Introducción: La aplicación sistemática de fórmulas matemáticas para estimar el filtrado glomerular (FGe) de la población general, atendiendo a la clasificación KDOQI de la enfermedad renal crónica (ERC), ha permitido conocer su prevalencia, considerándose un problema de Salud Publica. Muchos pacientes etiquetados de ERC (al menos estadio 3) son ancianos con un FGe disminuido sin otras manifestaciones típicas del daño renal, lo que está generando una preocupación entre los nefrólogos ante el incremento considerable de consultas no justificadas en esta población. Nuestro objetivo en este estudio es hacer un seguimiento prospectivo a 24 meses de ancianos con un FGe disminuido, para valorar qué ocurre tanto desde el punto de vista clínico como con su función renal (FR) y valorar si realmente nos tenemos que preocupar por esta “epidemia” de ERC en los ancianos. Pacientes y métodos: 80 pacientes clínicamente estables con una mediana edad de 83 años (rango 69-97) reclutados aleatoriamente en una consulta de Geriatría y en una de Nefrología General, entre enero y abril de 2006 fueron seguidos durante 24 meses. Hacemos dos grupos según niveles de creatinina sérica (Crs): grupo 1: 38 pacientes con creatinina sérica (Crs) £1,1 mg/dl (rango 0.7-1.1) y grupo 2: 42 pacientes, con Cr s > 1.1 mg/dl (rango 1.2-3). El 70% del total de pacientes tenía un estadio 3 ó 4 de ERC, de acuerdo con el FGe (MDRD abreviado). Clínicamente se estudiaron la morbi-mortalidad y los fármacos empleados. Analíticamente en sangre se determinó la Crs y se estimó el FG basal y 24 meses después según fórmulas de Cockroft y MDRD abreviado. En orina se realizó un sistemático a todos los pacientes para despistaje de proteinuria, cociente proteinas/creatinina en grupo 1 y cuantificación de proteinas en orina de 24 horas en el grupo 2. La estadística se realizó con el programa SPSS 11.0 usando medidas repetidas en el tiempo, chi-cuadrado y regresión logística. Resultados: Un 22,5% de los pacientes falleció antes de los 24 meses. Las patologías cardíaca e infecciosa fueron la comorbilidad más frecuente. No encontramos diferencias significativas entre ambos grupos en lo referente a morbimortalidad. La FR y la proteinuria permanecieron estables al cabo de los 24 meses, con independencia del grado de FGe previo. No hubo diferencias significativas en la evolución del resto de los parámetros analíticos estudiados salvo un descenso significativo del hematocrito en los ancianos del grupo 2 siendo sólo un 23,3% de los pacientes del grupo 2 los que continuaban con eritropoyetina al final del estudio Conclusión: en ancianos sin proteinuria, la estabilidad de la FR en el tiempo nos permite dar un mensaje tranquilizador a la hora de enfrentarnos a la “epidemia” de ERC en esta población.
Introduction: Introduction: Systematic application of mathematical formulae to estimate the glomerular filtration rate (eGFR) of the general population, according to KDOQI classification of Chronic Kidney Disease (CKD), has permitted to calculate its high prevalence, so as to be considered as a public health problem. Many patients with CKD according to this classification (at least stage 3) are elderly with a low GFR and without any other typical manifestations of renal damage, hich is generating a worry between nephrologists due to a significant increment in non justified referrals to their clinics. ur aim in this study is to follow-up during twenty-four months a group of elderly with a low eGFR. Patients and methods: 80 clinically stable patients, with a mediane age of 83 years (range 69-97), recruited alleatory in a consultation of Geriatric and Nephrology General, within January and April 2006, were followed up during twenty-four months. We separated them in two groups based in serum creatinine: Group 1: 38 patients with serum creatinine >_ 1,1 mg/dl (range 0,7–1,1), and Group 2: 42 patients with serum creatinine >1.1 mg/dl (range 1,2–3). Clinically we registered orbimortality and treatments received, and biochemically we measured in serum: creatinine and calculated eGFR at the time of recruitment and after twenty-four months of follow-up using two equations: Cockroft and abreviated MDRD. In urine we determinated basic urinalysis in all patients, protein/creatinine in group 1 and determinated protein in collection urine 24 hours group 2. Statistical comparisons were made using repeated measures, chisquare, and logistic regression of the SPSS 11.0 program. Results: 22,5% of the patients died during the follow up. Heart and infectious problems were the ind of morbidity more frequently found. Only a small proportion (23,3%) of group 2 patients were receiving erithropoietin treatment. Estimated GFR and proteinuria remained stable at the end of twenty-four months independently of basal eGFR. We found no significant differences between both groups in the rest of analytical parameters studied except a significant decrease of hematocrit in the elderly of group 2. Only a small proportion (23,3%) of group 2 patients were receiving rithropoietin treatment. Conclusion: In old patients without proteinuria, the stability of its renal function along the time llows us to give a soothing message at the moment of facing the so called CKD “epidemic” in this population.
