Acute kidney injury (AKI) conditions several short- and long-term complications. The aim of the present study was to analyse the impact of cardiac function and structure in the cardiovascular prognosis after an in-hospital AKI episode.
Material and methodsThis is an observational retrospective cohorts study including all in-hospital AKI episodes in 2013 and 2014 in our centre. At baseline, epidemiological values, comorbidities and echocardiography parameters were collected. During a follow-up of 49 ± 28 months, cardiovascular events (CVE) were collected, and associated factors were analysed.
Results1255 patients were included (55% male, age 75 ± 13 years). Of the 676 (54%) that had a previous echocardiogram, 46% had left ventricular hypertrophy, 38% pulmonary hypertension, 38% diastolic dysfunction and 22% systolic dysfunction. During the follow-up, 484 (39%) developed a CVE. Associated factors to VCE were male sex, age, diabetes mellitus, hypertension, dyslipidemia, coronary heart disease, heart failure, atrial fibrillation, neoplasia and chronic kidney disease (also, glomerular filtration rate at baseline and after the AKI episode). Survival curves demonstrated that all the echocardiographic parameters were associated to CVE. An adjusted Cox regression model showed that age (HR 1.017), diabetes (HR 1.576) and diastolic dysfunction (HR 1.358) were independent predictors for CVE.
ConclusionDiastolic dysfunction is an independent predictor for long-term cardiovascular events after an in-hospital acute kidney injury episode.
El desarrollo de un fracaso renal agudo (FRA) condiciona complicaciones a corto, medio y largo plazo. El objetivo de nuestro estudio fue analizar el impacto de las alteraciones cardiacas en el pronóstico cardiovascular de pacientes que presentan un episodio de FRA.
Materiales y métodosRealizamos un estudio observacional de cohortes retrospectivo incluyendo a todos los pacientes con FRA en 2013 y 2014. Basalmente recogimos variables epidemiológicas, comorbilidades y parámetros ecocardiográficos. Seguimos a los pacientes tras el ingreso durante una media de 49 ± 28 meses, recogiendo la incidencia de eventos cardiovasculares (ECV) y los factores asociados a los mismos.
ResultadosSe incluyeron 1255 pacientes (55% varones, edad 75 ± 13 años). De los 676 (54%) pacientes que disponían de un ecocardiograma previo, el 46% tenían hipertrofia de ventrículo izquierdo, el 38% hipertensión pulmonar, el 38% disfunción diastólica y el 22% disfunción sistólica. Tras la hospitalización por FRA, 484 (39%) tuvieron un ECV. Los factores asociados a presentar un ECV fueron el sexo (varón), la edad, diabetes mellitus, hipertensión arterial, dislipidemia, cardiopatía isquémica, insuficiencia cardiaca, fibrilación auricular, neoplasia previa y enfermedad renal crónica (y el filtrado glomerular estimado basal y tras el FRA). El análisis de supervivencia demostró que todos los parámetros ecocardiográficos se asociaban a ECV. Un modelo de regresión de Cox ajustado demostró que la edad (HR 1,017), la diabetes mellitus (HR 1,576) y la disfunción diastólica (HR 1,358) eran predictores independientes de ECV.
ConclusionesLa disfunción diastólica es un predictor independiente de ECV a largo plazo tras un episodio hospitalario de FRA.
The development of acute kidney injury (AKI) is a frequent complication in hospitalized patients that causes high morbidity and mortality.1,2 Beyond the immediate prognostic impact of renal function impairment, its importance extends to the medium and long term with complications such as cardiovascular events (CVD), development of chronic kidney disease (CKD) and mortality.3,4 Furthermore, considering AKI as a complex syndrome, other complications such as infections, neurological and cognitive alterations have been demonstrated.5
The association between renal and cardiac dysfunction is encompassed under the term cardiorenal syndrome.6 In both acute kidney injury and CKD, there is an increased incidence of CVD, which is due to numerous factors that are intricately related and include the activation of neurohumoral mechanisms (such as the renin-angiotensin-aldosterone system [RAAS], the sympathetic nervous system, and vasopressin secretion) capable of generating structural and functional changes at the cardiac level.7,8 In addition, certain factors such as arterial hypertension, water overload, acid-base balance, mineral metabolism disorders, inflammation or uremic toxins coexist in both pathologies, enhancing the deleterious effects on the renal and cardiac systems9–11 This means that many patients with renal dysfunction present structural and functional cardiac alterations that worsens their prognosis and whose early detection would allow their risk to be stratified.12,13
In the field of AKI, the usefulness of echocardiographic parameters and their prognostic impact have been less studied. For this reason, we present this study aimed at determining the association between cardiac ultrastructure and function and the incidence of cardiovascular events in patients who develop AKI.
