We present a new edition of the Hemodialysis Center Guide. It is part of the program of updating the Clinical Guides of the Spanish Society of Nephrology. It is a consensus document in which nephrologists with extensive experience in dialysis and experts in nephrological nursing have collaborated. The Guide has been corrected by a group of external evaluators. The opinion of the patients through the kidney diseases patient association (ALCER) has been reported and taken into consideration. This Guide includes in its ten chapters architectural, logistical and organizational aspects. It places special emphasis on human resources needs and their qualification. Review current hemodialysis modalities, dosage and adequacy, and intra and interdialysis monitoring. Hemodialysis is one of the forms of renal replacement therapy, so it mentions the need for transplant waiting list inclusion and the relationship with peritoneal dialysis units. The patient's quality of life on hemodialysis understands the need to relate and travel so care of transitory patients is reviewed and standardized. Quality management is a tool currently needed to achieve continuous improvement of any procedure such as hemodialysis. This Guide is intended to be an aid for the proper functioning of the Dialysis Units, for those responsible for them, as well as for health managers.
Presentamos una nueva edición de la Guía de Centros de Hemodiálisis. Se enmarca en el programa de actualización de las Guías Clínicas de la Sociedad Española de Nefrología. Es un documento de consenso en el que han colaborado nefrólogos con amplia experiencia en diálisis y expertos de la enfermería nefrológica. La Guía ha sido corregida por un grupo de evaluadores externos. Se ha informado y se ha tomado en consideración la opinión de los enfermos a través de la Asociación de Enfermos Renales (ALCER). Esta Guía incluye en sus diez capítulos aspectos arquitectónicos, logísticos y organizativos. Hace especial énfasis en las necesidades de recursos humanos y su cualificación. Revisa las modalidades actuales de hemodiálisis, su dosificación y adecuación y la monitorización y seguimiento intra e interdiálisis. La hemodiálisis es una de las formas de tratamiento renal sustitutivo, por lo que menciona la necesidad de la inclusión en lista de espera para trasplante y la relación con las unidades de diálisis peritoneal. La calidad de vida del paciente en hemodiálisis comprende la necesidad de relacionarse y viajar por lo que se revisa y estandariza la atención de los pacientes transeúntes. La gestión de calidad es una herramienta necesaria actualmente para lograr la mejora continua de cualquier procedimiento como la hemodiálisis. Esta Guía pretende ser una ayuda para el buen funcionamiento de las Unidades de Diálisis, para los responsables de las mismas, así como para los gestores sanitarios.
As described further below, renal replacement therapy in patients with advanced chronic kidney disease has undergone important changes since the Spanish Society of Nephrology (Sociedad Española de Nefrología [S.E.N.]) first published the Hemodialysis (HD) Centers Guide in the year 2006.
The same can be said of the typical patient profile, characterized by a gradual increase in age and patient changes in attitude towards the disease - with a demand for autonomy and full transparency regarding the decisions that are made.
In parallel to these changes in clinical aspects and therapeutic approach, there has also been an evolution in the search methodology, analysis and ranking of the scientific evidence upon which they are fundamented.
The above considerations explain and justify the decision of the S.E.N. to update the Hemodialysis (HD) Centers Guide 2006, adapting its methodology end editing, upgrading and monitoring policies to the current demands.
The Introduction to the 2006 edition1 sought to generate awareness of the epidemiological importance of renal replacement therapy (RRT), and in this regard we wish to take advantage of this opportunity to present the evolution of these data over the last 15 years.