INTRODUCTION
The ease of estimating glomerular filtration (GF) rate using mathematical formulae based on serum creatinine (SCr) with no need to laboriously collect urine over 24 hours enables us to ascertain renal function levels in the general population in a systematic way.1 In addition, the KDOQI guidelines define and classify chronic kidney disease (CKD) in various stages according to the patient’s estimated GF (eGF); for example, those patients whose eGF is between 30 and 60ml/min are at stage 3.2
Generalising this CKD classification to reach the entire population enabled us to know what the disease’s prevalence was.3,4 However, some studies5,6 find that a large proportion of patients who would be diagnosed with at least stage 3 CKD based on this classification are healthy elderly patients who simply have an eGF between 30 and 60ml/min and no other typical signs of chronic renal failure (CRF). The negative outcome of this phenomenon is the overload created when elderly patients with a low eGF,7 which is often physiological,8 and who have no other characteristic signs of CRF, are unnecessarily referred to nephrology departments.
Given that the prevalence of CKD is already known and a large percentage of the population with this diagnosis is elderly, and considering that this classification is already causing increased and often unnecessary demand for nephrology departments in several neighbouring countries,7 our objective in this study is to carry out a prospective 24-month follow-up on elderly patients with low eGF in order to evaluate both clinical and renal function outcomes.
PATIENTS AND METHODS
Patients
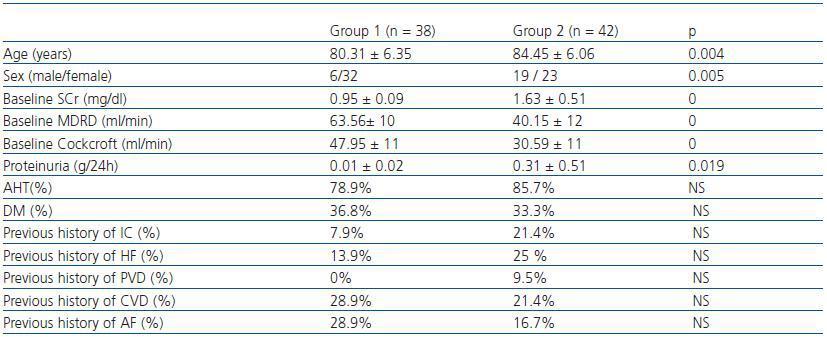
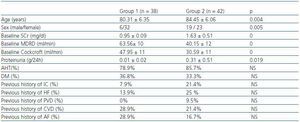
We studied 80 patients over 65 (median age: 83 years; range: 69-97 years) who were examined in a Geriatric and General Nephrology outpatient service during the period between January and April 2006. Patients were randomly chosen at these appointments. According to baseline SCr levels, we established two groups: group 1, n= 38 SCr ≤= 1,1mg/dl (range 0.7-1.1) and group 2, n= 42 SCr > 1.1mg/dl (range 1,2-3). Of the two groups combined, 68.8% of the patients were female; 37.3% had diabetes mellitus (DM) and 81.3% of all patients had arterial hypertension (AHT). The sociodemographic characteristics, the renal function and previous history of cardiovascular episodes per group are shown in table 1. Patients included in the study were clinically stable and received another clinical and analytical evaluation 24 months afterwards.
Patient distribution according to CKD stage (using the Cockcroft-Gault formula) in the baseline period was as follows: stage 1: 0%; stage 2: 7.9%; stage 3: 66.6%; stage 4: 20.6%; stage 5: 4.7%. Using the abbreviated MDRD formula: stage 1: 0%; stage 2: 30%; stage 3: 60%; stage 4: 10%; stage 5: 0%.
We recorded established treatments with anti-hypertensives, statins, calcium salts, iron, and erythropoietin.
