Secondary hyperparathyroidism is highly prevalent in kidney transplant recipients, and commonly results in hypercalcaemia; an association to osteopenia and bone fractures has also been observed. Paricalcitol has proved effective to control secondary hyperparathyroidism in chronic kidney disease in both dialysed and non-dialysed patients, with a low hypercalcaemia incidence. Currently available experience on paricalcitol use in kidney transplant recipients is scarce. Our main aim was to show the effect of paricalcitol on mineral bone metabolism in kidney transplant recipients with secondary hyperparathyroidism.
Material and methodsA retrospective multicentre study in kidney transplant recipients aged >18 years with a 12-month or longer post-transplantation course, stable renal function, having received paricalcitol for more than 12 months, with available clinical follow-up for a 24-month period.
ResultsA total of 69 patients with a 120±92-month post-transplantation course were included. Baseline creatinine was 2.2±0.9mg/dL and GFR-MDRD was 36±20mL/min/1.73m2. Paricalcitol doses were gradually increased during the study: baseline 3.8±1.9μg/week, 12 months 5.2±2.4μg/week; 24 months 6.0±2.9μg/week (P<.001). Serum PTH levels showed a significant fast decline: baseline 288±152pg/mL; 6 months 226±184pg/mL; 12 months 207±120; 24 months 193±119pg/mL (P<.001). Reduction from baseline PTH was ≥30% in 42.4% of patients at 12 months and in 65.2% of patients at 24 months. Alkaline phosphatase showed a significant decrease in first 6 months followed by a plateau: baseline 92±50IU/L; 6 months 85±36IU/L, 12 months 81±39IU/L (P<.001). Overall, no changes were observed in serum calcium and phosphorus, and in urine calcium excretion. PTH decline was larger in patients with higher baseline levels. Patients with lower baseline calcium levels showed significantly increased levels (mean increase was 0.5–0.6mg/dL) but still within normal range, whereas patients with baseline calcium >10mg/dL showed gradually decreasing levels. Fifteen (21.7%) patients had received prior calcitriol therapy. When shifted to paricalcitol, such patients required paricalcitol doses significantly larger than those not having received calcitriol. Paricalcitol was used concomitantly to cinacalcet in 11 patients with significant PTH reductions being achieved; clinical course was similar to other patients and paricalcitol doses were also similar.
ConclusionsParicalcitol is an effective therapy for secondary hyperparathyroidism in kidney transplant recipients. Overall, no significant changes were observed in calcium and phosphorus levels or urinary excretion. Patients having previously received calcitriol required higher paricalcitol doses. When used in patients receiving cinacalcet, paricalcitol results in a significant PTH fall, with paricalcitol doses being similar to those used in patients not receiving cinacalcet.
El hiperparatiroidismo secundario es muy prevalente en pacientes trasplantados renales. Cursa con frecuencia con hipercalcemia y se ha asociado al desarrollo de osteopenia y fracturas óseas. El paricalcitol ha mostrado su eficacia en el control del hiperparatiroidismo secundario en la enfermedad renal crónica con y sin diálisis, con una baja incidencia de hipercalcemia. La experiencia con paricalcitol en trasplantados renales es muy escasa. El objetivo de este trabajo fue mostrar el efecto sobre el metabolismo mineralóseo del paricalcitol en trasplantados renales con hiperparatiroidismo secundario.
Material y métodosEstudio retrospectivo multicéntrico con trasplantados renales de más de 18 años de edad y más de 12 meses de evolución postrasplante, con función renal estable, que hayan sido tratados con paricalcitol durante más de 12 meses, con seguimiento clínico hasta los 24 meses de tratamiento.
ResultadosSe incluyó a 69 pacientes, con 120±92 meses postrasplante, con creatinina inicial de 2,2±0,9mg/dl y FG-MDRD 36±20ml/min/1,73m2. La dosis de paricalcitol se incrementó progresivamente durante el estudio: basal 3,8±1,9μg/semana, 12 meses 5,2±2,4μg/semana; 24 meses 6,0±2,9μg/semana (p<0,001). Los niveles séricos de PTH descendieron de forma rápida y significativa: basal 288±152pg/ml; 6 meses 226±184pg/ml; 12 meses 207±120; 24 meses 193±119 pg/ml (p<0,001). Observamos una reducción sobre PTH basal ≥30% en el 42,4% de los pacientes a los 12 meses y en el 65,2% de los pacientes a los 24 meses. La fosfatasa alcalina descendió también significativamente en los 6 primeros meses para luego estabilizarse: basal 92±50 UI/l; 6 meses 85±36 UI/l, 12 meses 81±39 UI/l (p<0,001). Globalmente no hubo modificaciones en el calcio o fósforo séricos ni en la excreción urinaria de calcio. La reducción de PTH fue más importante en trasplantados con niveles séricos más elevados de partida. Observamos que los pacientes con calcio basal más bajo mostraron un incremento significativo de sus cifras de 0,5-0,6mg/dl en promedio aunque manteniéndose en rango de normalidad, mientras que pacientes con calcio basal>10mg/dl mostraron una reducción progresiva de sus cifras. Quince (21,7%) pacientes seguían tratamiento previo con calcitriol y al cambiarlos a paricalcitol precisaron dosis significativamente mayores que los pacientes que no habían recibido calcitriol. El paricalcitol fue asociado a cinacalcet en 11 pacientes, con reducciones significativas de PTH, con evolución similar al resto de la población y con dosis de paricalcitol también similares.