According to the latest National Dialysis and Transplantation Report, published in 20172, the data reflect a constant increase in prevalence with respect to the figures of the 2002 registry presented in the 2006 guide - with a current total of about 60,000 patients being subjected to one RRT modality or other. At present, the incidence is approximately 141 per million population (pmp), which corresponds to about 6500 patients a year.
There are still very marked differences among the different Spanish Autonomous Communities, and also among the different therapeutic modalities. With regard to this latter aspect, there has been a substantial increase in peritoneal dialysis and preemptive renal transplantation for incident cases. This first revision of the Hemodialysis Centers Guide of the Spanish Society of Nephrology (S.E.N.), published in the journal Nefrología1, aims to adapt its contents to the new current situation commented above.
Thirteen years have gone by since the first edition, and renal replacement therapy and its characteristics have evolved. In relation to the technical advances that have occurred in hemodialysis, mention must be made of aspects such as the use of ultrapure dialysis fluids and also citrate-based fluids; generalization of the use of biosensors; the marketing of membranes allowing different dialysis techniques (HD, expanded hemodialysis [HDx], hemodiafiltration [HDF]); and the application of integrated and two-directional software applications allowing for improved quality of care and clinical safety. In addition, there has been an increase in the use of convection techniques such as online hemodiafiltration (HDF-OL), that have resulted in a significant decrease in overall mortality versus conventional HD.
Likewise, new molecules have been added to our therapeutic repertoire, such as longer half-life erythropoiesis stimulating agents, different treatments for bone-mineral metabolism such as selective vitamin D receptor activators, calcimimetic agents, the new non-calcium based phosphate binders, and currently also the new potassium binders.
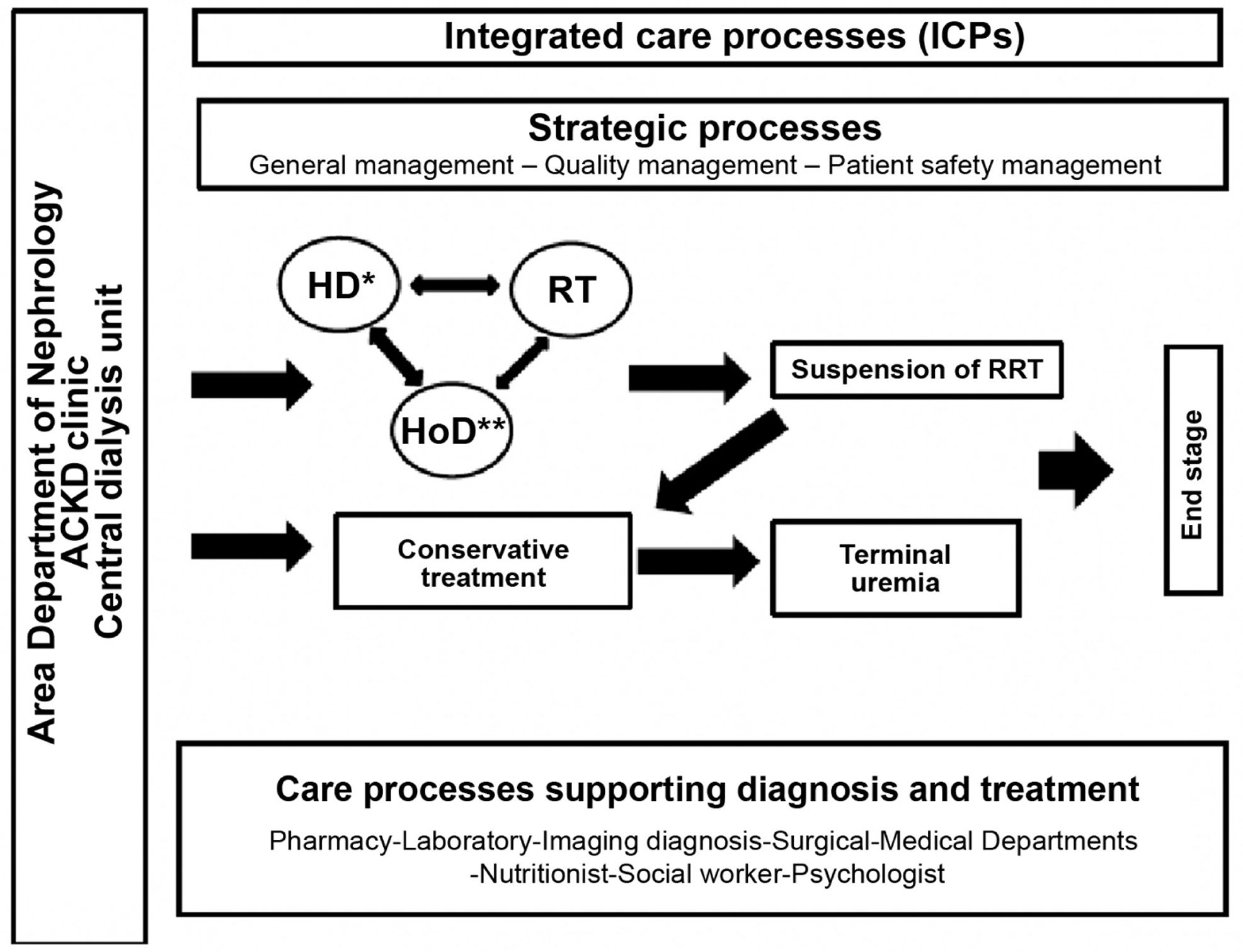
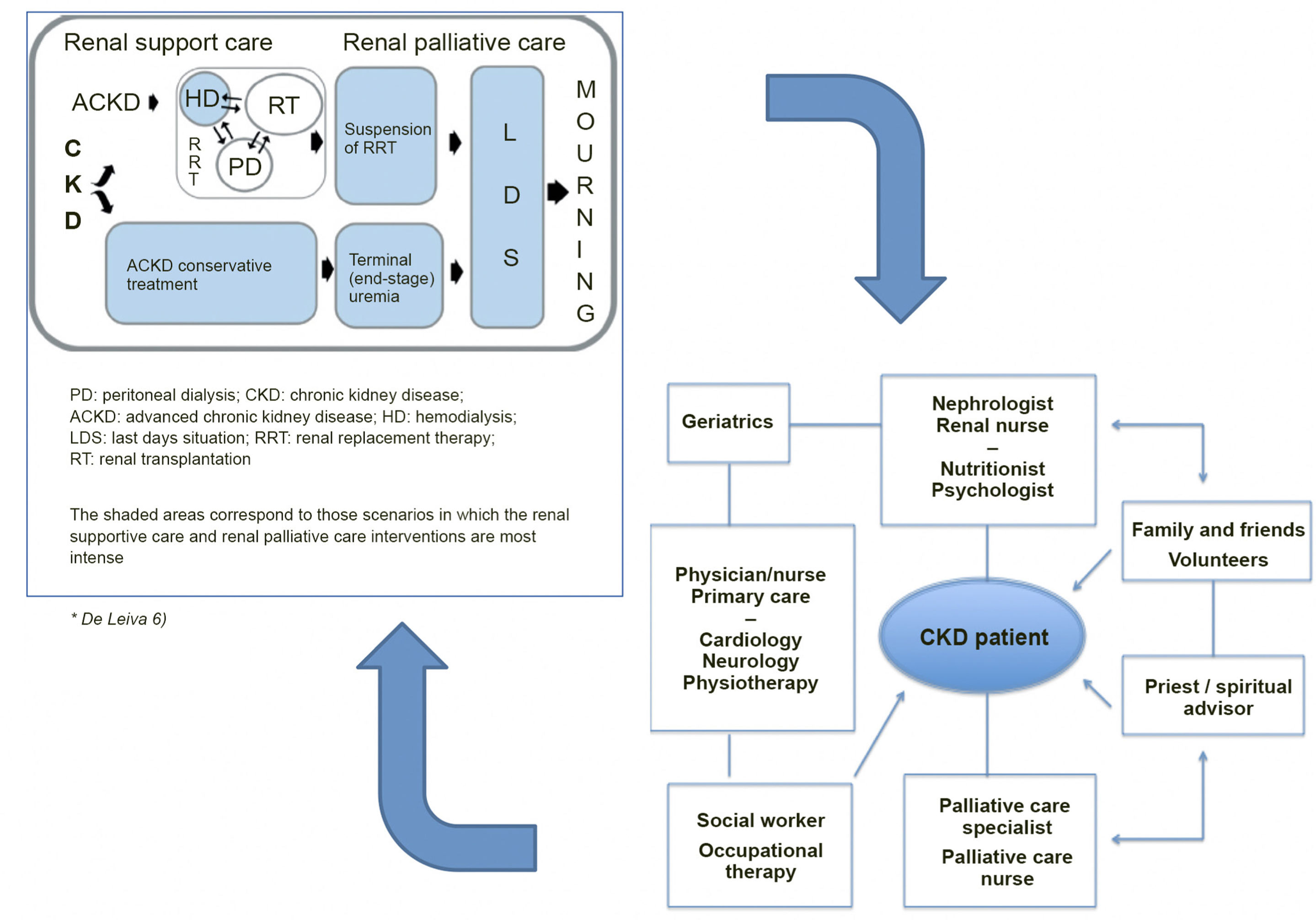
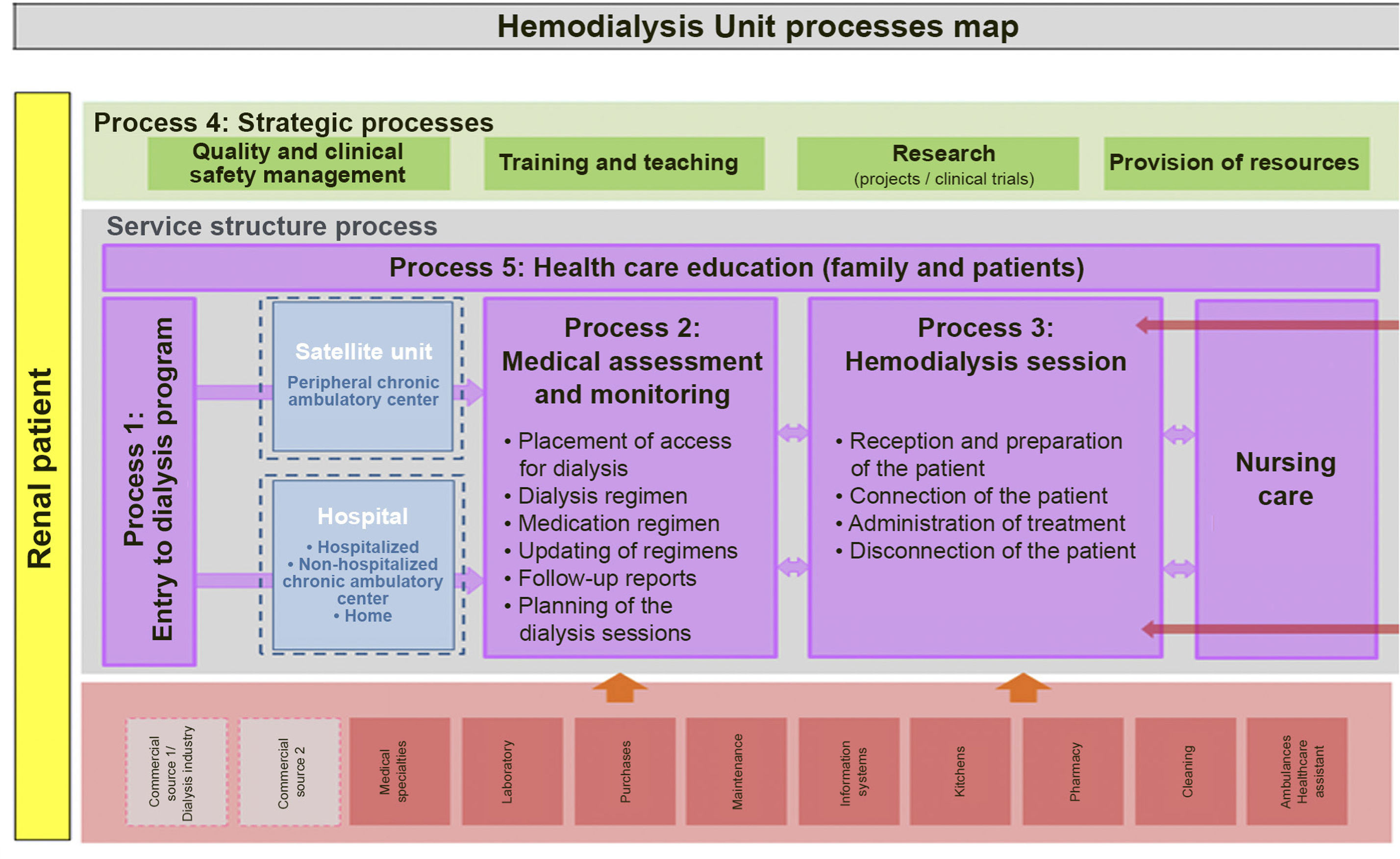
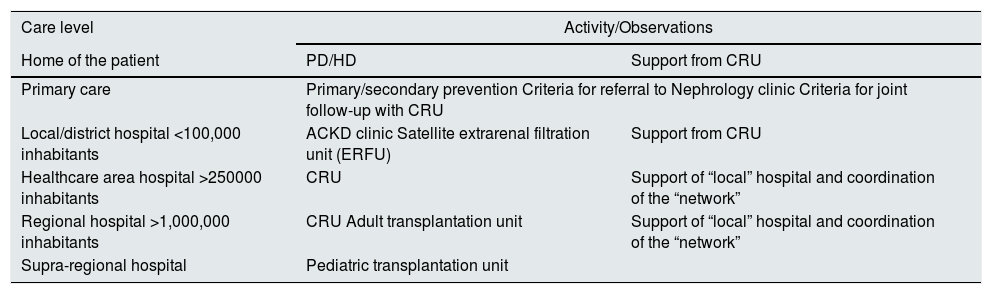
Definition and types of hemodialysis centers / unitsBefore describing the different types of dialysis centers or units (DUs), a definition should be provided of what such centers or units represent. A dialysis center or unit is an in- or out-hospital facility that provides dialysis for patients requiring such treatment. In this guide we focus on hemodialysis (HD); consequently, we will refer to the Hemodialysis Unit (HDU). Nevertheless, it should be clarified that in the case of hospital DUs, patients must be offered all the options of renal replacement therapy (RRT), i.e., transplantation (live and deceased-donor), HD, home techniques - both home hemodialysis (HHD) and peritoneal dialysis (PD), and conservative management. On the other hand, it must be underscored that patients have the legal right (Patient Autonomy Act, 41/2002)3 to receive information about their disease and the different treatment options, in order to be able to decide what adapts best to their personal lifestyle - provided there are no medical contraindications. In turn, the RRT modalities should not be viewed as sealed compartments: treatment planning should focus on the patient as a whole, taking into account all the possible options in each moment based on the concrete health conditions and preferences of the patient. It is therefore essential to favor patient education and training, facilitate shared decision making referred to RRT, and even develop a patient roadmap as guidance through the different RRT modalities4,5.
Home dialysis techniques are better options in terms of cost-effectiveness6,7; such options therefore should receive priority in care network resource planning.