Method
Prospective observational study. The first evaluation coincided with the patient’s scheduled visit to his/her primary centre during the period between January and April 2006. All patients were observed clinically and analytically during 24 months, and underwent a second evaluation between January and April 2008. In clinical observations, we recorded hospital admissions and their causes, as well as the occurrence of cardiovascular events and mortality. In analytical observations, we estimated the GF according to the Cockcroft-Gault9 and abbreviated MDRD formulae.10
The analytic calculations were made one week before the patients came in for their scheduled Geriatric and Nephrology check-ups, as a baseline measurement, and after 24 months. Venous blood was analysed according to routine hospital methodology to determine: creatinine, urea, uric acid, lipidogram, calcium, phosphorus, alkaline phosphatase, ionogram, haemoglobin and haematocrit. All patients underwent a systematic urine analysis to screen for proteinuria; for group 1, the protein/creatinine ratio was measured for a single void and in group 2, urine protein was measured over 24 hours.
In the baseline period: for group 1, no proteinuria was detected by the systematic analysis; the protein/creatinine ratio was < 0.05g, and for group 2, the baseline proteinuria was 0.31 ± 0.51g/24 hours, range 0-3g/24 hours. 87% of patients in group 2 had a proteinuria level below 0.5g/24 hours, 5.2% had proteinuria between 0.5-1g/24 hours and 7.8% had proteinuria between 2-3g/24 hours.
Statistical analysis
Statistical analysis was carried out using SPSS 11.0 software. Data is expressed as percentages, means and standard deviations. In order to evaluate the progression of renal function over time, we used a linear model for repeat measurements and percentage comparisons with Chi-square. Logistic regression was used to evaluate what factors predict mortality. Significance level is 95%.
RESULTS
Before completing the 24-month study period, 18 patients (22.5%) died (six of general deterioration, three of infections, two of complications due to fractures, two of cerebral ictus, one of a cardiac event and three, event unknown). Of the patients who died, 6 belonged to group 1 and 12 belonged to group 2 (not significant). There were no significant differences in mortality between the sexes (10 of those who died were women).
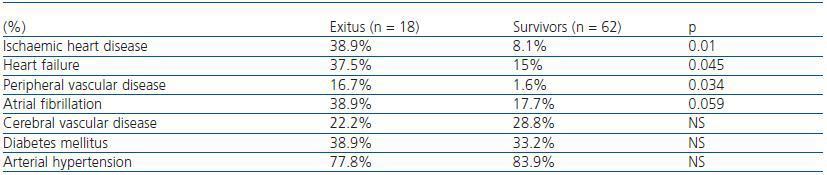
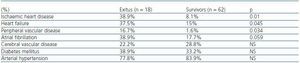
In table 2, we can see that the patients who died had a greater history of previous ischaemic cardiopathy (IC), heart failure (HF) and peripheral vascular disease (PVD). However, we found no differences in the history of other cardiovascular risk factors, such as DM or AHT. In the logical regression analysis, IC was shown as the principal factor determining mortality (exp[B] 20.83, 95% CI, 3.29-131.9, p = 0.001).
Regarding morbidity, 20 patients were admitted at least once and 5 patients were admitted twice throughout the 24-month period. We found no significant differences between both groups in the number of admissions (group 1: 10 admissions, group 2: 20 admissions) or for cardiovascular events (HF in group 1: 7.9%; HF in group 2: 25%: IC in group 1: 2.6%; IC in group 2: 5%). Cardiac causes and infections were the main etiological reasons for admission, comprising 52.2%.
Regarding drugs used after 24 months, 89.1% were on antihypertensives (71% of the same used diuretics), 21.7% were on statins, 21.7% were on calcium salts and 18.6% were on oral iron. Between the two groups, there were no significant differences in the frequency of use for these drugs. Significant differences only existed for use of erythropoietin: 23.3% of the patients in group 2 who were on this drug at the start of the study continued taking it during the following 24 months, while none of those from group 1 did (p=0.014); these patients had higher SCr levels (2.10 ± 0.29 vs. 1.14 ± 0.33mg/dl; p = 0.000) and lower eGF MDRD (30 ± 7 vs. 55 ± 14ml/min; p = 0.000).