ConclusionesParicalcitol es eficaz en el tratamiento del hiperparatiroidismo secundario de trasplantados renales. Globalmente no observamos modificaciones significativas de los niveles de calcio ni de fósforo, ni en su excreción urinaria. Los pacientes en tratamiento previo con calcitriol precisaron dosis mayores de paricalcitol. Cuando el paricalcitol se administra a pacientes tratados con cinacalcet, se observa un descenso significativo de la PTH con dosis de paricalcitol similar a pacientes sin cinacalcet.
Paricalcitol is a selective vitamin D receptor activator that has been demonstrated to be effective in the treatment of secondary hyperparathyroidism in advanced chronic kidney disease1–3 and in dialysis patients.4,5 In haemodialysis patients, paricalcitol has been shown to reduce serum parathyroid hormone (PTH) levels by more than 30% from baseline in 68–91% of cases, by more than 50% from baseline in 45–60% of patients,2,4,6 and to reach levels recommended in guidelines in 30–50% of patients. Some studies have shown paricalcitol to be as effective or more effective than cinacalcet.6 When paricalcitol was compared with calcitriol in patients on haemodialysis with resistant hyperparathyroidism, reductions in PTH were observed, even in those patients they did not respond to calcitriol.5–7 As compared with calcitriol, paricalcitol has less effect on calcium and phosphorus transporter proteins at an intestinal level, therefore the degree of increase in serum levels of calcium and phosphate is less after paricalcitol than calcitriol.8,9 This allows safer management in cases of more severe hyperparathyroidism.
Residual hyperparathyroidism is common in renal transplant recipients: various series have found that 30–50% of patients have abnormal PTH levels.10,11 It is particularly common to observe hypercalcaemia during the first year post-transplant and in up to 10–40% of patients after more than one year post-transplant.10,12 This may generate difficulties in the treatment of residual hyperparathyroidism with vitamin D derivatives, such as calcitriol. Paricalcitol could have a clear indication in the control of residual hyperparathyroidism in renal transplant recipients, aiming for a more ambitious reduction in PTH levels than what is often achieved in daily clinical practice.
There is little information about the use of paricalcitol in renal transplant patients. In one randomised trial in renal transplant recipients receiving paricalcitol from the third day post-transplant, prevalence of residual hyperparathyroidism at the end of the first year was reduced to 29%, compared with 63% in the control group.13 In one retrospective study in long-term transplant recipients, paricalcitol was shown to reduce serum PTH levels and to have a low incidence of hypercalcaemia.14
The aim of this multi-hospital study was to retrospectively collect information on the effect of paricalcitol on bone mineral metabolism in long-term transplant recipients, analysing the possible factors affecting changes in serum calcium and PTH levels.
Patients and methodsThis was a retrospective multicentre study with a single cohort of renal transplant recipients. Inclusion criteria were: renal transplant patients >18 years old, patients more than 12 months post-transplant, stable renal function, and treatment with paricalcitol for at least 12 months prior to the year 2012. We chose a population with more than 12 months post-transplant because during the first year, the progressive improvement in renal function or its deterioration due to rejection or other causes may cause changes in PTH levels. Such changes would add difficulties to a correct interpretation to the direct potential effect of paricalcitol on PTH. In addition, the administration of cinacalcet to control hypercalcemia is more common during the first year after transplantation; this treatment may also have affected the results.
All participating centres were asked to provide information on all patients who had received treatment with paricalcitol and who met the inclusion criteria. In each medical centre, patients received treatment with paricalcitol for secondary hyperparathyroidism in accordance with the standard clinical practice protocol, either as a primary medication, as a substitute for calcitriol treatment, or in conjunction with cinacalcet treatment. Paricalcitol was prescribed initially at a dose of 2–3μg per week in most cases, with a progressive increase in the dose to achieve the desired control of PTH. In patients previously treated from calcitriol to paricalcitol, the dose conversion ratio was lower than the recommended 4:1.