The HDU is a Unit offering multidisciplinary care within a specific area and which complies with a series of functional, structural and organizational requirements. The HDU guarantees the safety, quality and efficiency standards needed to offer correct dialysis treatment, based on the best available evidence. The Unit comprises both in-hospital and out-hospital facilities, and both must be perfectly coordinated (see the section on coordination of the centers). The requirements of the HDU will be addressed in the present guide.
From the beginnings of HD in Spain, and as a consequence of the limited number of treatment stations available in public hospitals, this particular treatment modality has coexisted with dialysis in the out-hospital setting. At present, many patients pertaining to the public healthcare system receives hemodialysis in out-hospital centers and are assisted by a likewise numerous body of nephrologists that perform their professional activities in these centers. A total of 319 both in- and out-hospital HDUs are distributed throughout Spain8.
Definition of the in-hospital dialysis unit or center
The in-hospital dialysis unit or center provides dialysis treatment within the hospital, addressing both its own demands and those derived from its operation within the healthcare resources network of its area. The hospital HDU (HHDU) is integrated within the Department of Nephrology, and this conditions its characteristics (structure, materials and human resources) and functions. The HHDU offers the service of hemodialysis treatment in the hospital and its area of influence. It includes patients who are on dialysis within the Spanish region (Autonomous Community) involved, as well as patients from other Autonomous Communities or countries who – through existing agreements and legislations – are to be treated by the Spanish National Health Service (Servicio Nacional de Salud [SNS]. The HHDU should be reserved for acute patients and hospitalized chronic patients undergoing RRT, as well as for patients with greater comorbidity.
The HHDU is in charge of care network coordination tasks, with the following objectives:
- •
Offer an advanced chronic kidney disease (ACKD) unit, including patient information and training regarding the disease and the different treatment options, with a view to facilitating the most appropriate technique in each case.
- •
Integration of the different types of renal replacement management: kidney transplantation (both deceased and live donor), PD and HD, including home hemodialysis (HHD), and conservative treatment.
- •
Provide the means required for the routine HD techniques: conventional HD, High-flow HD, expanded hemodialysis (HDx), online hemodiafiltration (HDF-OL).
- •
Provide care, training and teaching support for the out-hospital HDUs (OHDUs) of its area of influence.
- •
Provide dialysis care support for the rest of the hospital, referred to both acute and chronic patients.
- •
Provide special techniques support for the rest of the hospital (continuous dialysis and therapeutic apheresis).
The HHDU is to guarantee correct HD care for both scheduled and non-scheduled patients, management in the vascular access unit, and inclusion of the patients on the kidney transplantation waiting list of their own center or the reference center. There must be access to hospital admission beds and adequate follow-up by Nephrology.
The HHDU must ensure urgent management, with 24-hours a day care. A nephrologist must be present in person when any HD session is being carried out.
The functional or operational classification of the HHDU is described as follows:
- a)
Dialysis of chronic patients: Periodic maintenance HD of the patients reporting to the hospital. The architectural structure and technical and staff resources, as well as the treatment of patients with infections, are to be compliant with the current regulations.
- b)
Dialysis of acute patients: This unit is intended for patients who have already started RRT (chronic patients) and who require hospital admission due to an acute problem, or patients with acute disease who temporarily require dialysis. The unit may also make use of special techniques such as:
- •
Therapeutic apheresis.
