Despite current treatments, which include renin angiotensin system blockers and SGLT2 inhibitors, the risk of progression of kidney disease among patients with diabetes and chronic kidney disease (CKD) remains unacceptably high. The pathogenesis of CKD in patients with diabetes is complex and includes hemodynamic and metabolic factors, as well as inflammation and fibrosis. Finerenone is a highly selective nonsteroidal mineralocorticoid antagonist that, in contrast to current therapies, may directly reduce inflammation and fibrosis, thus adding value in the management of these patients. In fact, finerenone decreases albuminuria and slows CKD progression in persons with diabetes. We now review the mechanisms of action of finerenone, the results of recent clinical trials, and the integration of the kidney and cardiovascular protection afforded by finerenone in the routine care of patients with diabetes and CKD.
A pesar de los tratamientos actuales, que incluyen los inhibidores del sistema renina angiotensina y los inhibidores SGLT2, el riesgo de progresión de la enfermedad renal en los sujetos con diabetes y enfermedad renal crónica (ERC) continúa inaceptablemente alto. La patogenia de la ERC en el paciente con diabetes es compleja, e incluiría factores hemodinámicos, metabólicos, y de inflamación y fibrosis. La finerenona es un antagonista no esteroideo altamente selectivo del receptor mineralocorticoide que, a diferencia de los tratamientos actuales, podría disminuir directamente la inflamación y la fibrosis, aportando un valor añadido al abordaje de estos pacientes. De hecho, finerenona disminuye la albuminuria y enlentece la progresión de la ERC en personas con diabetes. La presente revisión aborda el mecanismo de acción de la finerenona, los resultados de ensayos clínicos recientes y la integración en práctica clínica de la nefroprotección y cardioprotección de la finerenona en el abordaje terapéutico integral del paciente diabético con ERC.
It is estimated that, in the year 2021, 537 million persons aged between 20 and 79 years had diabetes mellitus worldwide (approximately one out of 10). In addition, diabetes was responsible for 6.7 million deaths and was associated with very high healthcare costs, mainly related to the complications associated with the disease. However, due to both the aging of the population and lifestyle habits (sedentary lifestyle and overweight/obesity), it is estimated that these values will increase to 783 million people by 2045.1 Moreover, 850 million people have chronic kidney disease (CKD); in Spain one out of 6 adults has CKD, and it is estimated that before the end of the century this number will increase to one out of 4, making the disease the second leading cause of death after Alzheimer's disease.2,3 Approximately 2 out of every 5 patients with diabetes have CKD, and nearly 40% of cases of terminal CKD are attributable to diabetes.4
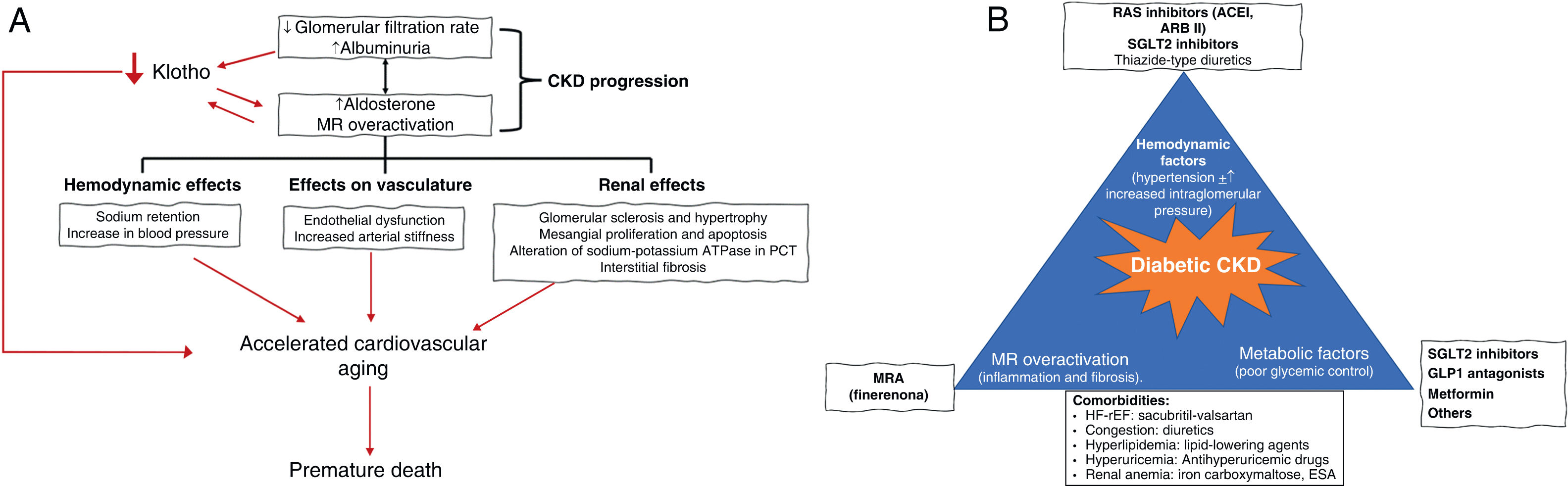
Although diabetes and CKD alone reduce life expectancy, when both conditions concur this reduction is much greater. Thus, in patients with diabetes and early CKD, life expectancy would be reduced by 16 years.5 Persistent albuminuria alone is sufficient to diagnose CKD because albuminuria increases the risk of developing renal and cardiovascular complications and death.6 In fact, albuminuria causes the loss of an important function of the kidney, the production of the anti-aging protein Klotho, long before the glomerular filtration rate decreases. Loss of Klotho causes hyperaldosteronism and accelerated aging characterized by hypertrophy, cardiac fibrosis and vascular calcification.7,8
Several factors, including hyperglycemia, arterial hypertension, abnormalities in lipid metabolism, activation of the renin angiotensin system (RAS), and inflammation, have been associated with the development of microvascular damage in patients with diabetes.3 Likewise, both aldosterone and the excesive activation of the mineralocorticoid receptor (MR) are involved in the development and progression of CKD. Finerenone, a new nonsteroidal MR antagonist, not only decreases proteinuria in patients with type 2 diabetes (DM2) and CKD, but also appears to slow the progression of CKD.9,10
This review analyzes the effects of finerenone in patients with DM2 and CKD, as well as practical aspects in the management of the drug in this population. For this purpose, a narrative review was performed by means of a search in PubMed (MEDLINE) until April 2022 using the MeSH terms [diabetes]+ [chronic kidney disease]+[Mineralocorticoid Receptor Receptor Antagonists]+[finerenone]+[albuminuria] without language restrictions.
