Frailty is a concept that has been mainly developed in geriatrics and it came from the need of identifying subjects at risk to develop complications when they faced a stressful event. Frail patients have higher risk of mortality, poor outcomes and disability, and this is independent from their age or comorbidities. Chronic kidney disease patients present with high prevalence of frailty, especially those who are in renal replacement therapy. Frail or pre-frail patients on the kidney transplant waiting list represent 20–30%, and these patients are proven to have poorer results after the transplant, which is a stressful event itself. Tools for frailty assessment, both scales or indexes, may be useful to identify which subjects might be at risk for complications after transplant, and this is necessary to adapt our clinical practice and minimize morbidity. The most used frailty scale in kidney patients is Fried scale, which is based in five phenotypic items. Besides that, knowing frail population allows potential interventions such as prehabilitation while the patient is waiting for the kidney transplant, which the aim of improving their vulnerability prior to transplant and, therefore, optimizing results after transplant. More studies are needed amongst kidney patients to improve and prevent frailty.
El concepto de fragilidad ha sido desarrollado principalmente en el campo de la geriatría y surgió para identificar a aquellos pacientes que presentaban vulnerabilidad al enfrentarse a un evento clínico estresante. Los pacientes frágiles tienen mayor riesgo de mortalidad, complicaciones y discapacidad, independientemente de su edad o comorbilidades. Se ha descrito una alta prevalencia de fragilidad entre sujetos con enfermedad renal crónica, sobre todo en terapia renal sustitutiva. El porcentaje de pacientes frágiles o prefrágiles en lista de espera de trasplante renal se sitúa en torno a un 20–30% y se ha demostrado que estos pacientes tienen peores resultados tras el trasplante renal, que constituye un evento estresante. Las herramientas para evaluar la fragilidad, escalas e índices, pueden ser útiles para identificar qué pacientes están en riesgo de padecer más complicaciones postrasplante, lo que resulta necesario para adaptar nuestra práctica clínica y evitar morbilidad. La más utilizada en pacientes renales es la escala de Fried, que se basa en la detección de cinco dimensiones fenotípicas. Además, conocer la fragilidad de nuestros pacientes permite plantear intervenciones prehabilitadoras durante el tiempo que estén en lista de espera, con el objetivo de mejorar esta vulnerabilidad pretrasplante, y así optimizar los resultados postrasplante. Se necesitan estudios en población renal para mejorar y prevenir la fragilidad.
Almost twenty years ago, the geriatrician Linda Fried defined frailty as a “physiological state of increased vulnerability to stressors, the result of a decrease or dysregulation of the reserves of multiple systems, which causes difficulty in maintaining homeostasis”.1 However, although this is the most widely accepted definition, it is also somewhat unspecific. Globally, frailty defines a state of vulnerability to health problems, but two conceptualizations of the term have been developed that have given rise to different approaches for its measurement.2 In the first place, frailty can be considered as a syndrome that gives rise to a phenotype that presents sarcopenia as one of its fundamental pathophysiological characteristics. This approach, already enunciated by the authors at the end of the last century,3 facilitates the measurement of frailty as a specific set of signs and symptoms: weight loss, slow gait, poor physical activity, weakness and reduced energy. This is the Fried1 frailty phenotype. The second approach arises almost at the same time in Canada from the hand of the geriatrician Kenneth Rockwood, who measures frailty using an index or scale, considering it as a state of accumulation of deficits that begins at the cellular level and leads to a loss of function of organs.4–6 In this case, frailty is conceptualized as a marker of biological age and, consequently, it is quantified by adding deficits of multiple systems. Regardless of how it is measured – at least 67 different scales have been used in the general population – frail patients are known to experience a decline in physical function and an increased risk of adverse health outcomes, disability, and mortality. In recent years, in the geriatric population, the measurement of frailty has been introduced as a variable associated with patient survival independently of age and the presence of other diseases. In the joint European action for prevention and management of frailty (ADVANTAGE), frailty is defined as a clinical condition that increases the vulnerability of an individual to develop dependence and/or increase mortality when exposed to a stress.7 It may be the result of a number of diseases and medical conditions (in fact, it is increased in patients with chronic diseases8) and, most importantly, its progression to disability can be delayed or prevented if it is identified and treated early.
Patients with chronic kidney disease (CKD) are good example of a frail patient. Therefore, it is not surprising that nephrologists have been eager to learn its pathophysiology and the potential intervention on modifiable factors that could reverse or at least improve the frailty of our patients. In the context of kidney transplantation (KT), the known benefit in overall survival as compared to dialysis, even in elderly patients,9 may be reduced by the presence of frailty; therefore, it is necessary an exhaustive evaluation of candidate patients to avoid complications in those more vulnerable in whom the stress of surgery and immunosuppressive treatment may increase their risk of death and/or complications.