- •
Continuous extrarenal filtration.
The unit should have specific nursing and assistant staff in accordance with the current regulations.
- •
- c)
Home dialysis: This therapeutic option seeks to control and train those patients who opt for dialysis at home, whether HHD or PD.
Definition of the out-hospital dialysis unit or center
These units or centers provide dialysis treatment outside the hospital (Spanish Organic Act 15/1999)9. They are generally found in strategic locations of the healthcare area: Primary Care Centers, District Hospitals or other facilities that meet the required health service criteria and are related to a Department of Nephrology.
These centers are regulated by Public Services Management contracts for the HD treatment in subsidized centers of patients belonging to the Public Healthcare system9. These contracts are awarded by the Health Services of the different Autonomous Communities, and cover patients on dialysis referred from the HHDU or other units, based on the existing legislation and agreements. The presence of a nephrologist is required during the dialysis sessions.
When the health authorities establish an agreement with a private center, the need to establish a functional or operational relationship between the Department of Nephrology and the out-hospital center must be considered, allowing the patients assisted in both centers to have the same opportunities regarding dialysis and other treatments, complementary tests, access to the transplantation waiting list and inter-consultations with other specialties.
In order to improve care continuity of patients on dialysis referred to the OHDU, it is advisable to establish an electronic communication link between the hospital and the OHDU, with a view to facilitating access to the information corresponding to the shared patients. This will favor the equity, safety, efficiency, reliability and confidentiality of the treatments of these patients.
The OHDU generally presents the same characteristics as the HHDU, except that it does not assist acute cases and cannot offer the full range of services and management modalities. Coordination between the OHDU and the HHDU is important to guarantee the equity and quality of HD.
Objectives of a hemodialysis unit
Both the in-hospital and the out-hospital HDUs aim to provide dialysis treatment for those patients who need it, in accordance with the criteria and “suitability” specifications recommended by the scientific evidence, as contemplated by the clinical guides, integrated care processes, protocols and other clinical management instruments.
Other specific objectives of an HDU are to10:
- •
Improve patient information and care referred to RRT.
- •
Promote live donor kidney transplantation.
- •
Guarantee the recommended technical quality, as well as due coverage of the necessary social aspects.
- •
Ensure an adequate vascular access, with the use of an internal arteriovenous fistula (IAVF) in all cases where this proves technically feasible.
- •
Increase the percentage of patients entering HD with a viable and operative arteriovenous fistula.
- •
Guarantee that all patients subjected to HD are treated with ultrapure dialysis fluid or dialysate.
- •
Encourage active patient participation.
- •
Apply the clinical protocols referred to the diagnosis and management of the complications of the HD technique.
- •
Maintain an adequate scientific and technological innovation level.
- •
Improve the capacities and skills of all the professionals.
- •
The HHDU is to provide technical and scientific support for the OHDU.
Coordination of the centers
As has been commented above, it is necessary to establish a functional or operational relationship and to ensure effective and continued communication between the out-hospital dialysis centers or units and the reference Department of Nephrology in order to guarantee the equity of patient care. The following is needed for this purpose:
- •
The use of easily accessible information technologies such as the telephone or e-mail. There should be a shared case history corresponding to each patient, and if this is not possible, there at least should be access to the different electronic systems in order to facilitate the exchange of relevant clinical information and avoid unnecessary patient displacements2. In relation to these two aspects, it is necessary to abide with the current regulations regarding personal data protection (Acts LOPD and RGPD).
- •
Establishment of the pertinent two-directional patient referral circuits.
- •
The definition of consensus-based clinical protocols on RRT, its complications and associated disease conditions (anemia, bone mineral metabolic disorders).
- •
The holding of periodic joint meetings to address clinical or organizational issues.
- •
The reference center will be in charge of the urgent cases, acute patients, chronic patients subjected to RRT and admitted to hospital due to any cause, or individuals requiring a modality of dialysis that cannot be provided by the out-hospital center.
- •
Quality criteria in common with the reference center (ISO 9001 standard).
In parallel to the evolution of kidney disease and its treatment over the years, there have also been developments in the concepts and strategies referred to the search for and communication of clinical information.
Although there are many controversial aspects, it is currently considered that rigorous clinical practice guides (CPGs) based on adequate methodology (Grading of Recommendations, Assessment, Development and Evaluations [GRADE]) play a key role in the transmission of clinical knowledge and in the improvement of care quality11,12.
The development of a CPG requires the following:
- a)
A clinical problem well defined by expert physicians and other pertinent individuals (patients, other healthcare professionals, etc.).
- b)
A structured and systematic search strategy of the published scientific-clinical literature, performing an analysis of its quality and probative capacity; this generally requires the collaboration of specialists in methodology and documentalists.
- c)
With the above elements, the group of experts establishes consensus-based recommendations addressing the different aspects of the mentioned problem.
- d)
Maximum transparency (literature evidence, methodology, conflicts of interest). Internal and external review controls.
- e)
Participation, at different levels, of all those implicated in the analyzed clinical procedure (nursing staff, other healthcare professionals, patients, industry).
In the development of a CPG, we find the following:
- a)
Descriptive or conceptual aspects (definitions, classifications, agreements, organizational aspects, etc.) that can be resolved using traditional narrative reviews.
It can be seen from the Index of the present work that most of its topics (chapters 1, 2, 3, 6, 7, 8, 9) are of a descriptive and/or conceptual nature, covering aspects such as the analysis of the architectural structure of a hemodialysis unit, its staffing, the description of the different HD modalities, transient patient care, etc.
These topics can be resolved with classical narrative review methodology. The studies made in this regard include a broad literature review of both the nephrological publications and any other type of information related to the subject (official documents and specifications, protocols of the Health Technologies Evaluation Centers, position statements of other societies, etc.).
The resulting document has been subjected to a review process by other nephrologists with expertise in the field (Annex 1), and nephrological nursing professionals (SEDEN) and renal patient associations (ALCER) have been consulted.
Lastly, the final draft of the guide was posted on the S.E.N. website for one month to allow associations and anyone interested to access and know the document and submit comments prior to definitive publication.
- b)
Aspects that analyze clinical problems of interest in HD (such as comparison of the outcomes of different therapeutic or diagnostic procedures, prognostic or epidemiological elements, etc.).
From the list of topics of our guide it can be seen that there are three basically clinical topics (chapters 4 and 5 and, partially chapter 10). These topics cover descriptive, conceptual and assessment aspects, and have been developed by expert nephrologists in narrative review format, preceded by an exhaustive literature search (PubMed, Cochrane) and developed with the methodology described in the previous section.
However, the S.E.N. and the professionals who developed the current guide are aware of the need to go into deeper detail in very concrete aspects of these clinical topics, in order to accredit the scientific rigor and probative capacity of the conclusions and recommendations, using methodology that guarantees systematic and rigorous compilation of the published evidence and analysis of the quality of the latter. Among these methods, the GRADE has been universally employed in systematic reviews and clinical practice guides13–15.
In order to improve the rigor and level of scientific evidence related to this area in our specialty, work is being done of a series of systematic reviews on clinical topics that will serve as the basis for the definition of recommendations, and which will be published in the journal NEFROLOGIA under the common name of Hemodialysis Guides.
Team developing the guideFor the update on the Hemodialysis Centers Guide of 20061, the Steering Committee of the S.E.N. has selected the most reputed specialists in each of the addressed topics; many of them had already participated in the drafting of the previous version of the guide.