Residual risk in patients with diabetic kidney disease despite traditional treatmentsFor decades, angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARB II)4,11–13 have been used to slow the progression of CKD in patients with diabetes. However, despite the benefit observed in diabetic nephropathy in terms of progression of kidney disease, in both the RENAAL (losartan) and IDNT (irbesartan) studies, 43.5% and 32.6% of patients, respectively, treated with ARB II reached the primary outcome measure (doubling of serum creatinine from baseline, end-stage renal disease or death).12,13
Sodium-glucose cotransporter type 2 (iSGLT2) inhibitors also reduce the risk of developing CKD and slow its progression. Nevertheless, in both the CREDENCE (canagliflozin) and DAPA-CKD (dapagliflozin) studies, approximately 10% of patients with CKD treated with iSGLT2 and RAS blockade experienced progression of their disease.14,15 Consequently, although the benefit of these treatments in the diabetic patient with CKD is evident, there is still a substantial residual risk of progression of renal disease.16
Patients treated with ACEI or ARA II are affected by aldosterone escape, which is associated with increased proteinuria and worsening renal function.17 Although combination of the classic antialdosterone drugs (spironolactone and eplerenone) reduces proteinuria, no benefit has been demonstrated in the progression of CKD.4,18 In contrast, finerenone not only significantly reduces proteinuria in subjects with DM2 and CKD, but it also slows the progression of CKD.9,10 Finerenone is indicated for the treatment of CKD (stages 3 and 4 with albuminuria) associated with DM2.19 Presently only RAS inhibitors (ACEI and ARB II), iSGLT2 inhibitors (canagliflozin and dapagliflozin), and finerenone are approved as renoprotective agents in patients with CKD and diabetes.4
In fact, the evidence obtained from the different clinical trials has influenced the recommendations of the KDIGO guidelines on the treatment of CKD in diabetes that have been updated in a short period of time. In 2020, the KDIGO guidelines were updated to include iSGLT2, and in 2022 they were updated again, including the new findings with iSGLT2 and also the new evidence from the finerenone trials.20–22 In addition, the latest update of the American Diabetes Association (ADA) guidelines in 2022 recommends the use of finerenone to slow progression of CKD and reduce CV events in diabetic patients with CKD at an increased risk of CV events or progression of CKD or in cases that cannot use iSGLT2; these recommendations had the highest level of evidence (class A).23
The mineralocorticoid receptor and the pathogenesis of chronic kidney diseaseThe understanding of the role of the RAS in the pathogenesis of CKD has been clarified over time. Thus, according to the old paradigm, upon a drop in blood pressure or a decrease in extracellular volume, the RAS was activated, increasing the release of renin by the kidney, increasing levels of angiotensin II, which has a direct effect on the blood vessels—by stimulating vasoconstriction—and increasing the release of aldosterone in the adrenal cortex. Aldosterone acts on the kidney, promoting retention of sodium and blood volume and, concomitantly, potassium excretion in the distal convoluted tubule. Thus, aldosterone played a central role in maintaining both extracellular volume homeostasis and blood pressure control. Hyperactivation of the RAAS was associated with an increased risk of arterial hypertension and CKD, among others.18,24–30
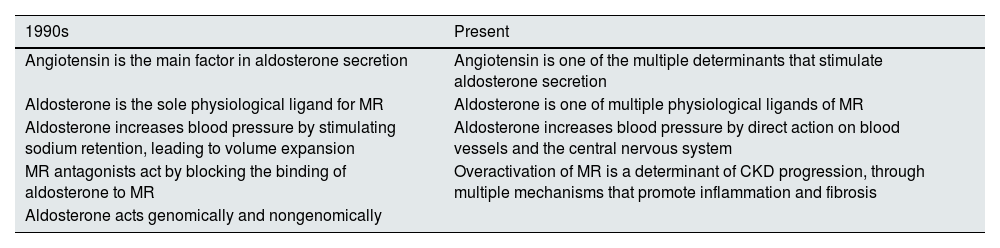
However, the greater complexity of the RAAS is now recognized. Thus, overactivation of the MR plays a crucial role in the genesis of CKD (Table 1). Previously, it was thought that angiotensin II was the main driver of aldosterone secretion and that aldosterone was the only physiological ligand of the MR. However, MR is also activated by the binding of other ligands (aldosterone, cortisol and progesterone), because of a loss of homeostasis (if there is elevated glycemia or excess dietary sodium ) or the presence specific proteins.18,24,25
Paradigm shift in the role of aldosterone and the mineralocorticoid receptor in the pathogenesis of chronic kidney disease.
| 1990s | Present |
|---|---|
| Angiotensin is the main factor in aldosterone secretion | Angiotensin is one of the multiple determinants that stimulate aldosterone secretion |
| Aldosterone is the sole physiological ligand for MR | Aldosterone is one of multiple physiological ligands of MR |
| Aldosterone increases blood pressure by stimulating sodium retention, leading to volume expansion | Aldosterone increases blood pressure by direct action on blood vessels and the central nervous system |
| MR antagonists act by blocking the binding of aldosterone to MR | Overactivation of MR is a determinant of CKD progression, through multiple mechanisms that promote inflammation and fibrosis |
| Aldosterone acts genomically and nongenomically |
CKD, chronic kidney disease; MR, mineralocorticoid receptor.
In addition, it was classically thought that the mechanism whereby aldosterone increased blood pressure was by stimulating sodium retention, leading to volume expansion. However, this is not the only mechanism; it also acts directly on vessels and on the central nervous system. Likewise, overactivation of MR decreases tubular production of the anti-aging protein Klotho and increases inflammation and fibrosis, promoting renal and cardiovascular damage.8 Thus, overactivation of MR favors glomerulosclerosis and glomerular hypertrophy, podocyte loss, and macrophage infiltration.4,18 In the proximal convoluted tubule, there is an increase in the expression of the sodium-potassium ATPase pump, the main consumer of energy in these cells.4,18 In addition, inflammation and oxidative stress promote tubulointerstitial fibrosis. Podocyte injury causes albuminuria and proteinuria, thus aggravating proximal tubular injury and loss of Klotho, generating a positive feedback loop that perpetuates tissue injury by favoring the progression of CKD, even after the triggering factor disappears (in the case of diabetes, the hyperglycemia).31 The loss of podocytes and the consequent glomerulosclerosis reduce the number of glomeruli by recruiting hemodynamic factors, such as increased intraglomerular pressure, which lead to hyperfiltration and dragging of more podocytes, thus increasing albuminuria and further overloading the renal tubules and worsening inflammation and fibrosis. In this way, we can understand the key role of MR overactivation in the genesis and development of CKD, and appreciate that it is much more complex than originally thought (Fig. 1A).16,18
Aldosterone and mineralocorticoid receptor in the pathogenesis of chronic kidney disease (A) and comprehensive protection of the patient with diabetic nephropathy (B).
ESA: erythropoiesis-stimulating agents; MRA: mineralocorticoid receptor antagonist; ARB II: angiotensin II receptor antagonist; ACEI: angiotensin-converting enzyme inhibitor; CKD: chronic kidney disease; GLP1R: glucagon-like peptide receptor type 1; HF-rEF: heart failure with reduced ejection fraction; ACEI: angiotensin-converting enzyme inhibitors; MR: mineralocorticoid receptor; SGLT2: sodium-glucose cotransporter type 2; RAS: renin angiotensin system; PCT: proximal convoluted tubule.
