Objective: The risk for tuberculosis (TB) is increased in patients with chronic renal failure and dialysis. Tuberculin skin test (TST) is the classical diagnostic method for screening despite its low sensitivity. New methods based on interferon-gamma have been developed. The aim of this study was to evaluate if Quantiferon® TB-gold In Tube (QFT-GIT) could be useful in the diagnosis of TB infection in patients on peritoneal dialysis (PD). Patients and methods: Fifty-four patients on PD were included in the study. They were evaluated for latent tuberculosis with QFT-GIT, TST and an assessment by an expert pulmonologist using patient’s medical history and x-rays. Agreement between test results was determined. Results:The prevalence of a positive TST was 29.6% for the first test and 31.5% for the second (booster effect). A positive chest x-ray increased the rate of detection of patients with latent TB infection up to 42.6% and the expert physician’s evaluation to 44.4%. The correlation between QFT-GIT and TST was fair (k=0.36; P=.006), as it was between TST and expert physician’s evaluation (k=0.257; P=.06). Conclusions: According to our experience QFT-GIT represents an important advantage in the diagnosis of latent TB infection in chronic renal failure patients on PD. It may complement but not replace TST.
Introducción: El riesgo de tuberculosis (TB) está aumentado en pacientes con insuficiencia renal crónica y en diálisis. La prueba de la tuberculina (PT) es el test de cribado clásico en estos pacientes, a pesar de su baja sensibilidad. En los últimos años se han desarrollado nuevos métodos diagnósticos que se basan en la producción de interferón gamma tras la estimulación con antígenos de M. tuberculosis. El objetivo de este estudio fue evaluar si el Quantiferon® TB-gold In Tube (QFT-GIT) puede contribuir en el diagnóstico de la infección tuberculosa en pacientes en diálisis peritoneal (DP). Pacientes y métodos: Se incluyeron 54 pacientes en DP. Se valoró la posibilidad de infección tuberculosa latente mediante el QFT-GIT, la PT y la valoración clinicorradiológica por parte de un neumólogo experto. Se estudiaron las concordancias entre los tests. Resultados: La prevalencia de un resultado positivo para el test de la tuberculina fue del 29,6% para el primer test y del 31,5% para el segundo (valorando el efecto booster). Una radiografía de tórax positiva aumentaba la detección de infección tuberculosa latente hasta un 42,6% y la del neumólogo hasta un 44,4%. El nivel de correlación entre el QFT-GIT y la PT fue moderado (kappa = 0,36; p = 0,006), al igual que entre la PT y la valoración del neumólogo (kappa = 0,257, p = 0,06). Conclusiones: El QFT-GIT aporta algunas ventajas en el diagnóstico de la infección tuberculosa en pacientes con insuficiencia renal crónica en DP, y puede complementar a la prueba de la tuberculina.
INTRODUCTION
The prevalence of tuberculosis (TB) in Spain varies depending on the region. The incidence of tuberculosis has dropped in Spain over the last decade; however, it is important to know that, even in areas of low prevalence, the occurrence of TB in immunocompromised patients is an important cause of morbidity and mortality.1
Uraemia is known to be associated with a large number of immune system disorders, most of them linked to impaired cellular immunity.2 Latent tuberculosis is characterised by a strong cellular immune response in the absence of detectable mycobacteria. As this infection is controlled by the cellular immune response, impairment in cellular immunity may lead to the reactivation of latent tuberculosis infection.1 At present the skin sensitivity to the tuberculin is the method used to detect latent tuberculosis infection. The response to this antigen depends on the infectious load and the condition of the individual’s cellular immunity. It has low sensitivity and specificity. Furthermore, in uraemic patients the delayed immune response to skin tests is reduced3 as well as the macrophage function.2 This may cause cutaneous anergy and alter the response to the tuberculin skin test (TST). For this reason, TST is not routinely performed on dialysis patients.
Given the fact that there is no gold standard test to diagnose TB, some groups have studied tests that use Interferon-Gamma Release Assays (IGRA). They have compared these with TST in order to determine their sensitivity and specificity in different subgroups of the population.1,4-6 The different regulations concerning the use of IGRA depend on each country. For example, the CDC (Center for Disease Control) in the USA recommends replacing TST with IGRA in all cases; while in the United Kingdom, the National Institute of Health and Clinical Excellence (NICE) recommends the use of IGRA in combination with TST only when the tuberculin skin test was positive. Other countries such as France or Canada have adopted these recommendations. However, they are based on cost-effectiveness studies which compare TST with one of the IGRA (QFT-GIT or T-SPOT.TB) available on the market. In a recently published study, Pooran et al7 compared the two types of IGRA. They reached the conclusion that it was cheaper to use a regimen combining TST/IGRA rather than using T-SPOT.TB or QFT-GIT or TST on their own for contact tracing. While T-SPOT.TB and QFT-GIT on their own prevent more cases of active TB, they do not better the lower cost of the combined use of TST/IGRA. However, these conclusions depend largely on the population studied.
The aim of our study was to compare QFT-GIT with the TST as a screening method to detect latent tuberculosis in patients on peritoneal dialysis (PD).