ReasoningWhy is frailty important in kidney transplant candidates? Pre and post kidney transplant resultsAlthough not fully understood, the relationship between CKD and frailty seems to be related to several factors: 1) muscle tissue damage, common in patients with advanced CKD due to uremia; 2) the alteration of taste and odor, which predisposes to anorexia; and 3) the loss of energy experienced as CKD progresses. Also contributes the inflammation, oxidative stress, anabolic/catabolic hormonal imbalance, metabolic acidosis, and other cellular alterations related with the uremic medium.10–12 A systematic review has shown the association between the severity of CKD and the fragility of the patient and their relationship with mortality and hospitalization.13 Therefore, frailty is more frequent in patients with advanced CKD (15–21%) than in the general population (3–6%).13 This prevalence is especially high in hemodialysis patients, up to 73%,14 and has been associated with multiple adverse health outcomes.14–19 Frail patients more often choose a conservative treatment of their advanced CKD,14 and if they enter the hemodialysis program they have more falls,15 hospitalizations16 and mortality.16–19 This fragility probably explains that less than 25% of hemodialysis patients access the transplant waiting list in our country.20 In a multicenter US study identified that 21% of candidates who initiated the study to be added to the KT waiting list were fragile, and only 12% were finally included.21 Despite these limitations, the age and comorbidity of KT recipients have progressively increased, so that age itself is no longer a limit for transplantation, candidates over 80 years of age are accepted.20
It is estimated that 20% of patients are fragile at the time of KT, with a decreased grip strength and low physical activity as its most prominent components.22 If a fragile patient undergoes a kidney transplant, the risk of delayed graft function is increased,23 as well as a longer hospital stay,24 or requiring readmission after transplantation regardless of age.25 Adjusting for other confounding variables, frail patients have a higher risk of dying after transplantation.26,27 Using the Fried1 phenotype as a measure of frailty, the Epidemiology Research Group in Organ Transplantation at Johns Hopkins Hospital has described that more than 50% of its KT receptors are fragile (20%) or pre-fragile (32%).27 But at the same time, those who overcome the KT procedure have a greater chance to improve their frailty. In a prospective study, 22% of KT candidates worsened their frailty status since evaluation and after KT, while 24.4% became less frail. Those who increased their state of frailty were more likely to have a long hospital stay ≥2 weeks and also more post-transplant mortality.28 The pretransplant fragility has also been associated with further cognitive deterioration at medium term29 and a greater probability of intolerance to immunosuppressive therapy.27 This may be particularly relevant, given the association between fragility and impaired immune system30–32 with its potential implication in graft survival and the individualization of immunosuppressive treatment in these recipients. Immunosenescence, defined as an altered immune state that predisposes to infections and cancer,33–35, has been associated with aging in KT recipients.36 Senescent cells are able to secrete various proteins, growth factors and cytokines, that contribute to the inflammatory state observed in older patients with renal disease.37 Given the profile of the current kidney transplant candidate, it is necessary to investigate mechanisms of action, dosage and pharmacokinetics of the different immunosuppressive drugs in immunosenescent patients. It is important to improve the balance between the containment of the immune response against the allograft and the adequate response in the case infection or cancer.