Source: Fernandez-Fernandez et al.,7 Barrera-Chimal et al.,16 Erráez et al.,18 Epstein,24 Epstein,25 Alicic et al.,26 Buonafine et al.,27 Bauersachs et al.,28 DuPont et al.,29 Alicic et al.,30 Ruiz Ortega et al.,31 Brown et al.32 and Mima.33
In short, the pathogenesis of CKD in the patient with DM2 involves multiple converging mechanisms, including poor metabolic control as a trigger and potential accelerator of the process, as well as MR-mediated inflammation and fibrosis and abnormal hemodynamics that, once established, are self-perpetuating, even after the correction of the triggering factor (Fig. 1B).16,18,24–33
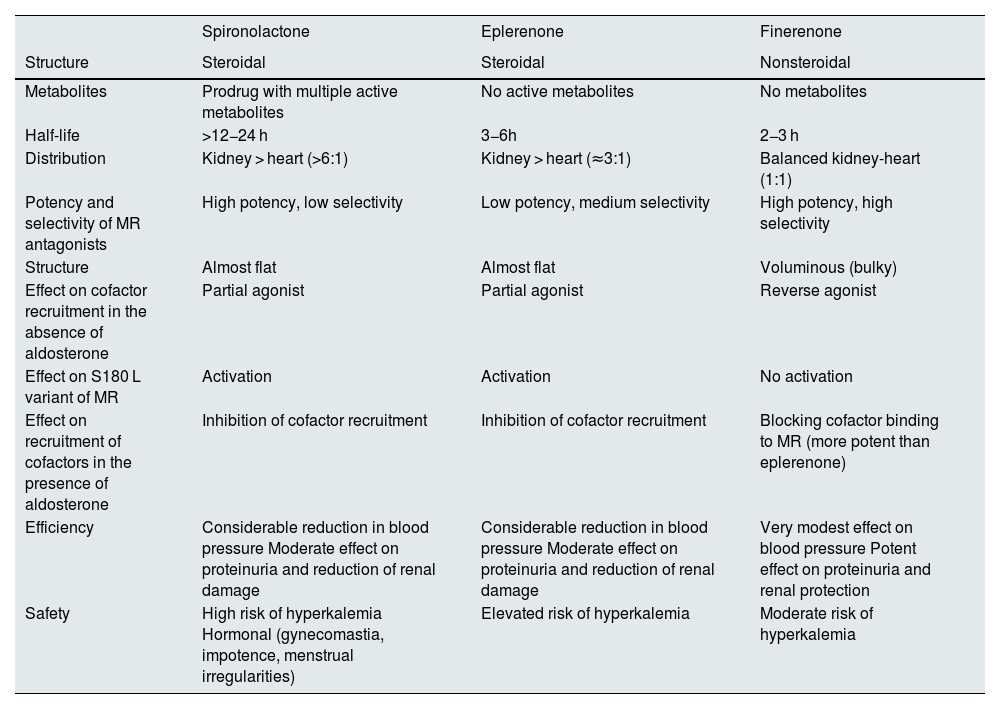
Differences between the various mineralocorticoid receptor antagonistsSpironolactone, eplerenone, and finerenone are MR antagonists. However, there are relevant differences between finerenone and the other two agents, and these have a fundamental impact on their efficacy and safety (Table 2).
Characteristics of mineralocorticoid receptor antagonists.
| Spironolactone | Eplerenone | Finerenone | |
|---|---|---|---|
| Structure | Steroidal | Steroidal | Nonsteroidal |
| Metabolites | Prodrug with multiple active metabolites | No active metabolites | No metabolites |
| Half-life | >12−24 h | 3−6h | 2−3 h |
| Distribution | Kidney > heart (>6:1) | Kidney > heart (≈3:1) | Balanced kidney-heart (1:1) |
| Potency and selectivity of MR antagonists | High potency, low selectivity | Low potency, medium selectivity | High potency, high selectivity |
| Structure | Almost flat | Almost flat | Voluminous (bulky) |
| Effect on cofactor recruitment in the absence of aldosterone | Partial agonist | Partial agonist | Reverse agonist |
| Effect on S180 L variant of MR | Activation | Activation | No activation |
| Effect on recruitment of cofactors in the presence of aldosterone | Inhibition of cofactor recruitment | Inhibition of cofactor recruitment | Blocking cofactor binding to MR (more potent than eplerenone) |
| Efficiency | Considerable reduction in blood pressure Moderate effect on proteinuria and reduction of renal damage | Considerable reduction in blood pressure Moderate effect on proteinuria and reduction of renal damage | Very modest effect on blood pressure Potent effect on proteinuria and renal protection |
| Safety | High risk of hyperkalemia Hormonal (gynecomastia, impotence, menstrual irregularities) | Elevated risk of hyperkalemia | Moderate risk of hyperkalemia |
Finerenone is a highly selective nonsteroidal MR antagonist. Its binding to the MR gives rise to a specific receptor-ligand complex that blocks the recruitment of transcriptional coactivators involved in the expression of proinflammatory and profibrotic mediators.4,19,24 This is because finerenone has a bulky nature that allows it to bind to the MR in a manner that occupies the entire receptor, preventing activation, and prevents the MR from converting or adopting an agonist conformation. In contrast, spironolactone is a steroid antagonist, with an almost flat, credit card-like structure, high potency and low selectivity for the MR, whereas eplerenone is a steroid antagonist with low potency and medium selectivity for the MR. Moreover, in the absence of aldosterone both spironolactone and eplerenone act as partial agonists on the recruitment of cofactors that can favor the transcription of proinflammatory genes and the expression of profibrotic genes. In fact, both activate the S810 L variant of MR, which causes early severe hypertension. However, finerenone is an inverse agonist (inhibits cofactor binding in the absence of aldosterone) that by its bulky conformation inhibits or decreases cofactor recruitment; therefore, a reduction in inflammation and fibrosis due to MR antagonism is to be expected. The MR antagonists (MRAs) also differ in their distribution, as shown in animal models: for spironolactone and eplerenone, distribution in the kidney predominates over the heart, which could increase the risk of hyperkalemia, whereas the distribution of finerenone is balanced (Table 2).4,19,24,25,34–39