We gathered analytical data and dose of paricalcitol used at baseline and at 6 month intervals during a 2 year of follow-up period. Information was collected on whether patients had received previous treatment with paricalcitol, and the dose they had been taking. Patients on treatment with cinacalcet continued taking it, and the dose used at each stage was noted. Parathyroid hormone was measured as intact PTH using Roche® Elecsys electrochemiluminescence in all centres, therefore values did not require conversion (normal range 15–65pg/mL). Our main objective was to collect and show the overall changes for all parameters: the increasing and decreasing trends in their values, with means, standard deviations, and standard errors (in graphs). Therefore, data are not shown for individual cases of, for example, hypercalcaemia, as these cases were managed at the discretion of each nephrologist. Serum calcium levels were not corrected for albumin because it was not available at all assessment times. Renal function was represented by serum creatinine values and by estimated glomerular filtration rate using the MDRD4 equation (GFR-MDRD).
Statistical analysis was performed with the statistical program SPSS 11.5. All continuous quantitative variables were assessed for distribution; if they did not follow a normal distribution, the variable was transformed using its natural logarithm. This occurred for PTH and alkaline phosphatase. Quantitative variables are expressed as mean and standard deviation, except in the graphs, which show the mean and standard error. Unpaired means of quantitative variables from two groups were compared using the Mann–Whitney test and the Friedman test when there were more than 2 groups. Paired means of 2 variables were compared using the Wilcoxon test. Paired means of more than 2 time points were compared using repeated measures ANOVA, either directly or after logarithmic transformation if they did not follow a normal distribution. For analysis of subpopulations of interest, we divided the population into tertiles, avoiding individual values that could have biased the results and with equal numbers in each stratum studied. For multivariate analysis, we used a linear regression analysis, checking if the remainders obtained followed a normal distribution in the model constructed. For correlation analysis, the Spearman coefficient was whenever variables did not follow a normal distribution. A P value <.05 was considered statistically significant.
ResultsThe epidemiological characteristics of the patients included in the study are shown in Table 1. Most patients were long-term renal transplant recipients: 84.1% of patients were more than 2 years post-transplant and 63.8% of patients were more than 5 years post-transplant. The distribution of cases by transplant follow-up centre were: Cádiz, 3 cases; Granada, 24 cases; Huelva, 11 cases; Jaen, 21 cases; Malaga, 4 cases; and Seville, 6 cases. Age and post-transplant follow-up time were similar for both sexes.
Epidemiological characteristics of the study population.
| N | 69 |
| Age | 55±12 |
| Sex, M/F | 34/35 |
| Months post-transplant | 120±92 (median 100) |
| Weight, kg | 75.2±17.5 |
| BMI, kg/m2 | 28.6±6.2 |
| SBP, mmHg | 134±18 |
| DBP, mmHg | 76±11 |
| Diabetes mellitus n (%) | 14 (20.2) |
| Immunosuppression % | |
| Ciclosporin A | 29.0 |
| Tacrolimus | 55.1 |
| Sirolimus | 5.8 |
| Everolimus | 5.8 |
| MMF/MPS | 84.1 |
| Azathioprine | 7.2 |
| Prednisone | 91.3 |
Initial PTH levels in the study population were as follows: PTH<150pg/mL, 17.2% of patients; PTH 150–249pg/mL, 26.6%; PTH 250–349pg/mL, 31.2%; PTH 350–500pg/mL, 18.8%; and PTH>500pg/mL, 6.2%. The prescribed starting dose of paricalcitol was 3.7±1.9μg/week, median 3μg/week, and range 1–10μg/week. A dose lower than 3μg/week was prescribed in 16 patients (23.2%); 3μg/week was prescribed in 30 patients (43.5%); 4–6μg/week was prescribed in 11 patients (15.9%); 7μg/week was prescribed in 11 patients (15.9%); and 10μg/week was prescribed in 1 patient. The PTH/dose ratio was 88±58pg/mL/μg/week (median 77pg/mL/μg/week). Serum calcium was >10.5mg/dL before starting treatment with paricalcitol in seven patients (10.1%). Three of those patients had had previous treatment with calcitriol, and one with cinacalcet.
Paricalcitol dose was increased progressively throughout follow-up: dose at baseline, 3.8±1.9μg/week; at 6 months, 4.4±2.2μg/week; at 12months, 4.9±2.4μg/week; at 18 months, 5.3±2.3μg/week; and at 24 months, 5.9±2.8μg/week (Wilcoxon P=.002 for 6 months and P<.001 for the other times).
