Sodium-glucose cotransporter 2 inhibitors (SGLT2i) have demonstrated cardiovascular and renal benefits in patients with type 2 diabetes mellitus, heart failure, or chronic kidney disease. Since the first studies with these drugs, an initial increase in hemoglobin/hematocrit levels was observed, which was attributed to an increase in hemoconcentration associated with its diuretic effect, although it was early appearent that these drugs increased erythropoietin levels and erythropoiesis, and improved iron metabolism. Mediation studies found that the increase in hemoglobin was strongly associated with the cardiorenal benefits of these drugs. In this review, we discuss the mechanisms for improving erythropoiesis and the implication of the increase in hemoglobin on the cardiorenal prognostic benefit of these drugs.
Los inhibidores del cotransportador sodio-glucosa tipo 2 (iSGLT2) han demostrado su beneficio cardiovascular y renal en pacientes con diabetes mellitus tipo 2, insuficiencia cardíaca o enfermedad renal crónica. Desde los primeros estudios con estos fármacos se objetivó un incremento inicial de los niveles de hemoglobina/hematocrito que se atribuyó a un aumento de la hemoconcentración asociados a su efecto diurético, aunque pronto se constató que estos fármacos aumentaban los niveles de eritropoyetina y la eritropoyesis y mejoraban el metabolismo férrico. Los estudios de mediación objetivaron que el aumento de hemoglobina se asociaba estrechamente con los beneficios cardiorrenales de estos fármacos. En esta revisión se discuten los mecanismos de mejora de la eritropoyesis y la implicación del aumento de hemoglobina sobre el beneficio pronóstico cardiorrenal de estos fármacos.
Sodium-glucose cotransporter type 2 (SGLT2i) inhibitors were initially introduced for the treatment of type 2 diabetes mellitus. Cardiovascular safety studies in type 2 diabetic patients demonstrated that these drugs decreased the risk of cardiovascular events (especially congestive heart failure [HF])1–5 and also reduce the progression of chronic kidney disease (CKD), as well as the reduction and regression of albuminuria or even preventing its development.6–10
Subsequently, studies were performed to demonstrate their benefit in patients with HF, both diabetics and non-diabetics. These additional studies showed that the use of SGLT2-i reduced the number of HF hospitalizations and also decrease the cardiovascular mortality in this population, both with reduced and with preserved ejection fraction11–15 regardless of whether or not they were diabetics or CKD patients.16
In both diabetic and non-diabetic CKD patients, iSGLT2 has also been shown to reduce CKD progression and albuminuria.17–20 Furthermore, in this setting iSGLT2 decrease the risk of HF hospitalization and cardiovascular mortality,5,20 as well as the risk of acute renal failure.20
Effect of sodium-glucose cotransporter type 2 inhibitors on erythropoiesisErythropoietinSince the first clinical trials in patients with type 2 diabetes mellitus, it was observed that SGLT2-i treatment produced an early and sustained increase in hemoglobin/hematocrit levels,21–24 a fact that has subsequently been confirmed in clinical trials in patiens with HF25–28 and CKD.29–31
Hemoglobin/hematocrit changes during iSGLT2 treatment were initially attributed to hemoconcentration due to its diuretic effect and the decrease in plasma volume,28 although it was soon suggested that other mechanisms might be involved, given that its increase exceeded what would be expected by the initial plasma volume contraction and its poor temporal relationship with volume contraction.22,29
In this regard, an initial increase in erythropoietin (EPO) levels and/or reticulocyte counts was observed with the use of these agents, both in patients with either normal24,28,32–38 or reduced renal function,29 confirming their direct positive effect on erythropoiesis. Recently, in a post hoc analysis of the Dapagliflozin on Renal Outcomes and cardiovascular Mortality in Patients With Chronic Kidney Disease (DAPA-CKD) study, it was found that the increase in hematocrit was gradual and reached a maximum about four months after the initiation of the treatment with dapagliflozin, which rules out its relationship with the initial decrease in blood volume.31 The increase in erythropoiesis with these drugs could be due to the reversal of relative tissue hypoxia in the proximal tubule as a result of the decreased activity in Na+/K+ ATPase pump, secondary to the decrease in sodium reabsorption by inhibition of sodium-glucose cotransporter type 2 (SGLT2). In diabetes mellitus SGLT2 expression is increased. Some studies have shown that repeated damage or hypoxia at the level of the renal proximal tubule may lead to dedifferentiation of EPO-producing cells and their conversion into myofibroblasts, which lose their ability to produce this hormone. Thus, the normalization of renal cortical oxygenation at this level would reduce metabolic stress and improve tubulointerstitial function, restoring the capacity of EPO production by peritubular fibroblasts, stimulating erythropoiesis.39 In addition, inhibition of glucose and sodium reabsorption by SGLT2i in the posterior segments of the nephron favors its distal recovery (e.g., SGLT1). This favors hypoxia at the renal medullary level, stimulating EPO production by interstitial fibroblasts at this location.39 Similarly, CKD due to nephroangiosclerosis is characterized by increased sympathetic nervous activity and activation of the renin-angiotensin-aldosterone system (RAAS), which increases sodium reabsorption in the proximal tubule and thus oxygen consumption; it also decreases sodium influx to the distal nephron, activating tubulo-glomerular feedback.
