of long-term survival is undisputed when it comes to assessing the success of transplantation.
CMV infection/disease decreased patient survival. However, there was no correlation with long-term mortality, which is similar to observations in other studies.29–31 For years there has been data suggesting of a possible correlation between CMV and cardiovascular disease.32,33 There was not a significant association between CMV and cardiovascular events, although there was a higher incidence of cardiovascular events after transplantation in patients with CMV infection/disease.
Activation of CMV after transplantation results in graft and patient injury which increases the risk of long-term graft loss. Any attempts prevent CMV will help to improve long-term outcomes. The first milestone in controlling and managing this complication was the emergence of potent antiviral drugs and the use of prevention strategies. To this day, these are the cornerstones of CMV prevention and should be used. Nevertheless, they are not sufficient to prevent viral replication. During the past two decades, it has become clear that both innate and CMV-specific immunity play a crucial role in the control of CMV,. Furthermore, monitoring for CMV is an important advance in predicting which patients are at high risk of developing CMV infection/disease after transplantation.34–36 Therefore, immunological monitoring before transplantation in moderate-risk patients and before completing prophylaxis in high-risk patients could be useful in controlling CMV after transplantation.
Moreover, based on the data from this study, as well as others, patients with CMV infection/disease should be considered as patients with an increased risk of graft loss and mortality. Their general condition and kidney function should therefore be closely monitored, increasing the number of warnings given to these patients on adherence to immunosuppressive treatment and monitoring their cardiovascular health more closely. This may help to improve long-term survival.
Our study has some limitations: first, its single-centre nature which, although it may favour uniformity of action, limits the extrapolation of results. This does not reduce the validity of the results, but their interpretation requires consideration of local conditions and circumstances. Second, its retrospective nature only allows us to speculate on potential reasons that explain the differences observed between the 2 groups. Nevertheless, the study is valuable in terms of the long follow-up period (median of 8 years of follow-up), which is fundamental when analysing the success of transplantation, and the uniformity of the medical team that treated the patients.
In summary, our study shows that viral replication after transplantation decreases graft and patient survival, acting as a risk factor for graft loss but not for long-term mortality. Close clinical monitoring of patients with CMV infection/disease would help to reduce the long-term damage caused by the virus on the graft and the patient. The study also confirms that in the Spanish population, CMV prevention strategies reduce the incidence of CMV infection/disease after transplantation, although there is still a non-negligible percentage of viral replication that needs to be controlled. Immunological monitoring is a useful tool that may help to control CMV after transplantation, although prospective studies are needed to confirm this.
AuthorshipMaría O. López-Oliva, Rafael Selgas, Teresa Bellón and Carlos Jiménez participated in the study design. María O. López-Oliva, Julio Flores and Carlos Jiménez collected the data from the patients’ medical records. Rosario Madero performed the statistical analysis of the data. Mª José Santana and Fernando Escuin treated the patients included in the study, while María O. López-Oliva and Carlos Jiménez are responsible for writing the manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: López-Oliva MO, Flores J, Madero R, Escuin F, Santana MJ, Bellón T, et al. La infección por citomegalovirus postrasplante renal y pérdida del injerto a largo plazo. Nefrologia. 2017;37:515–525.