Patients and methodsWe designed a single-center observational retrospective cohort study, which included all patients admitted with the diagnosis of AKI in 2013 and 2014. The aim was to analyze CVD after hospitalization and its relationship with cardiac ultrastructure and function. Patients with errors in the coding of AKI, those without data on renal function prior to admission, or those with a renal transplant were excluded. Likewise, those who died during admission, those who required renal replacement therapy at discharge or those who were lost to follow-up were excluded from the final analysis.
At baseline, we collected from electronic medical records epidemiological data (sex, age) and comorbidities (hypertension, diabetes mellitus, dyslipidemia, ischemic heart disease, heart failure, atrial fibrillation, peripheral vascular disease, stroke, and previous neoplasia). The presence of cognitive impairment was established and the functional dependence index was calculated using a modified Barthel scale (out of a total of 40 points, the higher the score the greater the independence).14 Regading the renal function, we recorded the presence of CKD and the estimated glomerular filtration rate (eGFR) (by Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI]) at baseline, the minimum value during the episode of AKI and the final value (which was established seven days after the maximum deterioration of renal function).15 The severity of the AKI episode was determined according to the Acute Kidney Injury Network (AKIN) scale.16 This scale uses an analytical criterion based on serum creatinine and urine flow to define the stage, such that the higher the stage, the greater the severity:
Stage 1: increase of 1.5–1.9 times the baseline creatinine value or increase greater than 0.3 mg/dL or presence of urinary flow less than 0.5 mL/kg/hr for more than 6 h.
Stage 2: increase of 2–2.9 times the basal creatinine value or the presence of a urinary flow of less than 0.5 mL/kg/h for more than 12 h.
Stage 3: increase greater than three times the baseline creatinine value, creatinine value greater than 4 mg/dL or the need for renal replacement therapy; or urinary flow less than 0.3 mL/kg/h in 24 h or anuria for 12 h.
Of the usual treatments, those patients that were prescribed diuretics and/or RAAS blockers were collected.
En los pacientes en los que estaba disponible un ecocardiograma en los seis meses previos, se registraron los siguientes parámetros17:
- -
Systolic dysfunction: defined as left ventricular ejection fraction (LVEF) less than 45%.
- -
Diastolic dysfunction: defined as a ratio greater than 14 between E and e' waves, left atrial volume greater than 34 mL/m2, septal e' velocity less than 7 cm/s or lateral e' velocity less than 10 cm/s and tricuspid regurgitation velocity greater than 2.8 m/2 (three or more parameters must be met).18
- -
Pulmonary hypertension: defined as pulmonary artery pressure greater than 30 mmHg.
- -
Left ventricular hypertrophy: defined as a left ventricular mass index greater than 130 g/m2 (men) or 105 g/m2 (women).
According to LVEF, three categories were established: severely depressed (below 40%), moderately depressed (40–49%), normal (above 50%).19
During follow-up of 49 ± 28 months, cardiovascular events (fatal or non-fatal) were recorded, defined as heart failure, ischemic heart disease, peripheral vascular disease, stroke or severe arrhythmias. Factors associated with cardiovascular events were analyzed, including the impact of echocardiographic parameters.
The study complied with current data protection regulations as well as ethical principles and was approved by the Ethics Committee of the Hospital Universitario de La Princesa (reference 3447, 11/2018).