Another possible effect could be the stimulation of vasopressin synthesis by SGLT2i,40 as this hormone has been shown to increase red blood cell count and stimulate erythropoiesis by an EPO-independent mechanism in experimental studies.41
Finally, the anti-inflammatory effects of SGLT2-i could enhance erythropoiesis by increasing EPO production and the response to EPO by bone marrow erythroblasts.42
Iron metabolismThere are many effects of SGLT2-i on iron metabolism.
Several studies have observed a decrease in hepcidin levels, which could favor intestinal absorption of iron and its mobilization from deposits, thus increasing its availability for erythropoiesis.28,34,35 Similarly, there are studies showing a significant reduction in ferritin levels.29,33–35,43,44 Erythroferrone, produced by erythroid precursor cells, acts in the liver decreasing hepcidin expression. In two studies the use of SGLT2i was associated to an increase in erythroferrone levels,34 although in one of these studies it did not reach statistical significance.28
Likewise, some studies have shown that treatment with SGLT2-i35,44 is accompanied by an increase in transferrin levels or total iron binding capacity34,35,43, soluble transferrin receptor, as well as a decrease in the transferrin saturation index,34,35,43 although not in all studies.29,33 Similarly, an increase in the expression of transferrin receptor types 1 and 2 has been observed in mononuclear cells.34
All these changes are compatible with an improvement in functional iron deficiency and an increase in intracellular iron availability/utilization, which would favor erythropoiesis and myocardial metabolic efficiency.45 In this regard, a recent analysis of a clinical trial in patients with HF and reduced ejection fraction showed that treatment with dapagliflozin was associated with decreased ferritin and increased soluble transferrin receptor. The magnitude of these changes was linked to the degree of the increase in short-term maximal functional capacity.44
Mediators of cardiorenal benefits of sodium-glucose type 2 cotransporter inhibitors in pivotal clinical trialsIn diabetic patients, anemia appears earlier in the course of renal disease and is more severe than in patients without diabetes.46 The presence of anemia is associated with a worse prognosis in diabetics47, HF26,27,48 and CKD patients.31,49–51 Likewise, anemia has been shown to be a factor in progression to end-stage CKD.31,50,51
The aforementioned studies found that treatment with SGLT2-i reduced the prevalence of anemia and the risk of its subsequent development in diabetic patients,22–24 with HF26,27 or with CKD.29–31
Interestingly, the mediation analyses performed in the different studies of cardiovascular or renal safety of SGLT2-i consistently indicate that the main mediators of cardiovascular52–55 and renal55,56 benefits of SGLT2-i are increases in hemoglobin/hematocrit, as well as decreases in uric acid, or urine albumin-creatinine ratio. Although being a potential mediator does not imply causality, it is noteworthy that most studies agree on the markers of cardiovascular and renal protection during treatment with these drugs. In patients with HF, the increase in hemoglobin with SGLT2-i9 precedes, predicts and is closely associated with reduced risk of events53–55 and correction of anemia with SGLT2i is associated with a better prognosis than in those in whom the anemia27 persists. In the DAPA-CKD study, the benefit on renal events with dapagliflozin was greater in anemic subjects than in non-anemic subjects.31 In terms of functional parameters, the increase in hemoglobin during treatment with SGLT2i has also been associated with improved oxygen consumption, improved quality of life and decreased natriuretic peptides in patients with HF.57
However, it is unlikely that the cardiovascular and renal benefits of SGLT2-i can be attributed to the increase in hemoglobin/hematocrit, since previous studies with erythropoiesis-stimulating agents have not demonstrated a benefit of hemoglobin normalization on cardiovascular events, including HF, or renal events in patients with CKD,58 however an increase in adverse effects hve been described. In the Reduction of Events by Darbepoetin Alfa in Heart Failure (RED-HF) study, treatment with darbepoetin did not reduce the risk of death or hospitalization for HF, and it was associated with an increase in thrombotic events,59 which is contrary to what has been observed with SGLT2-i.