PATIENTS AND METHODS
Patients
The study included patients with chronic renal failure (CRF) on PD from the Marqués de Valdecilla Hospital in Santander and Asturias Central Hospital in Oviedo. They had no signs or symptoms of active or extrapulmonary tuberculosis (between December 2007 and July 2008). They also had to accept to take part in the study.
The TST and the QFT-GIT were performed on all patients included in the study. Patients with a high risk of latent tuberculosis were taken to be any patient living in a TB endemic area, any patient that claimed to have been in contact with people infected with TB or any patient with previous history of TB and any patient who had a chest x-ray that was compatible with a previous TB infection.
Tuberculin skin test
The TST was performed by TB specialist nurses. All patients were administered 2 IU Rt-23 PPD on the inside of their forearm. The results were assessed after 72 hours in accordance with the established regulations. The test was considered positive with an induration of ≥5mm. In patients that had been previously vaccinated with BCG, the TST was considered to be positive with an induration of ≥10mm (except in patients with previous contact with tuberculosis, chest x-ray suggestive of TB, infected by the human immunodeficiency virus [HIV] or diagnosed with silicosis). The test was repeated 10 days later in patients that had no induration on the first test to rule out a possible booster effect.
Quantiferon® - TB Gold In Tube
The QFT-GIT test (Cellestis, Carnegie, Victoria, Australia) was performed according to the manufacturer’s instructions. The blood samples were processed 6 to 8 hours after being extracted. The blood was put into 3 different tubes: one did not contain antigens (control), the second tube contained TB antigens and the third contained phytohaemagglutinin (mitogen or positive control). The incubation time was 18-24h at 37ºC.
The results were considered positive, negative or inconclusive according to the criteria established in the manufacturer’s software. TST and QFT-GIT were performed on the same day.
Pulmonologist’s assessment
Two pulmonologists who are experts in TB (one in each hospital) assessed the risk factors for latent tuberculosis, including medical history of active TB or contact with an active case, vaccination, or born in a TB endemic area. The results of TST and chest x-ray were also assessed. All the data was assessed to determine whether the patient had been previously infected with M. tuberculosis.
Statistical analysis
The data analysis was performed with the statistical software SPSS (SPSS version 12.0, Chicago, IL) and P values less than 0.05 were considered significant. Cohen’s kappa coefficient was used to calculate the level of agreement between the two tests (TST and QFT-GIT). The following criteria were used to interpret the results (according to Landis and Koch): kappa below 0.00 poor, 0.00-0.20 slight, 0.21-0.40 fair, 0.41-0.60 moderate, 0.61-0.80 substantial and 0.81-1.00 almost perfect.
RESULTS
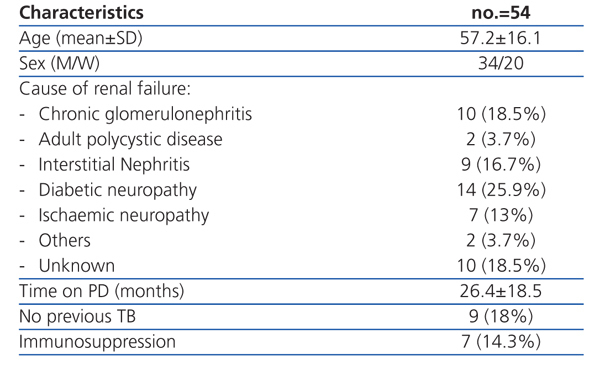
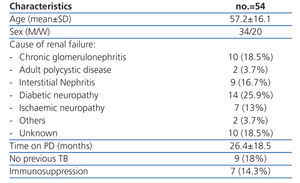
Characteristics of the population studied (Table 1)
A total of 54 patients were included between December 2007 and July 2008. All the patients were negative for HIV. Eight had serum albumin levels below 3.20mg/dl. The majority (31; 57.4%) had not had the BCG vaccine. Ten of the patients had a medical history of TB, two of them had not been treated correctly and one suffered a relapse of tuberculosis. No cases of primary infection or reactivation of tuberculosis were detected during the follow-up.
TST, QFT-GIT and pulmonologist’s assessment
TST had a 29.6% prevalence of positives (16 patients) for the first test and 31.5% (17 patients) for the second one. TST had 5.8% of false positives and three false negatives (8.1%).
A positive chest x-ray detected 6 additional cases of latent TB (42.6%) and the pulmonologist’s assessment detected 7 additional cases (44.4%). The pulmonologist found evidence of previous tuberculosis infection in 14 cases (26.5%).
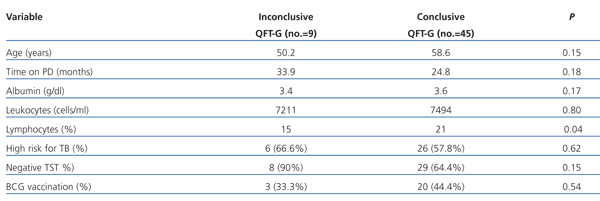
There were 10 positive cases (18.5%), 34 negative (62.96%) and 10 inconclusive results in the QFT-GIT. Nine (26%) of the negatives had a medical history of TB, a chest x-ray or a positive pulmonologist’s assessment. Five of the inconclusive cases were re-evaluated (1 stayed negative and the other 4 inconclusive). Of the 9 inconclusive results, 1 patient had a positive TST and the others had an induration of 0mm. Three of the patients had a low risk for TB and 6 had a high risk. The factors associated with an inconclusive QFT-GIT are shown in Table 2.