Statistical methodsIn the statistical analysis, numerical variables are expressed as mean ± standard deviation or median (interquartile range) according to the distribution of each variable. Given that we wish to establish the impact of diastolic dysfunction on cardiovascular events, we made a comparison between patients who presented this echocardiographic alteration and those who did not. For statistical inference, we used the X2 test or Fisher's F test and Student's t test or Mann–Whitney t test according to the results of normality (determined by the Shapiro–Wilk test). For continuous variables, if variances are not equal after using Levene's test, we applied a Welch correction to Student's t test. We performed a univariate analysis using Cox regression to establish the factors associated with the occurrence of cardiovascular events. To determine the independent predictors, we performed a multivariate model adjusted for the variables significant in the univariate analysis or those considered to be confounding variables. The model obtained was validated with discrimination tests (area under the Receiver Operating Characteristic [ROC] curve of the model and Harrell's C statistic) and calibration tests (using the Hosmer–Lemeshow test). We graphically represented the association between echocardiographic parameters and cardiovascular events using Kaplan-Meier survival curves. Statistical analyses and graphs were performed with SPSS 26.0®. A value of p < 0.05 was considered statistically significant.
ResultsBaseline characteristicsDuring the study period, 1,720 patients were admitted to our center due to an episode coded as AKI. Of these, 179 (10%) had exclusion criteria (poor coding or need for renal replacement therapy at discharge) and 286 (16%) died during the episode and were therefore excluded. Finally, 1,255 (73%) were analyzed, of whom 696 (55%) were male, with a mean age of 75 ± 13 years. As shown in Table 1, 943 (76%) had arterial hypertension, 379 (30%) diabetes mellitus, 560 (45%) dyslipemia, 543 (43%) CKD, 334 (28%) heart failure and 247 (20%) ischemic heart disease.
Baseline patient characteristics.
| Patients admitted with ARF | With diastolic dysfunction | Without diastolic dysfunction | p | |
|---|---|---|---|---|
| (n = 1,255) | (n = 205) | (n = 334) | ||
| Sex (male), n (%) | 696 (55) | 104 (51) | 177 (53) | 0.657 |
| Age (years) | 75 ± 13 | 78 ± 10 | 75 ± 13 | 0.004 |
| Diabetes mellitus, n (%) | 379 (30) | 66 (32) | 103 (31) | 0.775 |
| Hyprtension, n (%) | 943 (76) | 177 (86) | 258 (77) | 0.010 |
| Dislipemia, n (%) | 560 (45) | 116 (57) | 150 (45) | 0.010 |
| Heart falure, n (%) | 334 (28) | 114 (56) | 105 (32) | <0.001 |
| Left ventricular ejection fraction n (%) | <0.001 | |||
| Preserved | 453 (78) | 143 (71) | 275 (83) | |
| Moderately depressed | 77 (13) | 27 (13) | 48 (14) | |
| Depressed | 51 (9) | 35 (16) | 11 (3) | |
| Ischemic heart disease, n (%) | 247 (20) | 63 (31) | 74 (22) | 0.025 |
| Atrial fibrillation, n (%) | 299 (24) | 69 (34) | 103 (31) | 0.568 |
| Stroke, n (%) | 182 (15) | 40 (20) | 50 (15) | 0.191 |
| Peripheral vascular disease n (%) | 116 (11) | 30 (16) | 29 (9) | 0.031 |
| Previous neoplasm, n (%) | 299 (24) | 35 (17) | 73 (22) | 0.186 |
| Chronic kidney disease, n (%) | 543 (43) | 107 (54) | 159 (48) | 0.245 |
| Baseline eGFR (mL/min/1.73 m2) | 57 ± 25 | 51 ± 22 | 56 ± 24 | 0.030 |
| Minimum eGFR at ARF episode (mL/min/1.73 m2) | 30 ± 18 | 31 ± 17 | 30 ± 17 | 0.984 |
| GFR after ARF episode (mL/min/1.73 m2)a | 53 ± 27 | 46 ± 25 | 51 ± 25 | 0.015 |
| Diuretics, n (%) | 568 (46) | 129 (63) | 168 (49) | 0.001 |
| BSRAA, n (%) | 651 (53) | 136 (66) | 179 (54) | 0.005 |
| Cognitive impairment, n (%) | 217 (19) | 39 (20) | 39 (12) | 0.016 |
| Modified Barthel scale (score) | 34 ± 12 | 32 ± 11 | 35 ± 8 | 0.010 |
| Severity of ARF, n (%) | 0.059 | |||
| AKIN-1 | 758 (60) | 147 (78) | 214 (68) | |
| AKIN-2 | 209 (18) | 24 (13) | 56 (18) | |
| AKIN-3 | 151 (12) | 18 (10) | 45 (14) | |
| Aetiology of ARF, n (%) | 0.604 | |||
| Functional | 883 (70) | 152 (74) | 248 (74) | |
| Obstructive | 92 (7) | 10 (5) | 9 (3) | |
| NTA | 56 (5) | 7 (3) | 18 (5) | |
| Interstitial | 13 (1) | 1 (1) | 4 (1) | |
| Glomerular | 11 (1) | 2 (1) | 6 (2) | |
| Vascular | 12 (1) | 3 (1) | 2 (1) | |
| Unaffiliated | 188 (15) | 30 (15) | 47 (14) | |
| Echocardiogram available | 676 (54) | |||
| Left ventricular hypertrophy | 258 (46) | 133 (67) | 105 (32) | <0.001 |
| Pulmonary hypertension | 198 (38) | 86 (47) | 95 (29) | <0.001 |
| Systolic dysfunction | 128 (22) | 59 (29) | 54 (17) | 0.001 |
| Diastolic dysfunction | 205 (38) | – | – | – |
AKIN: Acute Kidney Injury Network; RAASB: renin angiotensin aldosterone system blockers; eGFR: glomerular filtration rate estimated by CKD-EPI; AKI: acute renal failure; ATN: acute tubular necrosis.