These differences have an impact on the efficacy and safety of these drugs. Thus, spironolactone and eplerenone reduce blood pressure considerably and have a moderate impact on inflammation, fibrosis, and proteinuria, thus limiting the beneficial effect on renal damage.4,19,34–37 By contrast, Finerenone, has a very modest effect on blood pressure. Animal models have shown that, by acting on MR, finerenone provides renal protection through multiple mechanisms, blocking the transcription of profibrotic and proinflammatory genes in several cell types at the renal level, such as podocytes, mesangial cells, macrophages and fibroblasts, as well as tubular cells. This results in a lower degree of injury, inflammation and fibrosis, and ultimately in reduced albuminuria and slower progression of CKD observed in clinical trials.40
Furthermore, the risk of hyperkalemia is clearly higher with spironolactone and eplerenone than with finerenone (Table 2).4,19,34–37
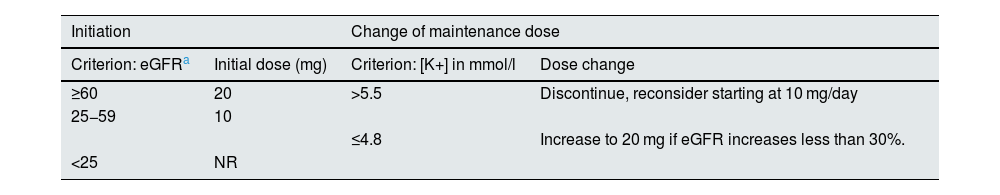
Finerenone in the treatment of diabetic kidney diseaseFinerenone is the only MRA approved to treat diabetic kidney disease. Specifically, it is indicated to treat adults with CKD stages G3 or G4 (glomerular filtration rate 15−60 ml/min/1.73 m2) and albuminuria A2 or A3 (urine albumin:creatinine ratio >30 mg/g) associated with DM2.22 Although finerenone, once started, can be maintained until the glomerular filtration rate falls below 15 ml/min/1.73 m2, it is not recommended to start it if the glomerular filtration rate is <25 ml/min/1.73 m2, owing to the lack of clinical data. The effect of finerenone is dose-dependent, and the drug is rapidly absorbed (time to peak concentration 0.5–1.0 h) and eliminated (terminal half-life ≈2−3 h). The recommended target dose of finerenone is 20 mg once daily, which is the maximum recommended dose. Table 3 shows the starting dose and how to adjust the dose during follow-up. Serum potassium and glomerular filtration rate should be measured at baseline and at 4 weeks after initiation, re-initiation, or an increase in the dose of finerenone. There is no need to adjust the dose according to age, body weight, or the presence of mild or moderate liver failure; moreover, another advantage of finerenone is that it requires only one dose per day. However, its use is not recommended in cases of severe liver failure, due to the lack of clinical data. Finerenone is metabolized via CYP3A4. It should not be used concomitantly with potent CYP3A4 inducers or inhibitors. In contrast, it can be taken with moderate or weak CYP3A4 inhibitors and with potassium supplementation, although renal function and potassium should be monitored more closely in affected patients.19,37
Finerenone: Starting dose and dose adjustment during follow-up.
| Initiation | Change of maintenance dose | ||
|---|---|---|---|
| Criterion: eGFRa | Initial dose (mg) | Criterion: [K+] in mmol/l | Dose change |
| ≥60 | 20 | >5.5 | Discontinue, reconsider starting at 10 mg/day |
| 25−59 | 10 | ||
| ≤4.8 | Increase to 20 mg if eGFR increases less than 30%. | ||
| <25 | NR | ||
eGFR, estimated glomerular filtration rate; NR, not recommended; K+, potassium.