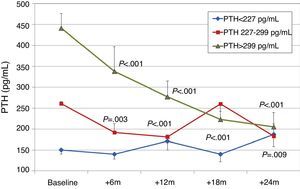
Effect on bone mineral metabolismChanges in parameters of bone mineral metabolism are summarised in Table 2. PTH and alkaline phosphatase levels decreased significantly at sixth month. Patients with higher initial levels of serum PTH levels had a more marked reduction in PTH, whereas patients in the lowest tercile had a moderate change PTH (Fig. 1). A PTH decrease of >60% from baseline value was seen in 11.9% of patients at 12 months, and in 23.9% of patients at 24 months. PTH was reduced by >30% from baseline in 42.4% of patients at 12 months and in 65.2% of patients at 24 months.
Changes after treatment with paricalcitol in renal function and parameters of bone mineral metabolism in blood and urine.
| Baseline | 6m | 12m | 18m | 24m | |
|---|---|---|---|---|---|
| Cr (mg/dL) | 2.21±0.93 | 2.29±0.92 | 2.38±0.97a | 2.41±0.95b | 2.41±1.05a |
| GFR-MDRD | 36±20 | 34±18a | 35±23 | 33±20b | 33±18a |
| iPTH (pg/mL) | 288±152 | 226±184c | 207±120c | 211±140d | 193±119c |
| Reduc. PTH (median) (%) | −24.1 | −23.7 | −29.7 | −35.6 | |
| Patients with reduc. >30% from baseline PTH (%) | 39 | 42.4 | 48.9 | 65.2 | |
| Alk. Phos. (IU/L) | 92±49 | 85±35b | 82±38d | 84±44e | 87±41f |
| Ca (mg/dL) | 9.7±0.7 | 9.8±0.6 | 9.6±0.7 | 9.6±0.6 | 9.6±0.6 |
| P (mg/dL) | 3.4±0.7 | 3.4±0.9 | 3.4±0.8 | 3.5±0.7 | 3.6±0.8 |
| Calciuria (mg/day) | 122±99 | 125±99 | 105±82 | 120±135 | 109±91 |
| Urinary Ca/Cr (%mg/mg) | 10.5±8.8 | 11.1±9.1 | 9.4±8.2 | 10.1±12.5 | 9.6±7.3 |
| Frac. Exc. Ca (%) | 1.81±1.22 | 1.96±1.64 | 1.80±1.30 | 1.89±1.21 | 1.95±1.17 |
| Phosphaturia (mg/day) | 744±305 | 704±256 | 706±316 | 667±255g | 629±245h |
| ClP (mL/min) | 16.3±8.5 | 15.9±7.7 | 15.4±7.7 | 13.9±6.7a | 12.8±6.7i |
| TRP (%) | 64±13 | 64±13 | 63±13 | 64±15 | 63±19 |
Overall, serum calcium and phosphorus levels did not change significantly, nor did urinary excretion of calcium. Phosphaturia and phosphate clearance decreased significantly at the end of follow-up (available measurements: n=48 patients at baseline and n=39 at 18 and 24 months).
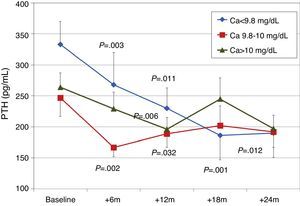
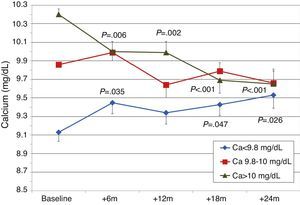
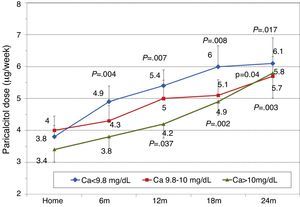
To analyse the effect of initial calcium levels on changes in PTH, we classified patients by terciles according to their baseline serum calcium levels. We found that patients in the lowest calcium tercile had higher baseline PTH values and that PTH decreased more markedly than in the other groups (Fig. 2). In patients who began with baseline calcium <9.8mg/dL (lowest tercile), serum calcium levels increased progressively but remained within normal range. In contrast, in patients who began with serum calcium >10mg/dL (highest tercile) calcium levels showed a significant progressive decrease (Fig. 3). Paricalcitol dose was progressively increased in all 3 calcium groups. This dose was increased more rapidly in the group of patients with the lowest initial calcium levels. At 24 months all groups were receiving similar doses of paricalcitol (Fig. 4). There were non-significant differences in renal function between the different calcium terciles: Ca<9.8mg/dL, Cr 2.4±0.9mg/dL; Ca 9.8–10mg/dL, Cr 2.1±0.7mg/dL; Ca>10mg/dL, Cr 1.9±1.1mg/dL. Cinacalcet was given to 6 of 21 patients (28.6%) in the lowest calcium tertile, 2 of 24 (8.2%) in the middle tercile and 3 of 24 patients (12.5%) in the highest tercile; and, the P value was not significant).