Data expressed as mean ± standard deviation or median (interquartile range).
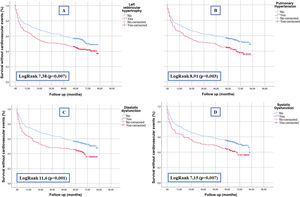
A transthoracic echocardiogram was available prior to the episode of AKI in 676 patients (54%) (some of them did not report all parameters). Of these, 258 (46%) had left ventricular hypertrophy, 198 (38%) had pulmonary hypertension, 205 (38%) had diastolic dysfunction and 128 (22%) had systolic dysfunction (Table 1).
Table 1 shows the differences between patients with and without diastolic dysfunction. We found a significant association between diastolic dysfunction and age, hypertension, dyslipidemia, history of heart failure, ischemic heart disease, peripheral vascular disease, cognitive impairment and functional dependence. In addition, those patients with diastolic dysfunction had a lower eGFR both at baseline and after the episode of AKI and were more frequently prescribed diuretics and RAAS blockers. We found a significant association between diastolic dysfunction and the rest of the echocardiographic parameters (systolic dysfunction, left ventricular hypertrophy and pulmonary hypertension).
Incidence and type of cardiovascular eventsAfter hospitalization for AKI, patients were followed up for a mean of 49 ± 28 months. There were 484 (39%) CVD, with heart failure being the most frequent (353 [73%]), followed by peripheral vascular disease (49 [10%]), stroke (40 [8%]), ischemic heart disease (32 [7%]), and severe arrhythmias (10 [2%]). Twenty-three patients (2%) died from cardiovascular event.
Factors associated with cardiovascular eventsWe performed a univariate analysis (Table 2) which showed that the incidence of CVD after an episode of AKI was associated with sex (male), age, history of diabetes mellitus, arterial hypertension, dyslipidemia, ischemic heart disease, heart failure, atrial fibrillation, and previous neoplasia. In terms of renal function, the development of cardiovascular events was associated with baseline CKD (and baseline eGFR) but also with eGFR after the episode of AKI.
Factors associated with developing CVD after an episode of AKI.