Several studies have shown that finerenone effectively reduces albuminuria in patients with CKD. Thus, in the Mineralocorticoid Receptor Antagonist Tolerability Study (ARTS)41 phase 2 clinical trial, finerenone at doses of 2.5–10 mg/day reduced albuminuria in patients with CKD, with a lower incidence of hyperkalemia than spironolactone in patients with heart failure with reduced ejection fraction and mild to moderate CKD. In the ARTS-Diabetic Nephropathy (ARTS-DN) study,42 whose primary objective was to evaluate the safety and efficacy of 7.5, 10, 15, and 20 mg per day of oral finerenone for 90 days in patients with DM2 and albuminuria >30 mg/g on treatment with ACEI or ARA II, finerenone significantly reduced albuminuria compared to placebo. But without doubt, the clinical trials, “Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD)”9 and “Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD)”10 have been the pivotal studies that have provided most evidence of the benefits of finerenone in renal protection.
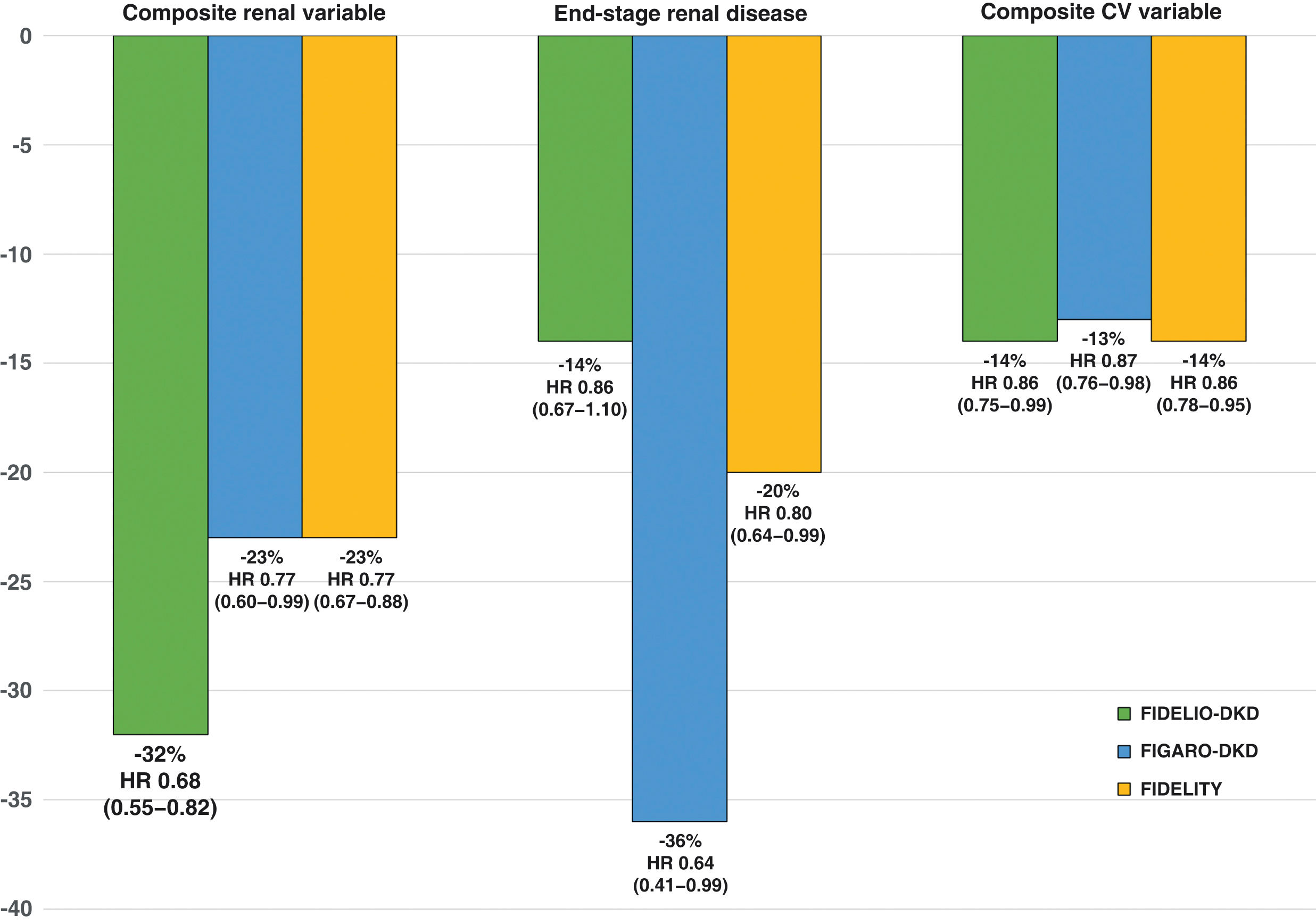
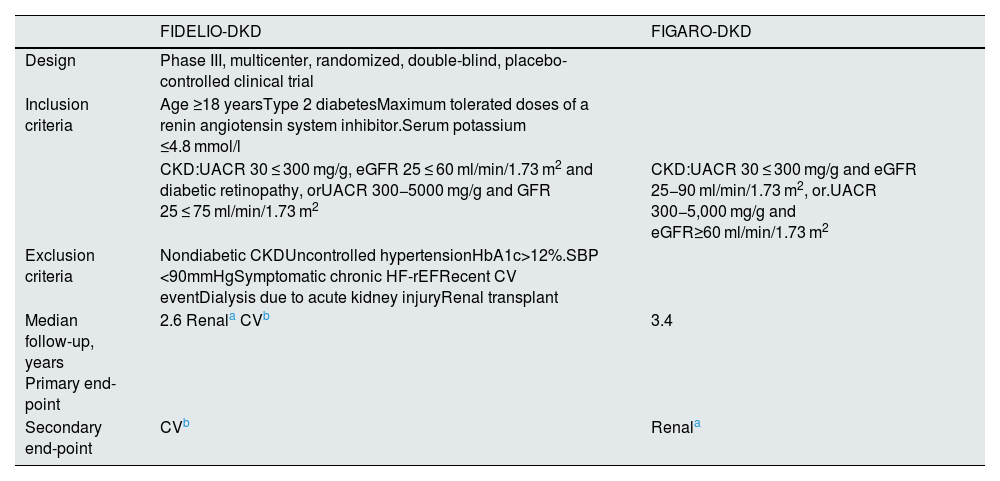
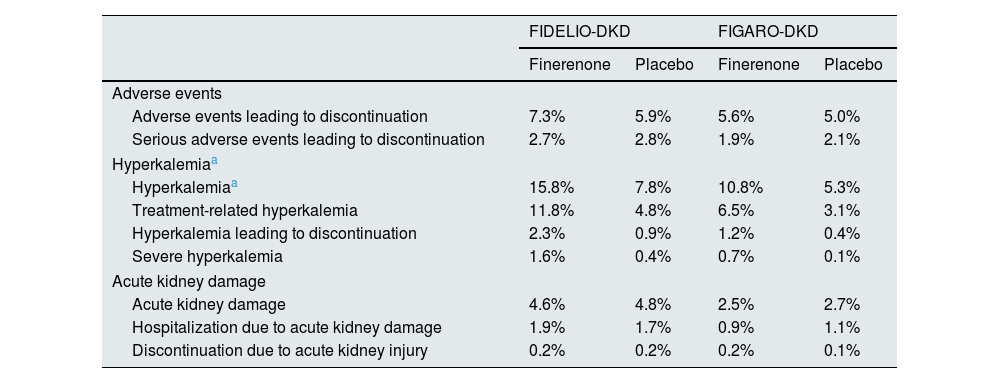
The FIDELIO-DKD study9 randomized to finerenone or placebo about 5,700 patients with DM2 and CKD, defined as an albumin creatinine ratio 30 ≤ 300 mg/g, estimated glomerular filtration rate 25 ≤ 60 ml/min/1.73 m2 and diabetic retinopathy, or as an albumin-creatinine ratio of 300−5,000 mg/g and estimated glomerular filtration rate of 25 ≤ 75 ml/min/1.73 m2, in treatment with maximum tolerated doses of ACEI or ARA II and serum potassium ≤4.8 mmol/l (Table 4). After a mean follow-up of 2.6 years, as compared with placebo, treatment with finerenone was associated with a significant reduction of 18% in the primary renal composite outcome and 19% in the sustained decline in glomerular filtration rate ≥40% from baseline levels (Table 5 and Fig. 2). In addition, finerenone reduced albuminuria by 31% at 4 months. In comparison with the placebo group, the reduction was maintained throughout follow-up. That is, finerenone slowed the progression of CKD in patients with DM2 and CKD. The risk of serious adverse effects leading to discontinuation, as well as acute kidney damage, were similar in both groups. By contrast, the incidence of severe hyperkalemia was higher in the finerenone group (1.6% vs. 0.4%), although there were few cases of hyperkalemia requiring discontinuation (2.3% vs. 0.9%) and no cases of death due to hyperkalemia (Table 6). The effects of finerenone on systolic blood pressure were very modest (−2.1 mmHg at one year of treatment vs. −0.9 mmHg in the placebo group).
General characteristics of the FIDELIO-DKD and FIGARO-DKD studies.
| FIDELIO-DKD | FIGARO-DKD | |
|---|---|---|
| Design | Phase III, multicenter, randomized, double-blind, placebo-controlled clinical trial | |
| Inclusion criteria | Age ≥18 yearsType 2 diabetesMaximum tolerated doses of a renin angiotensin system inhibitor.Serum potassium ≤4.8 mmol/l | |
| CKD:UACR 30 ≤ 300 mg/g, eGFR 25 ≤ 60 ml/min/1.73 m2 and diabetic retinopathy, orUACR 300−5000 mg/g and GFR 25 ≤ 75 ml/min/1.73 m2 | CKD:UACR 30 ≤ 300 mg/g and eGFR 25−90 ml/min/1.73 m2, or.UACR 300−5,000 mg/g and eGFR≥60 ml/min/1.73 m2 | |
| Exclusion criteria | Nondiabetic CKDUncontrolled hypertensionHbA1c>12%.SBP <90mmHgSymptomatic chronic HF-rEFRecent CV eventDialysis due to acute kidney injuryRenal transplant | |
| Median follow-up, years Primary end-point | 2.6 Renala CVb | 3.4 |
| Secondary end-point | CVb | Renala |
| Clinical characteristics and baseline treatments | ||
|---|---|---|
| Age, years | 65.6 ± 9.1 | 64.1 ± 9.8 |
| Male sex (%) | 70.2 | 69.4 |
| HbA1c, % | 7.7 ± 1.3 | 7.7 ± 1.4 |
| eGFR, ml/min/1.73 m2 | 44.3 ± 12.6 | 67.8 ± 21.7 |
| Urine albumin-to-creatinine ratio, mg/g g | 852 (446−1.634) | 308 (108−740) |
| ≥300 mg/g (%) | 87.5 | 50.7 |
| IECA/ARA II | 100 | 100 |
| GLP-1 receptor agonist | 394 (6.9) | 550 (7.5) |
| SGLT2 inhibitor | 259 (4.6) | 618 (8.4) |
ARA II, angiotensin II receptor antagonists; UACR, urine albumin-creatinine ratio; CV, cardiovascular; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; GLP1, glucagon-like peptide type 1; HF-rEF, heart failure with reduced ejection fraction; ACEI, angiotensin-converting enzyme inhibitors; MI, myocardial infarction; SBP, systolic blood pressure; SGLT2, sodium-glucose cotransporter type 2.