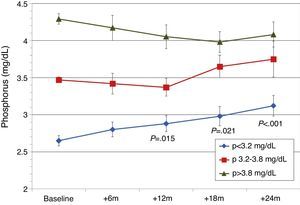
Patients were also classified according to their baseline serum phosphorus levels. Patients from the lowest tercile (P<3.2mg/dL) showed a significant and progressive increase in phosphorus levels, but levels remained within normal limits (Fig. 5). In the other tertiles, the changes in serum phosphorus were not significant. GFR was better in patients from the lowest tertile of phosphate: 1st (lowest) tercile, serum Cr 1.5±0.6mg/dL; 2nd tercile, serum Cr 2.4±1.1mg/dL; 3rd tercile, serum Cr 2.6±0.6mg/dL (P<.001). Mean baseline PTH levels were different. There were differences in baseline PTH levels among the different phosphorus tertiles: 1st tercile, PTH 238±100pg/mL; 2nd tercile, PTH 252±101pg/mL; 3rd tercile, PTH 362±215pg/mL (P<.001). In the 3 phosphorus groups, PTH decreased gradually and at 24 months the PTH values were similar for all Phosphate tertiles (154±78pg/mL; 186±89pg/mL; 189±114pg/mL).
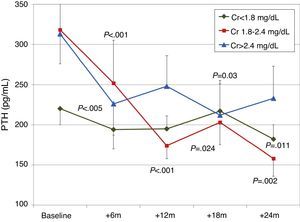
When patients were classified according to baseline renal function (terciles of creatinine), a more significant decrease in PTH was observed in patients with worse renal function (Fig. 6). There were no significant changes in calcium or phosphorus levels in any group during the study.
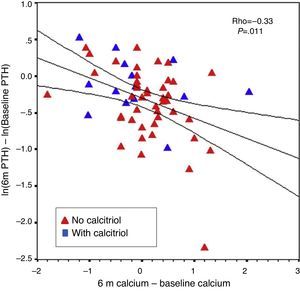
The percent change in PTH from baseline to six months correlated with baseline PTH level (r=−0.31; P=.016) and with changes in calcium from baseline to six months (r=−0.33; P=.011). This was observed even when patients without previous calcitriol treatment were analysed separately (Fig. 7). The percent change in PTH at 12 months showed a correlation with baseline PTH value (r=−0.47; P<.001) and with the starting dose of paricalcitol (r=−0.27; P=.035), but not with paricalcitol dose at 6 or 12 months or with changes in serum calcium at 6 or 12 months. Paricalcitol dose at 12 months and at 18 months showed a correlation with baseline PTH values (r=0.41 and P=.003, and r=0.31 and P=.030, respectively).
Factors predictive of PTH response (percentage change from baseline) were analysed using linear regression. The following factors were introduced as independent variables: paricalcitol dose at each time point, treatment with cinacalcet, previous treatment with calcitriol, age, sex, months post-transplant, baseline weight, calcium, phosphorus, baseline PTH, and baseline creatinine/GFR-MDMR. At 6 months the predictive factors were initial paricalcitol dose and prior treatment with calcitriol (r=0.37; P=.018). At 12 months the predictive factors were baseline PTH and initial paricalcitol dose (r=0.63; P<.001). At 18 months the only predictive factor was baseline PTH level (r=0.57; P<.001). We looked for predictors of paricalcitol dose at each time point, using linear regression, and found only baseline paricalcitol dose and baseline PTH.
When changes in calcium at 6 months were analysed using linear regression, the predictive variables were baseline calcium and phosphorus levels (r=0.64; P<.001). At 12 months, the predictors were baseline calcium and paricalcitol dose at 12 months (r=0.55; P<.001). At 18 months, the only predictor was baseline calcium level.
Changes in alkaline phosphatase levels maintained a correlation with PTH levels at 6 months (r=0.29; P=.046), at 12 months (r=0.41; P=.003), at 18 months (r=0.29; P=.048) and at 24 months (r=0.48; P=.003). There was no correlation with paricalcitol dose or with changes in calcium or phosphorus levels.
On linear regression analysis, changes in alkaline phosphatase values between baseline and six months were significantly related (r=0.77; P<.001) with baseline alkaline phosphatase values (beta=−.73; P<.001), with previous calcitriol treatment (beta=.32; P<.001), and with baseline creatinine (beta=−.19; P=.0321). Between baseline and 12 months, the predictors of change in alkaline phosphatase (r=0.74; P<.001) were baseline alkaline phosphatase value (beta=−.67; P<.001), baseline calcium (beta=.25; P=.008), previous treatment with calcitriol (beta=.25; P=.027), and baseline creatinine (beta=−.19; P=.042), with no association with changes in PTH.
