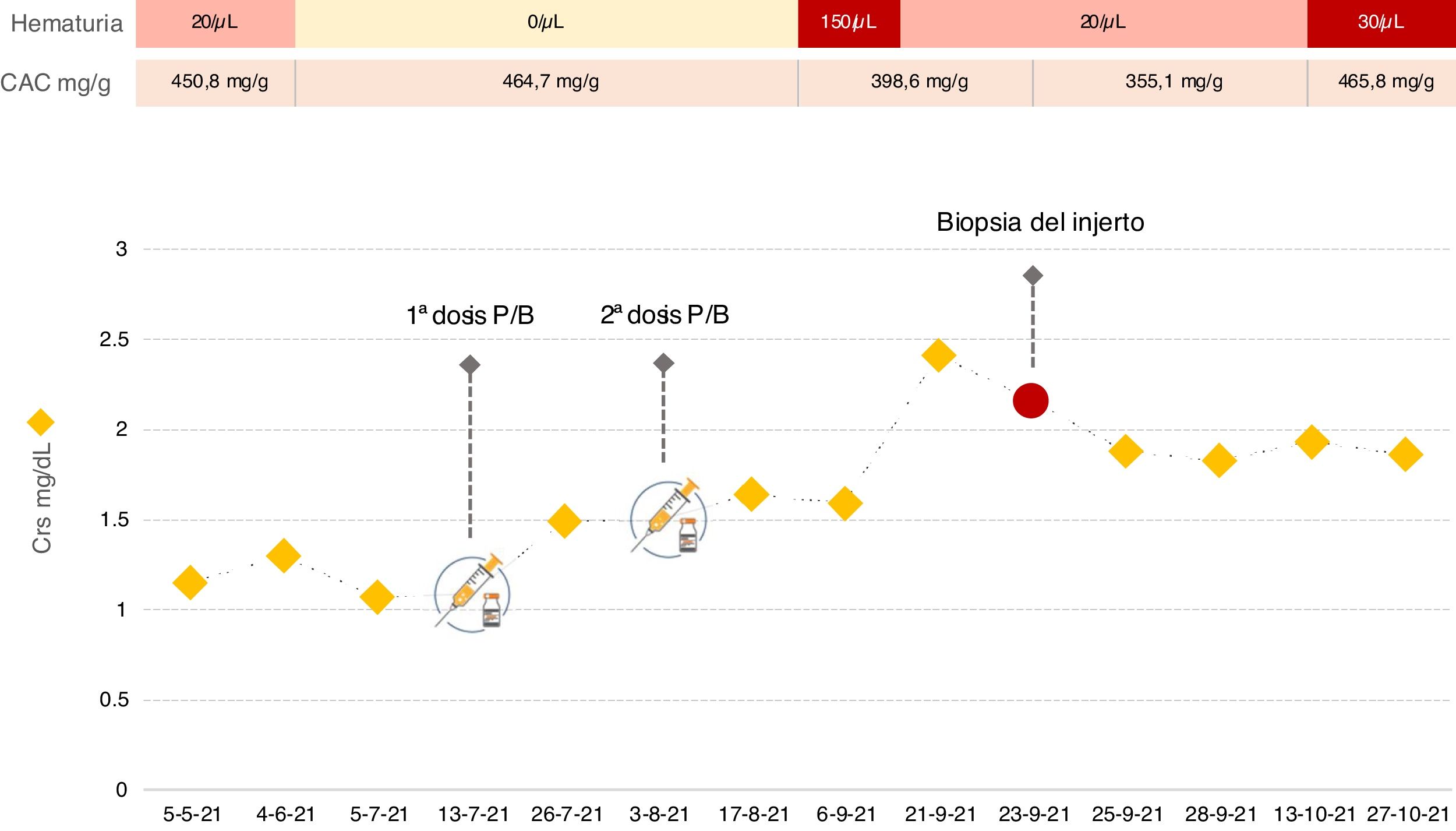
We present the case of a 30-year-old male diagnosed with membranoproliferative glomerulonephritis type 1 in 2011 and progression to end-stage chronic kidney disease. In 2019, he received a kidney transplant (KT), maintaining stable kidney function ever since, with baseline serum creatinine (sCr) around 1.1mg/dl and albumin/creatinine ratio (ACR) in urine of approximately 450mg/g, without any other urinary sediment abnormalities. Immunosuppressive treatment included tacrolimus, mycophenolate mofetil and steroids. In April 2021, due to the development of condyloma acuminata, mycophenolate was replaced by everolimus. On 13/07/2021, he received the first dose of the Pfizer-BioNTech COVID-19 vaccine, with no documented adverse effects. Thirteen days later, in routine blood tests, a deterioration of renal function was found, with sCr 1.5mg/dl. On 03/08/2021, he received the second dose and the deterioration in renal function progressed to sCr 2.4mg/dl. Simultaneously, the patient developed microhaematuria, with stable proteinuria (Fig. 1).