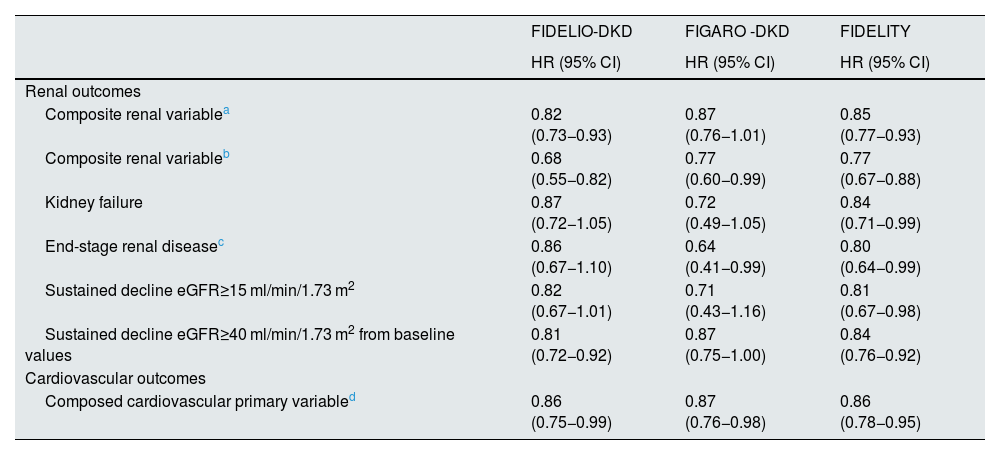
Main results of the FIDELIO-DKD and FIGARO-DKD studies.
| FIDELIO-DKD | FIGARO -DKD | FIDELITY | |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Renal outcomes | |||
| Composite renal variablea | 0.82 (0.73−0.93) | 0.87 (0.76−1.01) | 0.85 (0.77−0.93) |
| Composite renal variableb | 0.68 (0.55−0.82) | 0.77 (0.60−0.99) | 0.77 (0.67−0.88) |
| Kidney failure | 0.87 (0.72−1.05) | 0.72 (0.49−1.05) | 0.84 (0.71−0.99) |
| End-stage renal diseasec | 0.86 (0.67−1.10) | 0.64 (0.41−0.99) | 0.80 (0.64−0.99) |
| Sustained decline eGFR≥15 ml/min/1.73 m2 | 0.82 (0.67−1.01) | 0.71 (0.43−1.16) | 0.81 (0.67−0.98) |
| Sustained decline eGFR≥40 ml/min/1.73 m2 from baseline values | 0.81 (0.72−0.92) | 0.87 (0.75−1.00) | 0.84 (0.76−0.92) |
| Cardiovascular outcomes | |||
| Composed cardiovascular primary variabled | 0.86 (0.75−0.99) | 0.87 (0.76−0.98) | 0.86 (0.78−0.95) |
eGFR, estimated glomerular filtration rate; HR, hazard ratio CI, confidence interval.
Kidney failure, sustained decline in eGFR≥40% from baseline levels or renal death (secondary renal endpoint in FIDELITY study; primary renal endpoint in FIDELIO-DKD and FIGARO-DKD studies).
Main results of the FIDELIO-DKD, FIGARO-DKD and FIDELITY studies (finerenone vs. placebo).
*Composite renal variable: kidney failure, sustained decline eGFR ≥ 57% from baseline levels or renal death (primary renal variable in FIDELITY study; secondary renal endpoint in FIDELIO-DKD and FIGARO-DKD studies).
**Composite CV variable: time to first CV death, nonfatal MI, nonfatal stroke or hospitalization for HF.
CV, cardiovascular; eGFR, estimated glomerular filtration rate; HR, hazard ratio; CI, confidence interval.
Adverse effects in the FIDELIO-DKD and FIGARO-DKD studies.
| FIDELIO-DKD | FIGARO-DKD | |||
|---|---|---|---|---|
| Finerenone | Placebo | Finerenone | Placebo | |
| Adverse events | ||||
| Adverse events leading to discontinuation | 7.3% | 5.9% | 5.6% | 5.0% |
| Serious adverse events leading to discontinuation | 2.7% | 2.8% | 1.9% | 2.1% |
| Hyperkalemiaa | ||||
| Hyperkalemiaa | 15.8% | 7.8% | 10.8% | 5.3% |
| Treatment-related hyperkalemia | 11.8% | 4.8% | 6.5% | 3.1% |
| Hyperkalemia leading to discontinuation | 2.3% | 0.9% | 1.2% | 0.4% |
| Severe hyperkalemia | 1.6% | 0.4% | 0.7% | 0.1% |
| Acute kidney damage | ||||
| Acute kidney damage | 4.6% | 4.8% | 2.5% | 2.7% |
| Hospitalization due to acute kidney damage | 1.9% | 1.7% | 0.9% | 1.1% |
| Discontinuation due to acute kidney injury | 0.2% | 0.2% | 0.2% | 0.1% |
The FIGARO-DKD10 study included more than 7,000 patients with DM2 and CKD, defined as an Urinary albumin-to-creatinine ratio of 30 ≤ 300 mg/g and glomerular filtration rate of 25−90 ml/min/1.73 m2, or an albumin to creatinine ratio 300−5,000 mg/g and glomerular filtration rate of ≥60 ml/min/1.73 m2, on treatment with maximum tolerated doses of an ACEI or ARA II and serum potassium ≤4.8 mmol/l (Table 4). After 3.4 years of follow-up, compared to placebo, finerenone was associated with a significant reduction in the primary cardiovascular (CV) endpoint of 13% (HR: 0.87; 95% CI: 0.76−0.98; p = 0.03). In addition, it was associated with a nonsignificant 13% reduction in the renal composite outcome and a significant 36% reduction in the risk of end-stage renal disease (Table 5 and Fig. 2). In addition, finerenone reduced urinary albumin excretion by 32% at 4 months of treatment compared with placebo. The risk of serious adverse effects leading to discontinuation and the incidence of acute kidney damage were similar in both groups. However, the incidence of severe hyperkalemia was higher in the finerenone group (0.7% vs. 0.1%), although there were few cases of hyperkalemia leading to discontinuation (1.2% vs. 0.4%), and there were no cases of death from hyperkalemia (Table 6). The effects of finerenone on systolic blood pressure were modest (the difference between both treatment groups was 2.6 mmHg at 24 months).