Comparison of patients treated with cyclosporine with those treated with tacrolimus did not show differences in changes in PTH, alkaline phosphatase, calcium, phosphorus, excretion of calcium or phosphorus, or dose of paricalcitol used. Likewise, there were no differences in these variables when comparing patients who received anticalcineurin therapy or m-TOR inhibitors as immunosuppression. Sex, age, time post-transplant, and diabetic status did not affect immunosuppression choice.
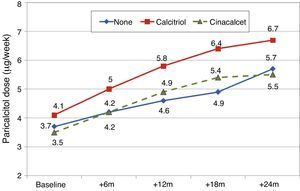
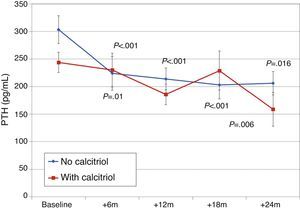
Patients previously treated with calcitriolFifteen patients (21.7%) had been receiving previous treatment with calcitriol, which was changed to paricalcitol. The initial dose conversion was 3.5±1.8μgparicalcitol/μg calcitriol (median, 3.4; range, 1–8μgparicalcitol/μg calcitriol).
Paricalcitol dose was progressively increased in patients treated with calcitriol, and these patients received higher doses than other patients, as can be seen in Fig. 8 (Friedman P<.001 for patients without calcitriol and P=.002 for patients with calcitriol). The most striking differences were between these groups at 12 months (Mann–Whitney P=.076) and 18 months (Mann–Whitney P=.019).
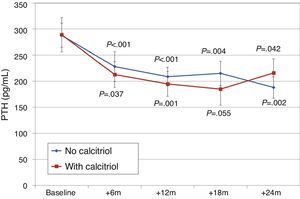
Changes in PTH, alkaline phosphatase, calcium, and phosphorus were similar, with similar final levels in both groups of patients (Fig. 9). There were no differences in urinary excretion of calcium or phosphorus.
Patients on treatment with cinacalcetEleven patients (15.9%) were on treatment with cinacalcet when paricalcitol was introduced. The initial dose of paricalcitol used in patients that were on cinacalcet treatment was 3.5±1.9μg/week. This dose was similar in patients not taking cinacalcet (3.8±1.9μg/week). The dose of paricalcitol was increased to the same extent in both groups (Fig. 8). The dose of cinacalcet did not change throughout follow-up: baseline, 53±36mg/day; 6 months, 60±40mg/day; 12 months, 60±47mg/day; 18 months, 71±41mg/day; 24 months, 71±48mg/day. In 7 patients there was no change in cinacalcet dose at any point. In one patient it was stopped at 12 months. In one patient the daily dose increased by 30mg at 6 months. In the other 2 patients the daily dose increased by 30mg from 12 months to avoid further changes.
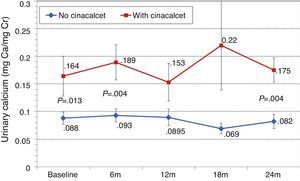
Changes in levels of calcium, phosphorus, PTH, and alkaline phosphatase, and changes in the excretion of calcium and phosphorus were parallel between patients with and without cinacalcet (Fig. 10). Baseline levels of calcium, PTH, and alkaline phosphatase were similar in both groups, as was the dose of paricalcitol used at the 6-month intervals. Patients with cinacalcet initially had slightly lower levels of phosphorus, although not significantly, and had parallel changes to patients without cinacalcet. Urinary excretion of calcium was higher in patients with cinacalcet, with no significant differences in its changes over the study period (Fig. 11).
Residual secondary hyperparathyroidism is common in renal transplant recipients. The factors associated with its persistence10–12,15–17 are length of time on dialysis, severity of secondary hyperparathyroidism prior to transplant, need for cinacalcet whilst on dialysis, degree of renal function achieved by the graft, vitamin D receptor genotype, and post-transplant circulating levels of vitamin D metabolites. Although secondary hyperparathyroidism has been associated with a higher incidence of post-transplant bone fractures18 and with loss of bone mass,19–21 there are no clear guidelines on the treatment of residual post-transplant hyperparathyroidism. The recommendations are general and are directed more towards prevention and treatment of post-transplant osteopenia than specific goals of secondary hyperparathyroidism treatment. Often, levels of PTH are maintained relatively high for the degree of renal function achieved, if compared with the recommended levels for patients with chronic renal failure who do not receive a transplant. Maintaining relatively high levels of PTH could be advisable to obtain a good degree of bone remodelling, counterbalancing the slowing effect of steroids and anticalcineurin therapy on bone remodelling .22 However, the maintenance of post-transplant secondary hyperparathyroidism even with hypercalcaemia does not guarantee a highly remodelled bone; often a low degree of bone remodelling, or even an adynamic bone, is seen.23 The approach of not starting active treatment unless there are complications could explain why paricalcitol was started in our series in 29.1% of patients with PTH of 250–349pg/mL and in 23.6% of patients with PTH>350pg/mL. The same can be observed in the series presented by González,14 in which the average PTH level was 333pg/mL at the time of starting paricalcitol.