| HR (IC 95%) | p | |
|---|---|---|
| Sex (male) | 1.214 (1.015−1.452) | 0.034 |
| Age (per year) | 1.025 (1.017−1.032) | <0.001 |
| Diabetes mellitus | 1.365 (1.133−1.646) | 0.001 |
| Arterial hypertension | 2.152 (1.670−2.774) | <0.001 |
| Dyslipidemia | 1.582 (1.322−1.894) | <0.001 |
| Heart failure | 2.706 (2.253−3.250) | <0.001 |
| Ischemic heart disease | 1.719 (1.405–2.103) | <0.001 |
| Atrial fibrillation | 2.223 (1.841–2.683) | <0.001 |
| Stroke | 1.274 (1.002−1.621) | 0.048 |
| Peripheral vascular disease | 0.948 (0.696−1.291) | 0.736 |
| Previous neoplasm | 0.599 (0.468−0.767) | <0.001 |
| Chronic renal disease | 2.132 (1.775−2.561) | <0.001 |
| Baseline eGFR (per mL/min/1.73 m2) | 0.985 (0.981−0.989) | <0.001 |
| Minimal eGFR at ARF episode (per mL/min/1.73 m2) | 0.997 (0.992−1.002) | 0.019 |
| GFR after the episode of ARF (per mL/min/1.73 m2)a | 0.988 (0.985−0.992) | <0.001 |
| Diuretic | 2.396 (1.995–2.877) | <0.001 |
| RAASB | 1.852 (1.534−2.235) | <0.001 |
| Metformin | 1.013 (0.781−1.314) | 0.923 |
| Cognitive impairment | 0.859 (0.735−1.005) | 0.057 |
| Barthel (per point) | 1.003 (0.995−1.011) | 0.490 |
| Severity of ARF (per category change) | 0.662 (0.568−0.772) | <0.001 |
| Etiology of ARF (functional vs. other) | 1.402 (1.138−1.727) | 0.001 |
| Echocardiographic parameters | ||
| Left ventricular hypertrophy | 1.371 (1.088–1.72) | 0.008 |
| Pulmonary hypertension | 1.435 (1.127−1.827) | 0.003 |
| Diastolic dysfunction | 1.500 (1.183−1.901) | 0.009 |
| Systolic dysfunction | 1.419 (1.093–1.843) | 0.001 |
RAASB: renin angiotensin aldosterone renin system blockers; eGFR: glomerular filtration rate estimated by CKD-EPI; AKI: acute renal failure; HR: hazard ratio; CI: confidence interval.
As for data from echocardiograms, left ventricular hypertrophy, pulmonary hypertension, systolic dysfunction and diastolic dysfunction were significantly associated with the development of cardiovascular events (Table 2). The survival curves shown in Fig. 1 demonstrate this association graphically and analytically.
Independent predictors of cardiovascular eventsWe performed a multivariable Cox regression model in which it was shown that age (HR 1.016, CI 95% [1.001–1.032], p = 0.037), diabetes mellitus (HR 1.815 CI 95% [1.281–2.527], p = 0.001) and diastolic dysfunction (HR 1.537 95% CI [1.096–2.157], p = 0.013) are independent predictors of cardiovascular events after an episode of AKI (Table 3).
Cox regression to determine independent predictors of cardiovascular events after an episode of acute renal failure.
| HR (95% CI) | p | |
|---|---|---|
| Age (per year) | 1.016 (1.001–1.032) | 0.037 |
| Diabetes mellitus | 1.815 (1.281–2.527) | 0.001 |
| Diastolic dysfunction | 1.537 (1.096–2.157) | 0.013 |
HR: hazard ratio; CI: confidence interval.
Model adjusted for sex, arterial hypertension, dyslipidemia, chronic kidney disease, systolic dysfunction, pulmonary hypertension, left ventricular hypertrophy, previous neoplasia, cognitive impairment, modified Barthel index, atrial fibrillation, stroke, severity of acute renal failure established by AKIN, type of AKI and glomerular filtration rate after the episode of acute renal failure.
The area under the ROC curve of the probabilities of the model was determined (Harrel's C statistic), obtaining a model with good discrimination (area under the curve 0.743, p < 0.0001). Likewise, the model showed good calibration (Hosmer-Lemeshow test 3.38, p = 0.640).
DiscussionOur study demonstrates that cardiac abnormalities, and specifically diastolic dysfunction, independently predict the development of CVD after an episode of AKI. Moreover, in patients in whom they were available, echocardiographic parameters are a useful tool for stratifying the risk of developing CVD.