More recently, the use of hypoxia-inducible factor prolyl-hydroxylase inhibitors (HIF-PHI) for the treatment of renal anemia have also failed to demonstrate a benefit on cardiovascular or renal events in CKD patients whether they were on dialysis or not, with respect to erythropoiesis-stimulating agents, despite improving ferrokinetics and achieving the same hemoglobin levels with circulating EPO levels within a more physiological range.60 All this suggests that the clinical benefits observed in patients on SGLT2-i treatment and their relationship with increased hemoglobin and hematocrit are due to multiple and complex pathophysiological mechanisms, well beyond the direct benefit of increased hemoglobin. Although it cannot be totally ruled out that the improvement in hemoglobin, and the consequent increase in tissue oxygen delivery, has a cardiovascular and renal benefit, which is attenuated by the confusing influence of the adverse cardiovascular effects of treatment with erythropoiesis-stimulating agents or HIF-PHI.
Very recently, an intervention study on the Canafliglozin and Renal Events in Diabetes With Established Nephropathy Clinical Evaluation (CREDENCE) study has observed that the benefit of increased hematological parameters is greater in patients with lower albuminuria, while in those with albuminuria > 300 m mg/g it would be associated to a reduction of albuminuria, although there is a complementary effect between the two.61
Potential mechanisms explaining the association between changes in hemoglobin/hematocrit and cardiorenal benefits of sodium-glucose cotransporter type 2 inhibitorsIn the very short term, hemoconcentration, secondary to its predominantly aquaretic effect, could be the mechanism that largely explains the initial increase in hemoglobin and hematocrit. In this line, a recent study performed in patients with HF and reduced ejection fraction showed an inverse relationship between the increase in hemoglobin and the initial decrease in glomerular filtration rate observed in these patients (another well-known parameter of hemoconcentration in patients treated with vigorous diuretic strategies).62 This relationship disappeared after three months, suggesting that there are other mechanisms involved in the increase of hemoglobin in the medium and long term, as previously discussed.62
Similarly, SGLT2-i would increase hemoglobin levels mainly by stimulating endogenous EPO production and enhancing iron kinetics through the stimulation of the hypoxia-inducible factor (HIF) pathway, partly sharing common mechanisms with HIF-PHI. However, SGLT2-i and HIF-PHI differ in some aspects.
HIF-PHI inhibitors act by inhibiting several PHIs (including PHD1, PHD2 and PHD3), thereby inhibiting the degradation of both HIF-1 and HIF-2.63 Of these, HIF-2 is the main physiological stimulus for EPO production and also controls the expression of genes involved in iron uptake and tissue distribution (such as bone marrow).64 Activation of HIF-1 would not be necessary and could be somewhat counterproductive, given that prolonged activation of this transcription factor favors proinflammatory pathways and accelerates the progression of cardiac and renal injury or angiogenesis.65
SGLT2-i decrease HIF-1 levels34,66 and by contrast they increase HIF-2 which reduces cellular stress and inflammation and has cytoprotective effects at the cardiac and renal levels.65 SGLT2-i increase sirtuin-1,67 that directly and selectively activate HIF-2. SGLT2i have been shown to increase HIF-2 at the cardiac level, potentially contributing to their antifibrotic actions.68