In view of the deterioration in kidney function, we performed tests. Donor-specific anti-HLA antibodies were negative. Determination of autoantibodies, proteins and complement were requested, which showed no abnormalities. Both cytomegalovirus and BK virus in blood were undetectable by PCR testing. Anti-SARS-CoV-2-S IgG antibodies and COVID-19 PCR were negative. In view of the microhaematuria, urine cytology was requested, which showed no abnormalities. Ultrasound of the graft also showed no ectasia of the urinary tract or abnormalities in vascularisation. For all these reasons, it was decided to perform a graft biopsy. On light microscopy, two out of nine glomeruli showed mesangial hypercellularity. Immunofluorescence study revealed intense granular mesangial IgA deposits (4+, on a scale of 0–4). Peritubular staining for C4d and SV-40 were negative and there were no findings consistent with acute rejection. Based on histological data, the patient was diagnosed with IgA nephropathy (IgAN). Although experience is limited in the treatment of IgAN in KT, given the deterioration in the patient's renal function, it was decided to start oral prednisone at a dose of 1mg/kg/day. After a month of follow-up, there has been no improvement.
We present the first case of de novo IgAN confirmed by pathology study in a kidney transplant recipient after vaccination against SARS-CoV-2. The COVID-19 vaccine has been linked to the development of glomerular disease, and IgAN is one of the most common histological findings.1–4 Cases of IgAN have also been described after recombinant Zoster or influenza vaccination.5,6 The pathophysiological mechanism is not fully understood, but it is probably due to an aberrant immune response of the spike protein or messenger RNA of SARS-CoV-2 in predisposed individuals.1 To date, there have been 15 published cases of IgAN after vaccination against SARS-CoV-2 in the general population. Ten are de novo diagnoses confirmed by biopsy and the remaining five were considered recurrences due to a flare-up of macroscopic haematuria in previously diagnosed patients.1–4 In KT, a single case of recurrence has been reported in a patient with IgAN as the underlying kidney disease and who developed haematuria after vaccination, although no graft biopsy was performed.4
IgAN can recur in KT, with an incidence of 20–53% and with a mean time of onset of seven years. Although rare, IgAN can also develop de novo in KT.7,8 Our patient developed this complication early after vaccination. Although a coincidental association cannot be ruled out, the temporal relationship seems to suggest causality.9 Moreover, he was a young man, on treatment with everolimus and HLA A2 and DR1, all of which are risk factors for IgAN in KT.10 In the case described, the vaccine could have been another stimulus for the development of the condition.
Almost all reported cases of IgAN associated with vaccination against SARS-CoV-2 occurred in young patients and between weeks one and three after receiving the vaccine.1–4 The main clinical manifestation was macroscopic haematuria. Most of the cases occurred after the second dose, but in the case of relapse in KT mentioned above, its onset was a few days after the first dose. In our patient, the deterioration in kidney function and the development of microhaematuria also began early, which may suggest that we need to be alert to the possibility of early development in KT, especially in patients at-risk.2,4,10
To date, 843 KT recipients at our centre have received at least two doses of the SARS-CoV-2 vaccine without having identified any more cases of IgAN (incidence 0.1% in our experience), which suggests that this is a rare event. However, although the follow-up of our patient has been short, this complication must be taken into account, as it could have serious consequences on the kidney graft function.
Conflicts of interestThis study received no specific funding from public, private or non-profit organisations.