Candidates for KT often have multiple comorbidities, which could be responsible for the increase in mortality. In fact, clinical experiences in Spain have been published in this regard. It has been shown that the recipient's comorbidity can predict the risk of post-transplant mortality.38,39 However, frailty is so important in mortality that comorbidity only increases mortality in non-frail patients.40 Out of 177 transplant candidates studied at Hospital del Mar using the Fried scale, 14 were frail (7.9%) and 41 were pre-frail (23.2%). Frailty was associated with older age, female gender, diabetes, a higher body mass index, and longer time on dialysis.41 In addition, data subsequently reported from this cohort confirmed that 30% of the patients who received a kidney graft were fragile, presenting higher mortality and graft loss after KT.42
It seems reasonable to think that interventions on the functional reserve of recipients before transplantation could improve post-transplant results and, therefore, in the latest revision of the KDIGO guidelines for the evaluation of candidates for KT, it is suggested that frailty should be evaluated at the time of inclusion, and periodically, once on the waiting list.43
MethodsHow to measure frailty in patients on the transplant waiting list? A practical approachHistorically, nephrologists have not been especially in favor of applying scoring systems, as other specialists such as intensivists, anesthesiologists or geriatricians that have used ASA, SOFA, APACHE, etc. CKD (and much more dialysis) already marks out kidney patients enough to add one more label. The issue is that some of these scores have been applied to the renal population and they do predict long-term results; therefore, to identify patients at risk and improve - or reverse if possible – this situation, becomes an obligation. In the medical history it should commented in a subjective manner, the impression about whether the kidney patient is fragile or not. In fact, the reliability of the nephrologist to identify the frailty of candidates for KT may fail in up to 37% of patients, emainly at the expense of the elderly population.44
As we have mentioned previously, there are various approaches to characterize frailty. The one that considers frailty as a phenotype of poor physical function relies mainly on objective measures of physical frailty (grip strength and gait speed). The scale most used with this orientation is the aforementioned Fried scale, described and validated in the Cardiovascular Health Study, which gives rise to the frailty phenotype.1 Unlike this approach, which understands frailty as a stage prior to dependence, others characterize frailty as a consequence of the accumulation of comorbidities, disabilities, symptoms and laboratory abnormalities, so in this case the measurement of frailty of includes comorbidity and dependence. Among the latter is the Frailty Index, which understands fragility as a continuum and it is one of the most used. In its original version included more than 70 items although it was later reduced to 4045 and, subsequently, a version has been made based on pictograms with the objective of facilitating its use in the clinic, the so-called Clinical Frailty Scale.46 Completion of some of the tests described is laborious and requires auxiliary instruments. For this reason, it has developed as a screening tool for the general population, the FRAIL scale which easy to apply (less than two minutes) and the patient has to respond questions without physical tests or laboratory parameters.47 This test assesses five components « fatigue, resistance, aerobic, illnesses, loss of weight » (FRAIL) and has been validated in different clinical contexts48–50 and in the renal population.51
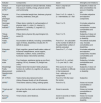
Table 1 shows the scales applied to the kidney population in different stages of disease, with their strengths and weaknesses.1,46,47,52–59 The Fried scale is the most used.1 Choosing between one scale or another will depend on several factors, mainly logistical. For example, scales that are filled out by the patient himself, according to his perception, such as the Kidney Disease Quality of Life Short Form Physical Component Subscale (SF-12 PCS) are obviously easily applicable. However, in front of the nephrologist who assesses the entry into the transplant waiting list, the patient may be reluctant to show his “weaknesses”, and in this case, purely objective scales such as the Short Physical Performance Battery56 may be more appropriate, although it will be necessary to take into account the time and resources used in each one of them.
Frailty scales and measures used in patients with chronic kidney disease.
| Indicator | Components: | Score | Strengths and limitations |
|---|---|---|---|
| Clinical Frailty Scale (CFS)44 | 8-point scale based on clinical interview. It takes into account: mobility, energy, physical activity and functionality. | From 1 (very fit) to 8 (severe frail). | Subjective. It does not take comorbidities into account. Disability, yes. |
| Physical frailty phenotype (Fried) 1 | Five: unintended weight loss, tiredness, physical inactivity, weakness, slow gait. | From 1 to 5: 0 = robust; 1–2 = intermediate; ≥3 = frail | It includes subjective and objective components. |
| Groningen Fragility Indicator52 | Fifteen items: mobility (4), self-perception of physical state (1), vision (1), hearing (1), hydration (1), morbidity (1), cognition (1), psychosocial (5). | From 1 to 15. | It includes subjective and objective components. Includes comorbidities and disability. |
| Tilburg Fragility Indicator53 | Fifteen items: physical (8), psychological (4), social (3). | From 1 to 15. | Subjective. It does not include comorbidities or disability. |
| Frailty Index45 | Accumulation of deficits, including: comorbidities, signs of illness, disabilities; includes 40–70 deficits to assess. | From 0 to 1. It is calculated as the number of deficits of the patient/total number of deficits evaluated. | The deficits evaluated vary. Includes comorbidities and disabilities. |
| Edmonton Frail54 | Eight: cognition, general health status, status of functional independence, social support, medication, nutrition, mood, continence, functional status instrumental activities. | From 0 to 17; >7 = frail | It includes subjective and objective components. Includes comorbidities and disability. |
| FRAIL47 scale | Five: tiredness, resistance (going up one floor), walking (100 m), illnesses (>5), weight loss (>5%). | From 0 to 5: 0 = not frail; 1−2 = pre- frail; 3 = frail. | Subjective. Includes comorbidities and disability. |
| Strawbridge Frailty Measurement Questionnaire 199455 | Sixteen items that include 4 fields: physical function (4), nutrition (4), cognition (4), sensory problems (6). | If the score ≥3 in any of them = problem or difficulty in that component. | Subjective. It does not include comorbidities or disability. |
| Frail = difficulty in ≥2 components. | |||
| SF-12 PCS | Twelve items about physical function | From 0 to 100 (the lower the worse). | Subjective |
| Kidney Disease Quality of Life-36 subscale. | |||
| SPPB56 | Three items: balance, sitting, walking speed. | From 0 to 12. | It does not include comorbidities or disability. |
| Timed up and go57 | Get up from the chair, walk a short distance, and sit down again. | Score in seconds. | It does not include comorbidities or disability. |
| Running speed58 | Time to travel a short distance. | Score in seconds or meters per second. | It does not include comorbidities or disability. |
Adapted from Harhay MN, et al. An overview of frailty in kidney transplantation: measurement, management and future considerations.59.