The selection was made on an independent basis, considering professional and scientific suitability criteria, and the absence of conflicts of interest.
In a first step, the coordinators were selected based on their research authority, professional experience and organizational capacity. With their intervention, and in accordance with the Steering Committee, a selection was made of the rest of the panelists in each of the topics, as well as of other internal and external collaborators – many of which had already participated in the previous version of the guide. Brief curricula vitae are included as evidence of the suitability of the professionals collaborating in the guide, along with the corresponding statements on conflicts of interest (Annex 2).
Guide target population- •
Physicians, nurses and assistant staff working in dialysis units.
- •
Managers and directors of hospitals and out-hospital centers with hemodialysis units.
- •
Public health administrations.
- •
Renal patient associations.
- 1)
To define the S.E.N. criteria referred to the structural and operational requirements of out-hospital hemodialysis units.
- 2)
To define the relations of the out-hospital Nephrology unit with the operational structure of the healthcare area (Hospital Unit / Department of Nephrology; Peritoneal Dialysis; Kidney Transplantation).
- 3)
To analyze concrete aspects of clinical practice, particularly those that are of mandatory and/or advisable application in all hemodialysis units. This is done based on the GRADE methodology, through consensus on the part of the document drafting group, the pertinent clinical (PICO) questions, the design of the systematic reviews and the decision-making processes (GRADE-Delphi methodology) for definition of the recommendations.
- 4)
The resulting documents have S.E.N. position statement status and will be generated over time under the common name of Hemodialysis Guides, with publication in the journal NEFROLOGIA, the website of the S.E.N., and Nefrología al día.
The facilities corresponding to the hemodialysis units (HDUs) must comply with the habitability and hygiene conditions required of all healthcare centers. The design of the building must be adapted to the climatologic, temperature and sonority conditions of the location16–28.
The current legal specifications referred to facilities of this kind in each Autonomous Community must be followed, with due application also of the pertinent Spanish national and European Union standards. This refers to both construction of the facilities and their operation, maintenance and posterior controls29–34.
The environment must be free of architectural barriers and should allow rapid, comfortable and safe access for the patients, while also ensuring adequate timing of care.
It would be advisable to size or scale the Unit to the theoretical demand, based on a demographic analysis of the setting (prevalence rates according to age groups, and characteristics of the population in the area), contemplating a 10-year population projection.
This chapter refers to both in- and out-hospital hemodialysis units. The former are to be located in a setting including the advanced chronic kidney disease (ACKD) clinic and home dialysis unit (both peritoneal and hemodialysis); some of the specified facilities (such as the waiting room) may be used on a shared basis.
Zones or facilitiesA home environment is to predominate over the hospital environment in the hemodialysis facilities for chronic patients, offering them a pleasant appearance, with good preservation and cleanliness.
The general design should take the versatility of the different environments into account, ensuring maximum comfort for the patients, their relatives and the healthcare staff, and offering intimacy in a dynamic and functional setting.
Administrative area (Admission / Reception / Secretariat)18
- •
Located at the entrance to the Unit, with visible and simple access.
- •
The reception desk should be designed with a low area to assist people in a wheelchair.
- •
Access and stay on the part of people with reduced mobility (wheelchairs, etc.) must be facilitated.
- •
The area should have a minimum surface of 9 m2 and may be integrated in or form part of other administrative areas or dependencies.
- •
The area must be equipped with communication media (voice and data). The reception desk is to be equipped with computers, copying machines, telephone and fax.
- •
The area is to allow functions of control, attention and general information for the users.
Waiting room18
- •
The waiting room is to be clearly indicated, with an information panel.
- •
It should allow access and stay by people in wheelchairs and patients with other disabilities.
- •
The furnishing should allow a comfortable and relaxed wait for all patients and accompanying persons of each shift.
- •
There should be a space for waiting in a wheelchair.
- •
The waiting room is to be located next to the patient dressing room, close to the hemodialysis room, and should have adjacent care services.
- •
Since the waiting room concentrates people, it should be sufficiently spacious, well ventilated and illuminated, and should offer a pleasant and relaxed environment for the patients and their relatives.
- •
The surface area should be over 1.5 m2 per patient corresponding to each hemodialysis shift. The minimum overall surface should be 20 m2.
Patient toilet facilities (restrooms)18
- •
These facilities are to be located close to the waiting room and patient dressing rooms.
- •
There should be at least one restroom for every 10 stations, with gender distinction.
- •
A restroom for patients with reduced mobility must be available, offering adequate toilet and washing facilities, and the absence of architectural barriers.
- •
All doors to the restrooms, showers and dressing areas are to open outwards, and it must be possible to open the locks on the doors from the outside in the event of an emergency, as a safety measure.
- •
The rooms should be equipped with buttons for triggering outside acoustic and/or visual emergency alarms.
Patient dressing rooms18,35
- •
These rooms should have areas differentiated by gender.
- •
Lockers should be available for personal belongings.
- •
There should be an individual restroom and dressing area for patients with positive hepatitis B virus serology.
Holding area for stretchers and wheelchairs
- •
Close to the hemodialysis room.
Treatment room18,26,28,35
- •
Each treatment station is to have a minimum area of 8 m2.
- •
The separation between treatment stations should allow easy circulation on the part of healthcare staff, wheelchairs and stretchers.
- •
Ideally, the patients should be distributed in such a way that some privacy is afforded while always remaining visible to the center staff.
- •
It is advisable to place portable screens (better than curtains on rails in the ceiling) between the different treatment stations in order to afford privacy without affecting the entry of natural light into the room.
- •
Comfortable automated chairs or beds are indicated, allowing the Trendelenburg position.
- •
A patient precision weighing scale is required, allowing the weighing of wheelchairs.
- •
In relation to nursing control, the electronic processing and reading and writing activities inherent to nursing activity must be possible. Telecommunication, patient communication systems, pneumatic tube transport and alarms must be available: fire protection, gases, and treatment and supply of water for dialysis.
- •
From each nursing station it must be possible to control all the hemodialysis stations dependent upon it.
- •
Each treatment station must be equipped with a nursing call system.
- •
The room should have space to allow hand-washing of the staff caring for the patients. Each washing sink must be easy to control by the staff (elbow, pedal or automatic).
- •
The clinical waste containers (sharp elements and others) are to be located alongside the staff washing area.
- •
There must be sufficient water-alcohol solution dispensers.
- •
It is advisable for the distribution of water, concentrates, electricity and electronics to be in the form of individualized modules that are easy to disassemble and access, thus allowing their repair or replacement without the need for masonry work.
- •
Each hemodialysis electrical station should be fitted with a differential switch.
- •
The electrical installation should allow illumination suited to the type of care, in both the room and in nursing control, and indirect lighting is to be available for patient rest.
- •
The station is to be fitted with a data input/output terminal linked to the server via a local/area network.
- •
The availability of Wi-Fi is advised.
- •
If audiovisual facilities are available, each hemodialysis station should offer individualized earphones.
- •
Oxygen and vacuum outlets (individual, portable or network) are to be available.
- •
The room should have heating and air conditioning affording a pleasant environment and temperature.
- •
There should be one reserve monitor for every 8 operative monitors.
Maintenance room / workshop36
- •
Electrical installations and water and drainage facilities are required.
- •
It may be located adjacent to the water treatment room.
Water treatment room37,38
- •
The surface should be consistent with the dimensions of the water treatment elements.