The clinical consequences of AKI include cardiovascular complications.20 Prospective studies, such as the recently published ASSESS-AKI, have shown that the simple fact of presenting an episode of AKI may increase the risk of presenting subsequent episodes of heart failure by up to 68%, establishing a cause-effect phenomenon. Despite the clinical evidence, the pathogenic explanation is not without controversy in the current literature. In a recent experimental study, based on a murine model, the authors demonstrate that an episode of AKI leads to the development of persistent diastolic dysfunction that could be considered as the triggering mechanism that conditions subsequent cardiovascular complications.21
Diastolic dysfunction is a pathology with a growing incidence and it is caused predominant by arterial hypertension, diabetes, and ischemic heart disease, most of which also produce renal alterations in an intricate mechanism that feeds back, enhancing the negative effects on the cardiorenal axis.22 In fact, our data confirm that age and diabetes worsen the cardiovascular prognosis of patients, both factors promoting increased myocardial stiffness and driving diastolic dysfunction itself.22
The description of diastolic dysfunction in patients with nephropathy has increased exponentially in recent years. In those with CKD, the prevalence of diastolic dysfunction increases with the deterioration of renal function and is even considered by some authors to be virtually universal in those requiring hemodialysis.13,23 Furthermore, the recently published population-based Atherosclerosis Risk in Communities Study establishes an independent association between numerous echocardiographic abnormalities and the incidence of CKD, suggesting the need to establish close monitoring of the functionality of both organs.24
In addition, the relationship between diastolic dysfunction and AKI presents little evidence at the present time. Cross-sectional clinical studies have been able to demonstrate that this cardiac alteration is associated with an increased risk of AKI during hospitalization, a situation that has been studied in specific populations such as cirrhosis, patients undergoing a contrast study, septic shock or elderly patients with hip fracture.25–28 The novelty of our study, beyond confirming that diastolic dysfunction is associated with greater severity of the episode of AKI (patients with diastolic dysfunction have a lower eGFR after the episode), is the demonstration of its impact on CVD-free survival after hospital discharge and in the long term.Undoubtedly, in the absence of confirmation of these data in prospective studies, the challenge is to establish closer monitoring of patients with diastolic dysfunction after AKI, but above all to determine specific therapeutic strategies to improve prognosis.
Impaired myocardial relaxation has also been shown to be an independent predictor of cardiovascular events in populations without AKI, which, due to its increasingly early detection and high prevalence, makes it necessary to establish specific measures to mitigate its consequences.29,30 To date, the only measures recommended have been based on the control of classic cardiovascular risk factors (diabetes, arterial hypertension, dyslipidemia, obesity) and non-classic risk factors (inflammation, uremia, hyperuricemia, among others).31 However, the publication of the EMPEROR-Preserved study opened the door to the first treatment with a potential prognostic improvement, empagliflozin.32 Although the results of this clinical trial are based on patients different from those presented in our study, it can be hypothesized its possible beneficial effect in patients with a higher cardiovascular risk after an episode of AKI.
Furthermore, as show in our study, the different echocardiographic abnormalities (systolic dysfunction, diastolic dysfunction, left ventricular hypertrophy [LVH] and/or pulmonary hypertension) are good markers for stratifying the risk of CVD in patients with AKI and could therefore be tools for establishing closer follow-up of certain subgroups of patients.
Our study is not without limitations. Firstly, it is a retrospective study, which entails the typical biases of this type of analysis. Despite the loss of data and the fact that, on occasions, these may not be completely homogeneous, the sample size we provided is sufficiently large to assess our objectives, and we were able to adjust the models for numerous variables. In this sense, we have applied the term AKI to define acute deterioration of renal function, although additional classifications are currently proposed that discriminate between AKI (renal damage up to seven days) and acute kidney disease (damage between seven days and three months).33 Secondly, as this was a real-life study, only 54% of the patients had an echocardiogram in the six months prior to the episode of AKI and these did not always describe all the alterations that we intend to study. To prevent certain alterations from going unnoticed, we decided to limit the validity of the echocardiograms to this time range, assuming that a greater number of patients would not have data on structure and renal function, but achieving greater accuracy as they were closer to the episode of AKI. Finally, the lack of an echocardiogram after AKI does not allow us to establish the effect of acute renal dysfunction on cardiac ultrastructure and function, so our data could be underestimated. However, in order to study this last limitation in depth, a prospective study with temporal homogeneity in the follow-up, at least in the performance of the control echocardiogram, would be required. In any case, we consider that the data provided by this study are sufficiently significant to be able to establish hypotheses that can be confirmed in studies with a more powerful design.
As a conclusion of the study, we can establish that diastolic dysfunction predicts the incidence of CVD after an episode of AKI. Furthermore, the risk of CVD can be stratified with the baseline parameters provided by echocardiographic studies, which would allow us to establish specific and close management of patients with structural or functional cardiac alterations.
FundingThis work has not received any type of funding.
Conflict of interestThe authors declare that they have no conflicts of interest.