Another effect suggested in recent studies would be the benefit on EPO production and erythropoiesis by an improvement of tubulointerstitial function.69
More recently, proteomic studies shed light on potential mechanisms involved in the cardiorenal protection of SGLT2-i, among which would be the increase in EPO levels, but also has beneficial effects on proteins involved in cardiac contraction/relaxation, iron metabolism, metabolic effects or cardiorenal protection mechanisms, such as the promotion of autophagy or the reduction of oxidative stress, inflammation or fibrosis.70–72
In this regard, in an analysis of the Myocardial-IRON study, myocardial iron repletion after IV iron carboxymaltose administration was superior in the subgroup of patients receiving SGLT2i,73 suggesting that they may also improve myocardial iron metabolism, supporting the results from previous studies.44
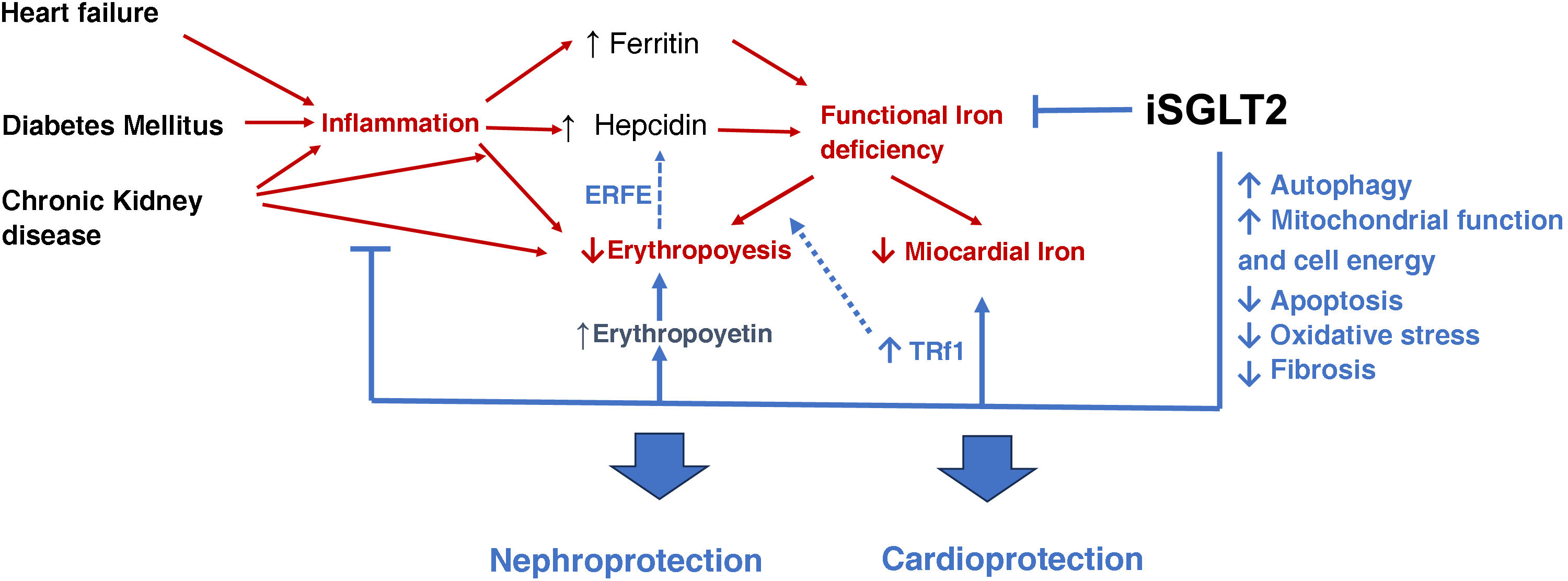
The beneficial effects of SGLT2-i, beyond increasing erythropoiesis are shown in schematic form in Fig. 1.74,75
Beneficial effects of SGLT2i beyond the increase in erythropoiesis. Indirect effects on kidney and heart.74,75 (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Heart failure, diabetes mellitus and chronic kidney disease favor inflammation, which increases hepcidin levels. This favors functional iron deficiency, decreasing iron availability in the bone marrow and at the myocardial level. Likewise, inflammation reduces erythropoiesis by inhibiting erythropoietin synthesis and its effects on erythropoiesis.
SGLT2i reduce inflammation, functional iron deficiency and increase erythropoietin levels, improving erythropoiesis. In addition, they optimize autophagy, mitochondrial function and decrease apoptosis, oxidative stress and fibrosis, resulting in nephro- and cardioprotection of these drugs.
SGLT2i: sodium-glucose cotransporter type 2 inhibitors; ERFE: erythroferrone; TRf1: transferrin receptor type 1.
Red arrows: deleterious effects. Blue arrows: positive effects.
In conclusion, treatment with SGLT2-i is associated with an increase in hemoglobin levels in the order of 0.5−0.7 g/dL, or its equivalent in hematocrit, probably due to multifactorial mechanisms; among these, improvement in erythropoiesis and in systemic iron metabolism. This increase in hemoglobin is associated in mediation studies with a better cardiorenal prognosis, suggesting that the increase in the hemoglobin and hematocrit could act as parameters monitoring response to treatment.
At the recent ASN (American Society of Nephrology) 2023 congress, the mediation analyses of the DAPA-CKD study were presented, confirming that the benefit on the primary renal event was mainly explained by the increase in hematocrit (35.5%) and the reduction in albuminuria (35.4%); the beneficial effect of the increase in hematocrit was observed in both diabetic and non-diabetic patients.76
Likewise, in a recent retrospective study in heart failure patients with reduced ejection fraction and iron deficiency, the increase in hemoglobin levels after IV iron administration was higher in patients treated with SGLT2-i than in those without treatment.77 It has been suggested that increased hemoglobin may increase blood viscosity and increase the risk of cardiovascular events.78 Alternatively, iron deficiency increases the number and activity of platelets. Similarly, transferrin has been shown to be involved in thrombosis,79,80 so studies are needed on the efficacy and safety of IV iron administration in patients with heart failure and iron deficiency treated with SGLT2-i.
Conflict of interestThe authors declare that they have no conflicts of interest.