Treatment of residual post-transplant hyperparathyroidism is based on the use of vitamin D derivatives and cinacalcet. The use of Cinacalcet is widespread and recognised in renal transplant patients with post-transplant hypercalcaemia, especially during the first post-transplant year.24,25 Often, when cinacalcet is stopped, new increases are seen in PTH and calcium, requiring continued treatment for an indefinite time period.17,26 In some series of renal transplant patients,27,28 supplementation with vitamin D derivatives reduced PTH, although the reductions obtained were moderate when initial PTH levels were very high. Increases in serum calcium were also observed.27,28 The ideal levels of 25-OH vitamin D are not well-established. A value of >30ng/mL, as in non-transplant patients, is recommended,27 although levels above 20ng/mL are probably sufficient. Calcitriol has been used in renal transplant patients, directed particularly at the treatment of bone mass loss, with significant reductions in PTH.19,29,30 Often, there is an associated increase in calciuria9 and in serum calcium.31 Alfacalcidol also achieves PTH reduction, but with an associated increase in serum calcium and reduction of bone mass loss in the spine and femur.32
Paricalcitol is a synthetic analogue of calcitriol. Paricalcitol has the advantage a reducing intestinal absorption of calcium and phosphorus,33,34 which explains the lower incidence of hypercalcaemia and hyperphosphataemia.35 There is little experience with paricalcitol in renal transplants. Amer13 studied paricalcitol as a treatment for post-transplant secondary hyperparathyroidism: in a randomised trial, 51 patients received 1μg/day of paricalcitol from the third day post-transplant, increasing to 2μg/day from 2 weeks until the study end. With this dose, the prevalence of patients with PTH >65pg/mL was reduced to 29%, compared with 63% in the placebo group. González14 performed a retrospective study on 58 patients who were prescribed paricalcitol at a dose of 1μg/day on alternate days, and observed an average PTH decrease of 30–40%. PTH levels decreased by ≥30% in 55–76% of patients. In our study, the population achieving a PTH reduction ≥30% at 12 months was 33.9%. As well as this milder response in our series, we also observed a progressive increase in paricalcitol dose used during the study, reaching double the final dose that was used in the study by González.14 The population of the study by Amer13 is not comparable to that of our study as the patients had a short period of dialysis (11 months), lower initial PTH levels, and half of the patients had a pre-emptive transplant.
In our study, the induced changes in PTH correlated inversely with the changes in serum calcium at 6 months; thereafter this correlation was lost. The main predictor of reduction in PTH was the starting value of PTH. There was no effect from the degree of renal function, initial phosphorus level, or dose of paricalcitol used. This relationship was to be expected, given that patients who start with higher PTH levels will have quantitatively greater reductions to meet the final target PTH level, compared with those with lower PTH levels, in whom a slight reduction will bring them into the desired PTH range.
Some studies have observed a relationship between paricalcitol dose and degree of PTH reduction.1 In our study, paricalcitol dose was directly related to the initial PTH level but not to the final PTH level, and was inversely related to calcium levels. This was probably because the paricalcitol doses used at each point were adjusted by the nephrologist according to initial PTH levels and serum calcium levels, in most cases aiming for a moderate PTH target rather than trying to normalise it completely. This was different to other prospective controlled studies, which had stricter, more uniform protocols with increasing doses and more ambitious PTH goals than those decided by the individual nephrologists in this study.