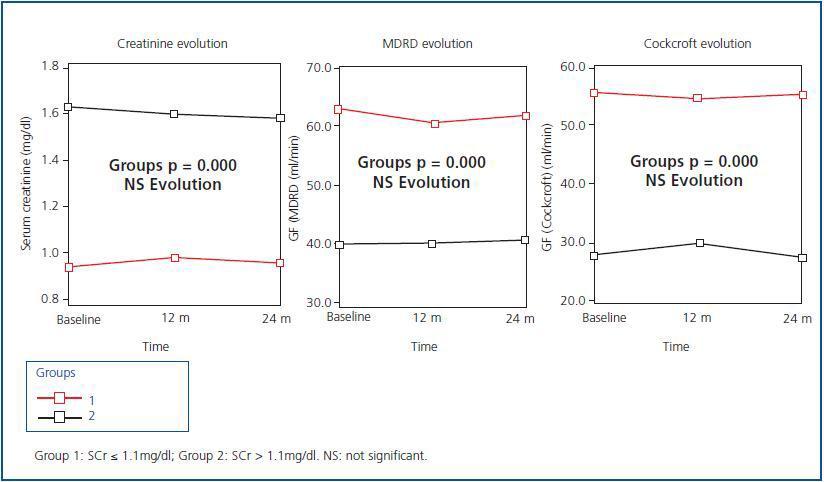
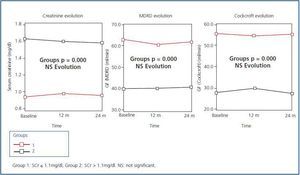
Figure 1 shows the evolution of renal function after 24 months of follow-up on the 62 elderly patients who finished the study. Although there are significant differences in SCr and eGF levels between the two groups (p = 0.000), we can see that during the evolution over 24 months, both SCr and eGF (MDRD and Cockcroft-Gault) did not vary significantly compared to baseline data. None of the patients required kidney replacement therapy (KRT)
In group 2, evolution of the proteinuria did not show significant changes after 24 months: baseline 0.31 ± 0.51g/24 hours vs. 24 months: 0.41 ± 0.81g/24 hours.
In the analysis by group, we found no significant changes in the progression of the analytical parameters that were studied (calcium-phosphorus, ions, lipids). Only 23.3% of patients on erythropoietin (group 2) had been on it since the beginning of the study, with baseline haematocrit: 42. 24 ± 5%, and at 24 months: 39.8± 4% (p = 0.017), and the same patients were still on erythropoietin at the end of the study. Patients in group 1 who did not need erythropoietin at baseline still did not need it after 24 months, as they managed to maintain their haematocrit levels; baseline: 41.6 ± 3% and 24 months: 42 ± 3%.
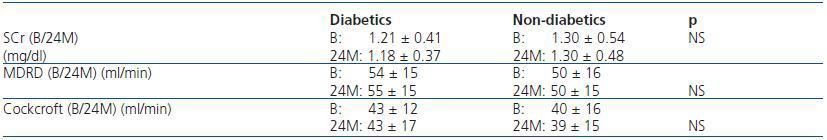
Furthermore, upon comparing the evolution of the eGF between diabetic and non-diabetic patients, we found no significant changes with the passing of time (table 3).
DISCUSSION
At present, CKD is considered to be a public health problem.11 Its recognition has been made possible in part by the acceptance of the conceptual model, definition and classification of CKD proposed by the National Kidney Foundation/Kidney Disease Outcome Quality Initiative (NKF/KDOQI).2,12
Applying this classification systematically to the entire population has enabled us to know what prevalence CKD has in its different stages.3,4 In numerous studies, based on the definition established by the KDOQI (stage 3: eGF between 30 and 60ml/min),13 we find that most of the population diagnosed with CKD is elderly. At the same time, the study Wetzels et al.5 carried out on healthy elderly individuals concludes that in most of these patients, the only sign of CKD is the finding of a low eGF which is not accompanied by other kidney-damaging changes, except in a low number of cases that present proteinuria.
In addition, after some countries introduced routine GF estimation (eGF) for the entire population seen by a primary care physician, there has been a considerable increase in referring patients to nephrology centres when their only sign of CKD is an eGF < 60ml/min. Many of these patients are women older than 65 with no other signs of CRF, which has caused patients and professionals to worry, aside from affecting costs.7,14
Therefore, the present study investigates how renal function evolves over 24 months in elderly patients whose baseline eGF is low and who should therefore be diagnosed with CKD according to that classification. Our purpose is to evaluate the real significance of this CKD «epidemic» in the elderly, and whether or not these patients truly benefit from referral to a nephrology specialist. In the follow-up study of renal function, after 24 months we confirmed the data that were already published regarding renal function at 12 months: renal function remained stable over time in elderly patients with no proteinuria.15 Our results are comparable to those from other studies such as Hemmelgarm’s,16 which describes CKD progression in a > 66 population throughout two years of follow-up, and observes that progression is slow, except in diabetic patients and patients whose GF is < 30ml/min. After seeing that renal function is stable over time and considering the increasing and often unnecessary demand for nephrology examinations, we believe that systematic GF estimation in the elderly population, which is simply used to define and classify CKD, might not be justified, which would enable us to dampen the increasing demand for referrals to nephrologycentres.