- •
The water treatment room should be as close as possible to the hemodialysis room.
- •
There must be sufficient ventilation / cooling to dissipate the heat and gases generated by the treatment and disinfections.
- •
The floor is to be waterproofed and with adequate drainage.
- •
The water supply storage capacity must be sufficient to cover the needs of one day of dialysis.
- •
The recommendations of the Guide on the management of dialysis fluid quality (Guía de gestión de calidad del líquido de diálisis)(second edition, 2015) should be followed.
Clean area39–45
- •
This area must be adequate and equipped with material allowing the storage and preparation of medications.
- •
The minimum surface should be 8 m2.
- •
Clean and/or sterile materials storage. Storage and preservation of medications according to Act 25/90 of 20 December, on Medicinal Products, and related regulations. Preparation of clinical material.
- •
The area is to house a refrigerator (4°C) with temperature control and an alert in the case of malfunction. There should be closets and devices appropriate for storages of this kind (shelves, cabinets, etc.). Information and protocol panels and adequate stands for pre-medication must be available.
Dirty area39–41,43–46
- a)
The minimum surface should be 8 m2, and the area must cover the needs referred to:
- ˆ
Temporary storage of dirty clothing and/or waste.
- ˆ
Cleaning of material.
- ˆ
Waste room47
- •
Both waste retrieval and provisional storage must abide with current legislation.
Storage facilities
- •
A general store is required for replacements and for the minimum consumables needed for one week.
- •
There should be spaces for clean clothing carts and the storage of textile materials (blankets, cushions, towels), to be separated from the dirty items (temporarily stored in the waste classification area).
- •
A specific closed area or zone for inflammable and volatile materials is required.
Medical office
- •
At least a consulting room is required, with a separate exploration zone to g uarantee patient intimacy, equipped with facilities for hand washing, an office desk with its chair and two consulting room chairs – the surface area being between 12-19 m2.
- •
The office may serve as a polyvalent space for other uses (psychologist, social worker, dietitian).
Nursing office
- •
The nursing office should be equipped with adequate furnishing and installations.
- •
It may serve as a polyvalent space for other uses (psychologist, social worker, dietitian).
Dressing rooms and toilet facilities (restrooms) for the healthcare staff
- •
Dressing rooms are required, with a locker for each staff member, located in the Dialysis Unit or in the General Centralized Units.
- •
The unit is to be equipped with one restroom for every 15 women or fraction per shift, and one restroom for every 25 men or fraction per shift.
- •
A clinical staff toilet facility is to be available for every 10 nurses or fraction per shift.
- •
These facilities may be those of the General Centralized Units.
Staff resting area
- •
A room adjacent to the hemodialysis room is required for the resting shifts of the healthcare staff.
- •
The minimum surface area should be 12 m2.
General Healthcare Services
- •
The general health regulations apply.
- •
The following should be available in the hemodialysis room or in a nearby zone of easy access:
- ˆ
Auxiliary or dressing carts or tables.
- ˆ
Cardiac arrest (crash) cart with portable vital signs monitor.
- ˆ
Portable 12-lead electrocardiographic equipment.
- ˆ
- •
Ultrasound is recommended for control and management of the vascular accesses.
Other zones and areas
Availability of the following is advised: public restrooms near the waiting room; a wound dressing room that may be covered by an office; meal service that may be assigned to the staff resting area; and a polyvalent meetings / library / sessions / teaching room.
General conditions29–34,48,49Compliance with the current local, Autonomous Community, Spanish state and European Union regulations referred to the construction, functioning and elimination of architectural barriers of installations of this kind is mandatory. The same applies to the general regulations, Spanish Royal Decree 556/1989, of 19 May, specifying the minimum measures referred to accessibility in buildings, Act 8/1993, of 22 June, on the Accessibility and Suppression of Architectural barriers of the Community of Madrid, and Act 51/2003, of 2 December, referred to the equality of opportunities, non-discrimination and universal accessibility for people with disabilities.
The facilities corresponding to the hemodialysis units (HDUs) must comply with the habitability and hygiene conditions required of all healthcare centers.
Most of the center, destined to patient care, is to be located on the ground floor, and if this is not possible, an elevator must be available in which wheelchairs and stretchers can be transported.
The floors of the hemodialysis unit must be waterproof and resistant to acids and other chemical products used in HD.
The specifications of the general structure of the Unit regarding the circulation of people and materials are as follows, according to the type of transport involved:
- •
Chronic patients circuit: 1.50 m in width.
- •
Potential stretcher turning zones: 2.00 m free width.
- •
Potential bed turning zones: 2.40 m free width.
The electrical installation should include an alternative power circuit to guarantee operation of the Unit in the event of a power failure of the main circuit. The Unit is to have an emergency power supply in the case of a mains power supply shutdown. The mentioned circuit at least will include the hemodialysis monitors, the water treatment plant, the medication and biological sample refrigerators, and environmental illumination. The power setting should be no less than 4000 W per dialysis station.
The electrical installation should afford maximum guarantees of power supply, with an auxiliary power generator unit (general or pertaining to the Unit) capable of operating for at least one full hemodialysis session (5 hours).
All the electrical power outlets are to be equipped with individual differential switches, and there must be a general power panel with sections differentiated by hemodialysis station.
The applicable low-voltage regulations of the Spanish Ministry of Industry must be followed in full.
The provision of water to the HDU must comprise a double water circuit, a non-treated water deposit sufficient for one day and, in the case of a hospital HDU, a corresponding storage cistern.
Correct protection, signaling and evacuation facilities, with emergency lights and exits, are required in accordance with the current regulations, to ensure effective evacuation and fire alert or other emergency procedures.
Technical certification is required of compliance with the current regulations referred to safety in buildings, emergency exits and protection in the case of fire alerts.
It is advisable for the rooms to remain empty between shifts in order to facilitate their cleaning and disinfection before the start of the next treatment shift. In this regard, the ceilings, walls and floors are to be made of materials suitable for the periodic cleaning routines and which allow the use of cleaning and disinfectant products.
The healthcare center must identify and classify the sanitary waste, guaranteeing its adequate removal and elimination.
The maintenance staff of all the installations will hold the pertinent technical certification and will strictly comply with the applicable regulations.
Absence of architectural barriers18,35,50,51
The Unit should be designed to guarantee easy and safe access to all the zones, taking into account that:
- •
There must be no architectural barriers for people with mobility problems. All the applicable norms in this respect (Autonomous Community and state) are to be followed.
- •
It is advisable for the building in which the HDU is located to have public and medical transport accesses, with a safe patient transfer zone.
- •
There must be easy, comfortable, safe and direct access to the HDU, including transfer by wheelchair or stretcher through all areas (corridors, elevators, etc.).
- •
The access routes (both exterior and interior) are to be clearly and simply signaled and identified. They must be free of furniture or other elements and should not be used as storage zones that may complicate the fluid circulation of people or equipment. The access routes also should be equipped with railings and other elements of support, as well as non-slipping floors needed to guarantee adequate circulation.
There must be an Emergency Evacuation plan, with an appropriate architectural design meeting the pertinent regulations.
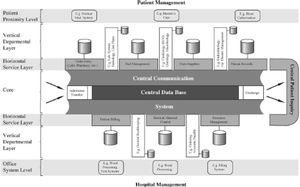
Electronic and software facilities of the hd unitsAn internal electronic communication network is required, connecting the different computers of the center, the electronic scales, dialysis monitors and other medical instruments and devices capable of generating and exporting electronic data.