Patients that were previously on treatment with calcitriol showed changes in PTH similar to patients without calcitriol, but required higher paricalcitol doses. Patients on treatment with cinacalcet experienced progressive decreases in PTH, with doses of paricalcitol similar to those of patients without cinacalcet. Combined treatment with paricalcitol and cinacalcet was appealing from a pathophysiological point of view in these patients. In transplant patients, cinacalcet has been shown to be capable of resetting the set point of calcium36,37 and increasing vitamin D receptors in parathyroid cells.38 In patients on haemodialysis, cinacalcet has been shown to reduce parathyroid gland size independently of the initial size. This would suggest that it could be effective in cases of nodular hyperplasia.39 In the removed parathyroid glands of transplant patients with hypercalcaemia, there is reduced density of calcium sensing receptors and VDR only in glands with nodular hyperplasia, whereas this is almost normal in cases of diffuse hyperplasia.40 The rate of parathyroid cell apoptosis is much higher in glands with diffuse hyperplasia than in nodular hyperplasia, these rates being even higher than those in patients on haemodialysis.40 This apoptosis, along with lower proliferation, could explain the post-transplant reduction seen in the size of parathyroid glands.41,42
The number of vitamin D receptors and calcium sensing receptors in parathyroid cells is reduced in renal failure40,43 and also immediately following renal transplant,43 with subsequent normalisation if there is no nodular hyperplasia.16,40 Paricalcitol, unlike other vitamin D analogues, stimulates the expression of calcium-sensing receptors and exerts an antiproliferative effect on parathyroid cells.44 Paricalcitol can potentiate the effect of cinacalcet on parathyroid cells and promote a further decrease in PTH. In our short series of patients we did not observe any differences in calcium level in patients with cinacalcet compared to those without. If there was an increase in calcaemia, cinacalcet probably helped to control this increase; thus allowing the use of higher paricalcitol doses in cases of severe hyperparathyroidism to slow glandular production of PTH. The dose of cinacalcet used did not change in most patients and in one patient cinacalcet was stopped. It is not yet known if the added reduction in PTH levels with paricalcitol could facilitate the gradual withdrawal of cinacalcet in some patients.
As was found in the other 2 series,13,14 overall, we observed no increase in calcium levels. This could be due in part to the nephrologists’ caution with regard to increasing paricalcitol doses, increasing them gradually according to the calcium levels at each point (Fig. 3). In the series by Amer, the incidence of hypercalcaemia was 20%. This forced them to stop paricalcitol in 10% of patients. This hypercalcaemia could probably be explained by the higher doses of paricalcitol used (2μg/day), the concurrent administration of calcium supplements along with paricalcitol, and the tendency towards hypercalcaemia that is seen in the first year post-transplant in a significant proportion of patients.
We observed that changes in serum calcium depended on the baseline calcium level: patients that began with lower levels (Ca<9.6mg/dL) experienced a small increase in their levels, but they remained within normal limits, whereas patients with Ca>9.9mg/dL had no changes in their levels or even showed mild decreases. In addition, a baseline calcium level of <9.6mg/dL was associated with higher baseline PTH levels and a more significant PTH reduction than in patients with a higher calcium level. Patients with lower baseline calcium had slightly worse renal function, higher baseline PTH levels, and higher paricalcitol doses, which could explain the increasing trends in serum calcium. Patients belonging to the highest tercile of calcium showed a reduction in calcium levels. This was a consequence of reduced renal tubular reabsorption and reduced bone resorption of calcium due to a decrease in PTH.
Levels of alkaline phosphatase decreased progressively, accompanying the PTH decrease, with a partial relationship between changes in PTH and changes in alkaline phosphatase values. The VITAL study also found this relationship, suggesting that the decrease in alkaline phosphatase was a consequence of not only PTH reduction, but also a direct suppressive effect of paricalcitol on osteoblasts.1 In patients who had had previous calcitriol treatment, alkaline phosphatase levels were higher and decreased with paricalcitol in a parallel way to that of patients who had not previously received calcitriol. These decreases were not observed to be related to any factor, and other authors have found similarly.1 Immunosuppressor type did not show an effect on changes in alkaline phosphatase, although the sample size of patients with m-TOR inhibitors was too small to be able to compare with patients on anticalcineurin therapy and draw definitive conclusions.
In summary, in this study we describe the changes in PTH in different subpopulations of long-term renal transplant patients with secondary hyperparathyroidism treated with paricalcitol, with a significant reduction in PTH levels for all PTH ranges. The initial PTH reduction was partially related to an increase in serum calcium levels, especially in patients with lower initial levels, although later this relationship was lost. The response did not appear to depend on renal function or initial calcium level. Patients who had previously received calcitriol treatment required higher doses of paricalcitol than those who had not. Patients on cinacalcet treatment showed a good response on paricalcitol doses similar to those of patients without cinacalcet. The use of paricalcitol appears promising in renal transplant patients. A more ambitious treatment of residual secondary hyperparathyroidism could be established, to see what effects it would have on bone mineralisation, especially in long-term transplant patients. In patients on cinacalcet treatment, a combined treatment with paricalcitol could be used, directed at better control of PTH, and potentially even allowing subsequent withdrawal of cinacalcet. There remains to be studied the potential beneficial effects of paricalcitol on bone mineralisation when PTH levels are successfully reduced to suitable levels.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Borrego Utiel FJ, Bravo Soto JA, Merino Pérez MJ, González Carmelo I, López Jiménez V, García Álvarez T, et al. Efecto de paricalcitol sobre el metabolismo mineralóseo en pacientes trasplantados renales con hiperparatiroidismo secundario. Nefrologia. 2015;35:363–373.