High-risk patients compared with low-risk patients
A total of 32 patients (59%) were at high risk and the other 22 (41%) were at low risk of latent tuberculosis. Neither of the two tests (TST/QIT-GIT) was able to distinguish between the high-risk and low-risk patients.
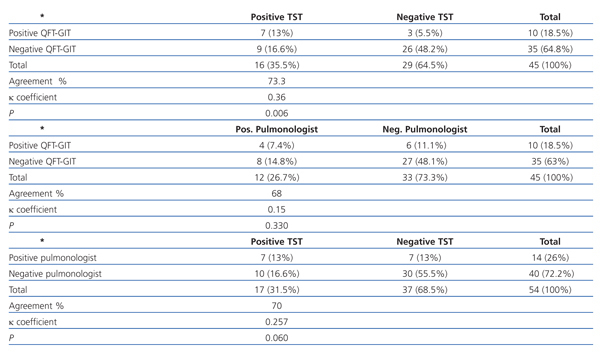
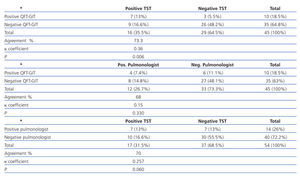
Agreement between the TST, QFT-GIT and the pulmonologist’s assessment
The agreement between the three diagnosis methods is shown in Table 3. A posterior analysis between the conflicting tests showed that QFT-GIT was positive in 3 patients that were negative for tuberculin and was negative in 9 patients that were positive for tuberculin. The TST was positive in 10 patients who were found to be positive by the pulmonologist and negative in 7 patients considered positive for TB by the pulmonologist.
DISCUSSION
As there is no gold standard diagnosis technique for latent tuberculosis, several authors have compared the use of IGRA with TST in different groups of patients (general population, children, hospitalised patients at-risk and immunocompromised patients).1,4-5 TST has been used routinely despite its low specificity, high number of false positives (previous vaccination with BCG or infection by non-tuberculosis mycobacteria), and low sensitivity (high number of false negatives in immunocompromised patients and patients with cutaneous anergy). Woeltje et al studied 307 patients on HD, 32% had cutaneous anergy to three different allergens and 9% of the patients without anergy tested positive for TST.8 In our study, the positive response to TST was similar to that described for patients on HD.2 The finding of 6 TST-negative patients with positive chest x-ray can be explained by the rate of cutaneous anergy described above in HD patients. Immune system impairment has been described in uraemic patients leading to a high rate of infections and mortality.9 This delayed immune response leads to interleukin-2 deficit, B-cell lymphopenia, increased cell apoptosis, impaired T-lymphocyte activation and more antigen-presenting cells.10-15 All these changes in the host’s responses may explain the negative results for TST, regardless of whether they had cutaneous anergy or not.
The use of IGRA in HD patients has been researched with similar results to ours. One of these studies was specifically designed to compare TST with QFT-GIT in 203 patients on HD and found a reasonable correlation between them. Therefore, TST for TB screening without a physician’s assessment was not recommended for this population, but rather using a combination of IGRA with a physician’s assessment.16 More recently, Torres et al found a moderate level of agreement between both tests with a similar sample size to ours. This study included hospitalised patients with a high risk of suffering from TB (some on HD). They recommended using QFT-GIT routinely in this type of patients, at least in cases on HD, to try and establish when to treat the tuberculosis.4 These results in PD patients can be explained by cutaneous anergy as well as the low production of QFT-GIT and other cytokines.
In this study, 16% of patients were inconclusive for QFT-GIT. Our results coincide with those of Manuel et al, although these authors studied a different group of immunocompromised patients (with chronic liver disease).5 By analysing all these data, we could come to the conclusion that the inconclusive results are probably related to immunodeficiency.
This study investigated for the first time the contribution of QFT-GIT to improving the diagnosis of tuberculosis in PD patients. Its main weakness is the number of patients who had a positive skin test and/or QTF-GIT. Our results correlate well with the percentage of positive results found in the few publications there are, and the number of patients studied is relatively high given the low incidence and prevalence of PD patients in Spain.17
To conclude, we believe that IGRA represent an important advance in the diagnosis of tuberculosis, and at this moment they can complement the TST but not replace it. The role of IGRA in the screening of at-risk people, including PD patients diagnosed with CRF, still has to be defined; therefore, longitudinal studies that provide solid evidence on their prognostic value in the long-term development of TB are needed.
Table 1. Patients¿ characteristics
Table 2. Univariate analysis of the risk factors for inconclusive results of Quantiferon-TB gold (QTF-G)
Table 3. Agreement between the tuberculin skin test, Quantiferon-TB Gold and assessment by a pulmonologist, excluding patients with inconclusive results for QFT-GIT*