Frailty, as we have discussed, is frequent in patients with CKD and confers a worse prognosis in hemodialysis patients. Being fragile predicts the post-transplant evolution of patient included in the transplant waiting list. Therefore, is fragility another criteria to be taking into consideration to include or not patients in the waiting list for KT? Should this classification be applied, as cardiologists do with the EuroSCORE, to determine if a patient is a candidate for cardiac surgery?60 Certainly, this is not the right approach. In fact, the only available evidence in this regard is that for frail patients transplantation is a better therapeutic option than dialysis. A retrospective study performed in almost 20,000 KT recipients with prior registration of the SF-12 PCS health questionnaire revealed that after three years,patients from the last quartile had worse survival than those from the first quartile (84 vs. 92%). However, the benefit of KT over dialysis persisted in all quartiles, even the most fragile.61 Therefore, identifying frail subjects not only has a prognostic purpose, but, most interesting, it has a therapeutic purpose. Today we know that the fragility can be improved and/or reversed. Actually, it is possible to intervene.
A level of functional capacity of an individual predicts outcomes associated with elective surgical interventions. This is how the concept of "prehabilitation" was born: a therapy program based fundamentally on exercise, which aims to improve preoperative functional capacity to improve tolerance to the upcoming stressor, leading to better results after surgery.62–65 In addition to physical exercise, prehabilitation also consider the nutritional64 and psychological optimization of the patient.65 Improving the preoperative global functional status is important because a poor functional status is associated with adverse peri- and postoperative outcomes, also in KT.66 The effect of exercise-based prehabilitation has been studied in various surgical populations.67–69 Increased physical activity and functional capacity before a major surgical stressor contributes to a reduction in the period of postoperative recovery and a more rapid return to previous functional capacity.67–70 Typically, efforts to improve outcomes after KT have focused on post-KT interventions. It may be more useful to act before KT, since candidates usually wait months and years for the arrival of the kidney.71 Recommendations of exercise after KT may not be useful, given the sharp drop in physical activity during the first year after KT and the poor compliance with the prescribed rehabilitation treatment.72,73 By contrast, candidates to KT may be more motivated to do so, knowing that they will undergo major surgery within the next few months.74 Only one small study has evaluated the possibility of prehabilitation in candidates of KT.75 The intervention consisted of weekly physiotherapy sessions in an outpatient center and with home exercises. With difficulty, due to low reception, 18 participants were included, 31% of them fragile. After two months of prehabilitation, the participants improved their physical activity by 64% (p = 0.004). Participants reported high satisfaction, increased physical function and energy, sustained endurance, and a better attitude. In the five participants who received a KT during the study, the length of hospital stay was shorter than in controls.
However, prehabilitation before KT is more difficult than in other surgical modalities, because the date of transplantation is often unknown. Also, probably not all KT candidates are able to attend weekly pre-rehabilitation sessions at the center, as they have to be reconciled with HD schedules. This should not prevent the patients from making attempts and search for options to improve the functional status of kidney patients. In fact, there are low-intensity exercise programs for frail subjects that have shown benefits in both outpatient and home patients and during hospital admission for acute illness.76–78
ConclusionsFrailty is a common condition in kidney patients, since advanced CKD stages to dialysis and KT. Being fragile is associated with poorer outcomes after transplantation. In candidates for KT, measuring frailty may facilitate the development of strategies to reverse it while on the waiting list and to minimize peri and postoperative morbidity and mortality. Intervention studies are needed to analyze whether early intervention can improve these results.
FinancingMJPS and JP are sponsored by the ISCII project FIS-FEDER PI19/00037. MJPS has support from a Spanish Society of Transplant scholarship.
Please cite this article as: Pérez-Sáez MJ, Gutiérrez-Dalmau Á, Moreso F, Rodríguez-Mañas L, Pascual J. La fragilidad en candidatos a trasplante renal. Nefrologia. 2021;41:237–243.