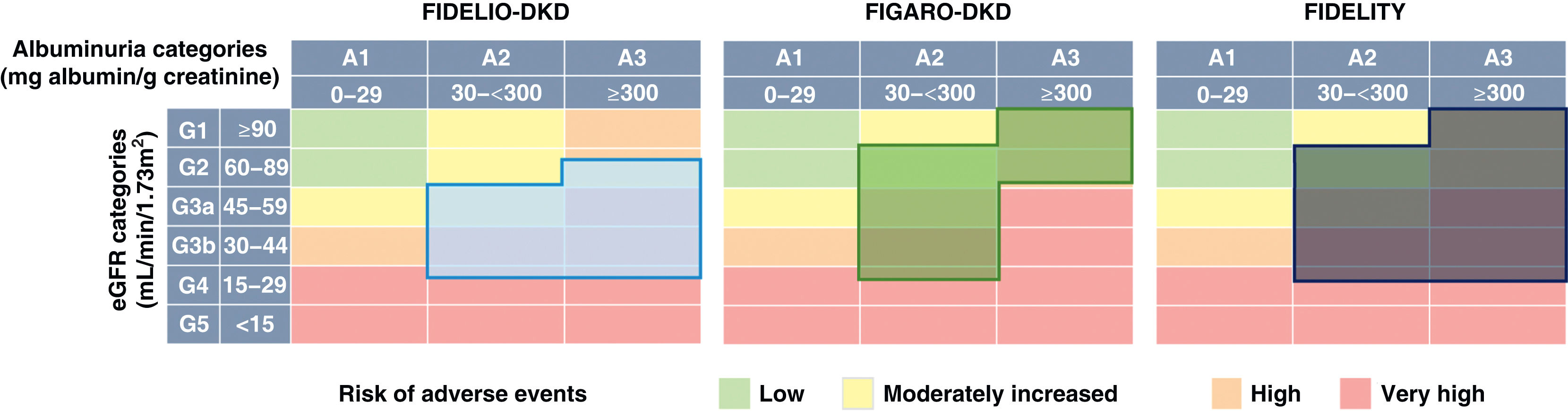
In Finerenone in chronic kidney disease and type 2 diabetes: Combined FIDELIO-DKD and FIGARO-DKD Trial program analysis (FIDELITY),43 a combined analysis of the FIDELIO-DKD and FIGARO-DKD studies was performed to achieve a more robust estimation of the results of both studies. Overall, according to the KDIGO risk categories, which combine estimated glomerular filtration rate and baseline albuminuria, 48% of participants were at very high risk, 41% at high risk, 10% at moderate risk, and 0.5% at low risk (Fig. 3). After a median follow-up of 3 years, compared with placebo, finerenone reduced the composite endpoint of renal failure, sustained ≥57% decline in glomerular filtration rate from baseline levels, or renal death by 23% (HR 0.77; 95% CI: 0.67−0.88), and the composite renal variable of renal failure, sustained decline in glomerular filtration rate ≥40% from baseline levels, or death from renal cause by 15% (HR 0.85; 95% CI: 0.77−0.93). Likewise, finerenone was also associated with significant reductions in the risk of progression of kidney disease. Furthermore, there was a significant 14% reduction in the risk of developing the composite endpoint of CV death, nonfatal myocardial infarction, and nonfatal stroke (Table 5 and Fig. 2). Consequently, finerenone slowed the progression of CKD and decreased the risk of CV events. Although the risk of adverse effects was similar between finerenone and placebo, hyperkalemia leading to discontinuation of treatment was more frequent with finerenone, although it was equally low (1.7% vs. 0.6%).
In a systematic review of 4 clinical trials (n = 7,048), which included FIDELIO-DKD, compared with placebo, finerenone significantly reduced the albumin-creatinine ratio in patients with CKD, without increasing the risk of adverse effects, except for an increased risk of hyperkalemia compared with placebo.44 Another meta-analysis of 13,945 patients with DM2 and CKD, which included FIDELIO-DKD and FIGARO-DKD, among other studies, confirmed the same reduction in the albumin-creatinine ratio. In addition, there were fewer patients with loss of glomerular filtration ≥40% from baseline compared to placebo (RR 0.85, 95% CI 0.78−0.93), with no increased risk of adverse effects, except for an increased risk of hyperkalemia.45
In short, clinical trials with finerenone have shown that this drug reduces albuminuria and delays the progression of CKD in patients with DM2, helping to reduce residual risk.
Finerenone in the global approach to the patient with diabetic kidney diseaseAt present, the only drugs specifically approved to slow the progression of CKD in diabetes mellitus are ACEI, ARB II, finerenone, canagliflozin, and dapagliflozin.22 CKD has multiple causes, and only an integrated approach, involving treatment with drugs with complementary mechanisms of action, can provide complete protection (Fig. 1B).
In the FIDELIO-DKD9 and FIGARO-DKD10 studies, all patients were being treated with ACEI or ARB II. Consequently, these studies show that in patients with DM2 and CKD, finerenone added to ACEI and ARA II provides additional benefits in progression of CKD. The question is, could combined therapy with finerenone and SGLT2 inhibitors produce an additional cardiorenal benefit? Unfortunately, in these studies, only a small percentage of patients were treated with SGLT2 inhibitors. In the FIDELITY analysis,43 approximately 7% of patients were taking SGLT2 inhibitors. The benefits of finerenone were independent of treatment with SGLT2 inhibitors, in terms of both the decrease in albuminuria and the progression of CKD and cardiovascular events.43,46 The fact is that, the best outcomes were observed in patients who combined SGLT2 inhibitors and finerenone. In this regard, in the ROTATE-3 study, which was carried out in patients with albuminuria ≥100 mg/24 h and eGFR 30−90 ml/min/1.73 m2 receiving the maximum tolerated doses of ACEI or ARA II, the combination of dapagliflozin with eplerenone had a robust additive effect on the reduction in albuminuria.47 Furthermore, in the DAPA-CKD study, as in the heart failure clinical trials conducted with iSGLT2, the benefit in terms of reducing events was independent of the use of aldosterone antagonists.15,48–51 Supporting the results of the clinical trials, in an animal model of organ damage associated with hypertension, the combination of finerenone and empagliflozin was associated with reductions in proteinuria, blood pressure, and renal and cardiac histological lesions, suggesting an additional benefit with the combination of both drugs.52 There is biological plausibility for these observations, as finerenone and SGLT2 inhibitors probably have complementary mechanisms of action, which decrease the risk of CKD progression. Thus, finerenone has anti-inflammatory and antifibrotic properties according to preclinical models, and SLGT2 inhibitors reduce glomerular hyperfiltration and have direct effects on metabolic and cellular function.
In addition, the risk of hyperkalemia from finerenone may decrease with the addition of SGLT2 inhibitors. For example, in the CREDENCE study, canagliflozin reduced the risk of hyperkalemia in patients with DM2 and CKD treated with an RAS inhibitor.49 Furthermore, in the FIDELIO study, no patient concomitantly treated with finerenone and an SGLT2 inhibitor had serum potassium >6.0 mmol/L, indicating that combined treatment would facilitate the more frequent use of MR antagonists, as they present a lower risk of hyperkalemia.15,46,49 Consequently, the data indicate that both drugs are complementary and could provide additional benefits when used concomitantly.
Ongoing clinical trials are specifically analyzing the impact of the combination of finerenone and SGLT2 inhibitors on safety and efficacy (CONFIDENCE trial, NCT05254002).