Upon analysing the presence of typical signs of CRF in patients with low eGF, we found that in our study, overall, patients with low eGF did not show the typical signs of CRF, either at baseline or after the 24-month follow-up. Fewer than 25% of patients in group 2 were being treated with erythropoietin from the beginning, and they were in fact the only ones to show a significant decrease in haematocrit levels over time.
Furthermore, numerous clinical studies show a correlation between the level of proteinuria and the progressive loss of renal function, and proteinuria is considered to be the most important predictor of CKD progression.17 In this study, we cannot demonstrate the harmful effect that proteinuria has on FR, since only a small number within group 2 has a proteinuria level above 0.5g/24 hours. For this reason, even though proteinuria is a modifiable factor that can be counteracted, many elderly patients referred to nephrology centres do not have proteinuria, as is true in our case. These patients will not benefit from being referred to a nephrology specialist, because they do not possess that factor requiring action on our part.
Additionally, when we analyse morbidity and mortality in these patients, we find that there were no significant differences between the groups that were studied, and that part of the mortality rate can be attributed to the effect of old age (progressive deterioration). We must take into account the fact that traditional risk factors such as AHT,18 dyslipidaemia and diabetes are very frequently present in the elderly, and that this also contributes to their developing cardiovascular disease.19 Likewise, we must keep in mind that for CKD patients, whether they are on dialysis or have received a kidney transplant, cardiovascular diseases are the principal cause of death.20 Nevertheless, in this study, the predominant final cause of death was progressive deterioration and not cardiovascular disease (this may be due to the low number of patients). When we analyse the presence of a prior history of cardiovascular disease, however, we can see that patients who died are those with the most history of those events, and according to the logical regression analysis, the presence of a history of HF was the most important mortality predictor in these patients.
With a cohort of 396 patients with stage 4 CKD, Conway et al.21 studied their mortality predictors and the need for KRT and found that most of them, the elderly most of all, died before beginning KRT, and that those patients with a low risk of renal progression can be observed from a primary health centre. Similarly, none of our patients who died needed kidney replacement therapy, and renal function follow-up for the patients who were still alive after 24 months showed that renal function remained stable over time, with no patient needing to go on KRT.11
If we consider that RF in these patients remains stable over time, with a low risk of progressive renal damage, whereas a high risk of mortality does exist (which is not directly related to kidney disease), the approach to CKD in the elderly should focus on intensive primary cardiovascular disease prevention, which can be done from a primary health centre. In this study, we observe how nearly 90% of patients remaining in the study received anti-hypertension treatment, which allows us to speculate on the possibility of this being one of the key elements explaining the stability of renal function over time, as well as patient survival.
In conclusion, we see that many healthy elderly patients, including those with SCr within the normal range, are labelled with CKD on the sole basis of having a low eGF according to the KDOQI’s classification when they show no other signs associated with kidney damage (proteinuria, anaemia, etc.). Our results indicate that not only do many of these patients not present these signs (despite having an eGF < 60ml/min, but also, in elderly patients with a low eGF without proteinuria, renal function remains stable over time. This allows us to conclude that the current established classification method should not be applied systematically to the geriatric population. Likewise, a significant number of English nephrologists support Glassock and Winearls’ proposal7 of being more careful and avoiding routine estimation of GF until the classification proposed by KDOQI guides is more defendable from a scientific viewpoint. This would avoid scaring patients unnecessarily and contribute to reducing the increasing worries among nephrologists due to unnecessary referrals to their centres and the high costs resulting from that situation.
To conclude, for elderly patients without proteinuria, stability of renal function over time sends a soothing message when it comes to facing the CKD «epidemic» in the geriatric population. Patients who, in addition to having a low GF, also show other signs of kidney damage (proteinuria, anaemia, etc.) are the ones who should be referred to a nephrology specialist.
Table 1. Socio-demographic characteristics and baseline renal function and co-morbidity in the groups under study.
Table 2. Percentage of prior cardiovascular history
Table 3. Renal function evolution according to presence of diabetes mellitus
Figure 1.