A software system capable of hosting the required electronic applications is needed.
The facilities should allow access from each workstation and/or room. The network and machines (PCs or servers) will be technologically up to date and are to meet the safety and maintenance requirements documented in the management plan.
The computer system managing the Dialysis Unit must be able to receive (through continuous, automated and configurable downloading protocols) the data corresponding to the hemodialysis sessions from the monitors installed in the HDU, in order to allow due monitoring from a single software application.
The availability of “open” electronic systems allowing the two-directional connection of monitors of different commercial brands is highly advisable.
The software system should include the possibility of tracing the products and consumables used in the sessions automatically, recording products, batches and expiry dates.
The software facilities should be able to generate quality indicators, serving as support for care and operating protocols.
An integral management computer system should be available, including a contingency plan to guarantee operability in the event of software or network problems.
Connection to the applications of the electronic case history implemented in the reference hospital under an HL7 standard through events, web services, etc., should be considered.
The computer system of the Dialysis Unit should contemplate access to the database and renal patient registry of the health authorities of the different Autonomous Communities.
Isolation zones18,43–46In the case of patients with positive hepatitis B serological testing, an independent location with a separate dirty area is required.
Potentially contagious patients with hepatitis C virus (HCV) and human immunodeficiency virus (HIV) markers do not have to be dialyzed in an isolation unit. Dialysis in a specific zone is recommended in these cases, with the adoption of careful universal preventive measures.
In the case of patients with other infectious and potentially contagious diseases, the isolation recommendations of Preventive Medicine should be followed.
The rooms for the treatment of infected patients require a separate dirty area, as well as the equipment and facilities described for each dialysis station in the general treatment room. The useful surface should be no less than 16 m2.
In some hospital HDUs it would be advisable to habilitate a negative-pressure room for dialysis.
Economy and ecology52,53Location of the HDUs in “green” buildings that are respectful with the environment, optimizing water supply and waste processing while also complying with sustainable economy principles (e.g., involving the use of renewable energy sources such as solar energy) should be viewed as a positive element.
Zones or areas
- 1.
Administrative area (admission / reception / secretariat)
- 2.
Waiting room
- 3.
Patient restrooms
- 4.
Patient dressing rooms
- 5.
Stretcher and wheelchair station
- 6.
Treatment room
- 7.
Maintenance room / workshop
- 8.
Water treatment room
- 9.
Clean area
- 10.
Dirty area
- 11.
Waste room
- 12.
Storage room
- 13.
Medical consultation or office
- 14.
Nursing consultation or office
- 15.
Healthcare staff dressing and restroom facilities
- 16.
Staff resting area
- 17.
General healthcare services
- 18.
Other zones and areas
Patients enrolled in periodic hemodialysis programs are increasingly of older age and present greater complexity and comorbidities in both in- and out-hospital units. According to the annual reports of the kidney patient registries, the mean age of the incident cases has increased by over two years in the last decade, reaching 64.8 years54. Individuals over 65 years of age represent 70% of all new patients on renal replacement therapy2. In subjects over 65 years of age, diabetes and vascular nephropathy - associated to increased comorbidity – account for 65-70% of all incident cases over 75 years of age2.
Although in theory patients treated in out-hospital hemodialysis centers are less disabled and more autonomous, the progression in recent years of other renal replacement modalities such as pre-emptive live donor renal transplantation (5% of all incident cases), the development of deceased (non-heart beating) donor renal transplantation programs, and the growing use of peritoneal dialysis in Spain, imply that younger patients with fewer comorbidities are enrolled in lesser proportion in periodic hemodialysis programs2.
Definition of the staff requirements in dialysis units in this changing environment is a complex issue. The best approach is to develop a series of general recommendations to ensure care efficiency by establishing a balance among the patient needs, the work of the staff members, and the costs of treatment55.
It should be understood that these recommendations are established in reference to out-hospital dialysis centers18. The health care organization of the hospital, characterized by dialysis treatment carried out in parallel with other activities such as hospitalization, outpatient consultations or emergencies, together with the staff needed to guarantee continuous (on duty) care, imply that the needs referred to dialysis staff cannot be addressed in an isolated manner.
General organization of human resources18The staff working in out-hospital hemodialysis units must be organized as a multidisciplinary team oriented in all cases towards the needs of the patient and not to those of the professionals, in which all the team members maximize their contribution through genuine teamwork - independently of the professional categories involved.
Each dialysis unit should keep an updated record of the healthcare professionals employed, with the corresponding training certification, professional category, degree of qualification, professional certificates or credentials, training needs and training activities completed.
A training plan should be implemented on an annual basis, including the detected training needs in each professional group, the planned activities, and the evaluation of compliance.
Each Unit should have a procedure for the reception of newly incorporated professionals. In turn, each newly incorporated professional should have access to the most relevant information needed to perform his or her activities.
The dialysis center will adopt the measures needed to guarantee the identification of its staff, with stratification according to academic degree and professional category. Each healthcare professional must be correctly identified by name and professional category.
Clinical directorEach hemodialysis unit must have a care supervisor, who by definition has to be a Medical doctor with specialist medical training in nephrology. The nephrologist is in charge of the dialysis treatment provided in the center.
The clinical director might not be exclusively dedicated to the center, though his or her working time in the center must suffice to plan, organize, and direct the care services of the dialysis center. The clinical director may also fulfill the role of the Manager of the Center.
The responsibilities of the position include:
- •
Participation in selection of the renal replacement therapeutic modality best suited to each patient.
- •
Assurance of adequate monitoring of the patient and of the dialysis process.
- •
Assurance of ongoing training of the staff working in the center, and the promotion of teaching and research activities.
- •
Adequate coordination with the reference Department of Nephrology.
- •
Assurance of the development and implementation of quality systems and of a dialysis procedures manual. This manual must address the different types of dialysis performed in the center, the procedures for carrying out dialysis, protocols for the prevention of infections, policies for the management of infected patients, and a risk prevention plan.
- •
Promotion of the patient safety plan of the center and accreditation of the Dialysis Unit.
- •
Assurance that all candidates for kidney transplantation are included in an active waiting list.
The recommendations from other countries on the physician staff requirements cannot be extrapolated to the situation found in Spain. In order to establish these recommendations, the French legislation on this issue has been used as a reference, as it establishes a mimimum staff ratio per post and centre19, along with the different out-hospital hemodialysis public procurement agreements implemented in the different Spanish Autonomous Communities that define a minimum ratio per number of patients56–58.
By definition, the physician in charge of the prescription and supervision of dialysis treatment must be a medical doctor with completed specialist training in nephrology.
Each dialysis center must have one full-time nephrologist for every 40 patients undergoing treatment in the center - the minimum for a dialysis center being two specialists in Nephrology. When the clinical director has full-time dedication to the center, he or she will be regarded as one of the nephrologists of that center.
During treatment of the patients, the center will require the physical presence of a nephrologist.
Maintenance of the professional capacity of the nephrologists should be ensured by means of a personalized annual continuing medical education plan.
It is advisable for the physicians of the hemodialysis center to create stable relationships regarding care delivery, education, and research with the reference Department of Nephrology. These relationships are to be mentioned in written care protocols available to all the staff members.
In the case of public procurement out-hospital dialysis centers, ongoing training of the nephrologists is also required; periodic rotation through the reference hospitals is the most appropriate strategy in this regard.