Although some glucagon-like peptide type 1 (GLP1) receptor agonists have shown a nephroprotective effect (mainly antiproteinuric), we need studies specifically designed to assess the effects of combined treatment with finerenone.53–55 In the FIDELIO-DKD study, 6.9% of patients were taking GLP1 receptor agonists, and the effects of finerenone on albuminuria and renal events were independent of treatment with GLP1 receptor agonists or insulin use.56
From a cost-effectiveness point of view, although finerenone is a priori more expensive than spironolactone and eplerenone, only finerenone has been shown to slow the progression of CKD. This is associated with a significant reduction in costs, particularly hospitalizations, end-stage renal disease, and dialysis, which are the major determinants of health care costs associated with the management of patients with CKD. Therefore, it is to be expected that greater use of finerenone in clinical practice would translate into a reduction in the health care costs associated with CKD.57,58
Consequently, triple therapy comprising RAS inhibitors, SGLT2 inhibitors, and finerenone represents a change in the natural history of patients with CKD and DM2. However, we must ask how access to proven cardiorenal therapies can be guaranteed for those patients that need them most? It is necessary to overcome the barriers that prevent their implementation. In this regard, every patient with DM2 should undergo albuminuria screening at least once a year, regardless of treatment, as recommended by the latest ADA guidelines (2022).23 Better coordination between levels of care (primary care and nephrology) could help to implement an optimal approach for patients with diabetes mellitus and CKD. This should involve early detection of CKD in patients with DM2. Both initiation and titration of finerenone are simple, and, if simple guidelines are followed, there should be no major complications in day-to-day use. Its reduced effect on blood pressure, the lower risk of hyperkalemia than spironolactone and eplerenone, and the benefits demonstrated in clinical trials on the progression of kidney disease, should facilitate implementation of finerenone in clinical practice, regardless of the level of care at which the patient is being treated.59–61
However, there are still doubts that need to be resolved. What is the position of finerenone in the clinical practice guidelines for patients with DM2 and CDK? effects will finerenone have on patients with nondiabetic nephropathy? Can finerenone be initiated in patients with a glomerular filtration rate <25 ml/min/1.73 m2 or does it offer benefits to patients on dialysis undergoing renal transplant, or without albuminuria? Finally, it should be remembered that there is also another nonsteroidal MR antagonist, esaxerenone, which, while known to increase the probability of normalizing albuminuria and decreasing progression to higher levels of albuminuria in patients with DM2 and microalbuminuria treated with RAS inhibitors, has not yet proven able to reduce the progression of CKD in this population.62
ConclusionsFinerenone is a potent and selective nonsteroidal MR antagonist that, in patients with DM2 and CKD, reduces albuminuria, slows CKD progression and offers cardiovascular protection. This benefit appears to be independent of other nephroprotective treatments, such as RAS inhibitors and SGLT2 inhibitors, with a good safety profile and a low risk of hyperkalemia. The greatest benefit in the therapeutic approach to these patients will be achieved by means of a comprehensive treatment that acts on the multifactorial pathogenesis of diabetes-associated CKD. Once treatment with ACEI or ARA II and SGLT2 inhibitors has been consolidated in the clinical practice guidelines for the management of patients with diabetes and CKD, finerenone could prove to be a highly innovative option in the therapeutic armamentarium, coming to be a key element in the treatment of this population. Thanks to the mechanism of action of finerenone, the benefits of the drug could extend beyond diabetic kidney disease, so that in the future it could be prescribed in other forms of CKD and even for other therapeutic indications.
Key points
- -
Despite current treatments, patients with diabetes are still at high risk of progression of CKD.
- -
The pathogenesis of CKD in the patient with diabetes is complex, involving hemodynamic, metabolic, and inflammation- and fibrosis-related factors.
- -
The mineralocorticoid receptor (MR) plays a key role in the genesis and development of CKD.
- -
Despite decreasing proteinuria, spironolactone and eplerenone have not been shown to reduce the progression of CKD.
- -
Finerenone is a highly selective nonsteroidal MR antagonist characterized by a large and bulky structure.
- -
Binding of finerenone to the MR results in a specific receptor-ligand complex that blocks the recruitment of transcriptional coactivators involved in the expression of proinflammatory and profibrotic mediators.
- -
Finerenone has been shown to reduce albuminuria significantly in patients with DM2 and CKD as early as the fourth month of treatment.
- -
In patients with CKD and DM2, finerenone reduces both the progression of CKD and the risk of CV events.
- -
The risk of hyperkalemia is lower with finerenone than with spironolactone and eplerenone, thus facilitating its use in clinical practice.
- -
The approach to patients with diabetes and CKD should be multifactorial and would include ACEI or ARA II, SGLT2 inhibitors, and finerenone.
The authors received no honoraria and/or financial compensation for their participation in this article. The authors thank Content Ed Net for collaboration in the writing of the article and editorial assistance. These tasks were funded by Bayer Hispania.
Conflict of interestJLG reports personal fees from NovoNordisk (fees, advisory), Boehringer (fees, advisory and consulting), Eli Lilly (fees), AstraZeneca (fees, advisory and grants), Esteve (fees), Bayer (fees, advisory and consulting), and Vifor (fees and advisory).
JRGJ reports fees for lectures and for participating on advisory boards from Bayer.
LF has received fees from Novonordisk, Boehringer, AstraZeneca, Bayer, Esteve, and Eli Lilly.
AO has received grants from Sanofi and consulting or speaking fees or travel support from Advicciene, Astellas, AstraZeneca, Amicus, Amgen, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Menarini, Mundipharma, Kyowa Kirin, Alexion, Freeline, Idorsia, Chiesi, Otsuka, Novo-Nordisk, Sysmex, and Vifor Fresenius Medical Care Renal Pharma and is Director of the Mundipharma-UAM Chair in diabetic kidney disease and the AstraZeneca-UAM Chair in chronic kidney disease and electrolytes.
MJS has received personal honoraria from NovoNordisk (honoraria, expert advice Janssen (honoraria), Boehringer (honoraria, expert advice grants and consultancy), Eli Lilly (honoraria), AstraZeneca (honoraria and expert advice Esteve (honoraria), Fresenius Medical Care (honoraria), Mundipharma (fees and expert advice), NovoNordisk (fees, expert advice and consulting), Bayer (fees, expert advice and consulting), Travere Therapeutics (fees and expert advice), GE Healthcare (expert advice), and Vifor (fees and expert advice). MJS is also Editor-in-Chief of the Clinical Kidney Journal.
AV has received speaking fees from Bayer, Boehringer-Ingelheim, Daiichi Sankyo, and Pfizer-BMS.
AO's research is supported by the Instituto de Salud Carlos III (ISCIII) RICORS program to RICORS2040 (RD21/0005/0001) and SPACKDc PMP21/00109, FEDER funds. MJS's research is supported by the Instituto de Salud Carlos III (ISCIII) RICORS program to RICORS2040 (RD21/0005/0016) and Marató TV3 2020 421/C/2020, Marató TV3 2021 215/C/2021, (PI21/01292) FEDER funds, and EIN202020-1123381.