The Health Administration must include a rating of merits applicable to public employment offers (OPES) for each year of work completed in a public procurement contract out-hospital dialysis center.
Nursing staffThe nursing staff assisting the patients during the dialysis session constitute a key element for ensuring quality care.
Observational studies suggest that a lower nurse-to-patient ratio can worsen the outcomes of dialysis and lead to a greater number of adverse events59,60 – though this has not been confirmed by adequately designed prospective studies61.
The nursing staff needed is mainly conditioned by the patient care requirements. In this regard, many factors can modify the workload of the professionals, particularly:
- •
The degree of patient dependency and comorbidity.
- •
The architectural design of the Unit: number of treatment stations per room and session, and the presence of architectural barriers.
- •
Special isolation requirements (Guides on viral diseases in hemodialysis of the S.E.N.).
- •
The characteristics of the technique employed, and the type of vascular access involved.
At present, no regulations have been established in Spain regarding the minimum required number of nursing professionals per dialysis station and shift – in contrast to the situation found in other countries such as France or in some parts of the United States62,63. As a reference, there are also recommendations such as those of the British Renal Society64, although they are based on a different type of healthcare organization and even include healthcare professionals not found in our setting.
Other relevant references are the technical bases of the public procurements of the Spanish administrations for the provision of hemodialysis services in out-hospital centers, which all involve more demanding specifications regarding the minimum ratio of nursing professionals to patient56,65. Taking into account the recommendations of the previous edition of these guidelines55, the evolution of the dialysis population, the legal recommendations found in our neighbouring countries59 and the existing public procurement agreements with out-hospital hemodialysis centers56,60, the minimum specifications recommended are as follows:
- •
One certified nurse is required for every four operating and occupied treatment stations or fraction thereof.
- •
The center should have at least two certified nurses for every dialysis shift.
- •
A certified nursing care technician is required for every eight operating and occupied treatment stations or fraction thereof.
However, the staff requirements are dynamic and vary over time according to the complexity of the patients. For this reason, it is advisable to develop and use validated dialysis workload assessment scales18,66,67.
It is advisable for each dialysis center to have a nursing supervisor, who in collaboration with the clinical director will design the dialysis care protocols and define the continuing training of the nursing staff of the center. The nursing supervisor must have certified experience in dialysis.
The nursing staff directly assisting the patients on dialysis must have a proven experience in the hemodialysis unit of at least three months before assuming the responsibility of patient treatment.
It is recommended that a proportion of professionals with extensive experience in dialysis (no less than two years) should be available so that each shift can count on the presence of a nurse capable of solving technical problems and dealing with nursing care activities of particular complexity.
An continuing nursing education program should be defined to ensure that the nursing staff continues to be professionally well prepared.
Non-healthcare staffProprietary or externally contracted staff must be available for the following services:
- •
Cleaning.
- •
Preventive and corrective maintenance of the equipment and installations (monitors and water treatment plant) of the center.
If the center treats over 60 patients, the administrative processes associated to such a volume of patients advise the availability of a full-time equivalent person in charge of the administrative / secretarial activities on a stable basis.
Supporting staffEach out-hospital dialysis center should offer the added service of a dietician or nutritionist for personalized counseling regarding the dietary needs of patients on dialysis. Although the existing public procurements define no specific needs per number of patients, the availability of nutritional support is effectively contemplated as a quality criterion.
Likewise, the sociosanitary and psychological problems associated with patients of this kind advise the support of a social worker and a clinical psychologist. In this case, the existing public procurements again define no specific needs per ratio of patients, though the availability of these professionals is indeed specified.
These supporting staff members may belong to the center itself, to the reference hospital, or to the renal patient associations.
As an indicator of the importance of these professionals, the recommendations of the British Renal Society establish the need for one dietician and a social worker for every 100 patients on hemodialysis, taking into account the particularities of the organization of their healthcare system64.
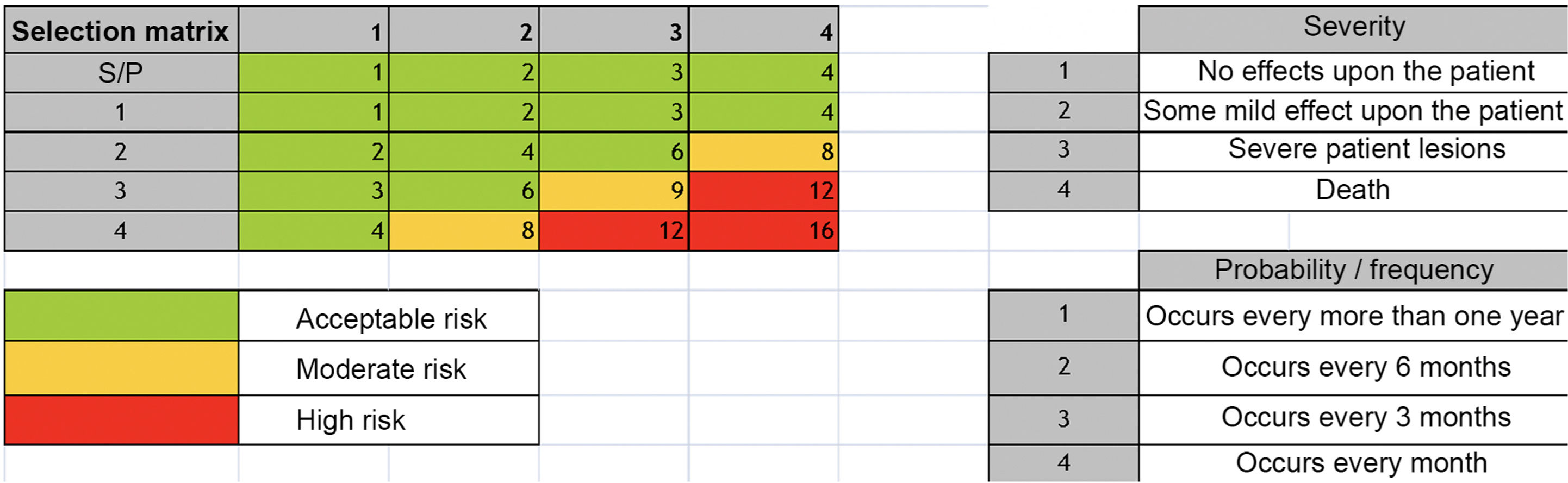
Overall level of evidence: C
Hemodialysis modalitiesIntroductionHemodialysis was introduced 60 years ago as a life-preserving technique for highly selected patients. Since then, it has been developed to become an effective and well contrasted method that has been used in millions of patients with kidney failure throughout the world. Over these years, the dialysis procedures have evolved in parallel with the technological developments, there being at present many types of dialysis membranes with increasingly improved biocompatibility. The standards referred to dialysis fluids are increasingly strict, and the dialysis monitors are automated – this allowing precise control of numerous parameters that influence the quality and tolerability of dialysis treatment (temperature, ultrafiltration, composition of the dialysis fluid, etc.).
Over the last decades, these technological improvements have led to changes in the profile of the population on dialysis, allowing the incorporation of older patients and those with increased comorbidities to the hemodialysis programs. As a result, in the 1980s, the mean age of the population on hemodialysis was 45 years68, though in 2017 this age had increased to 70 years. Despite this notorious and progressive increase in patients' age, the annual mortality rate among prevalent cases has not changed over the last 10 years, remaining high (12.5-15.5% annually, according to the Dialysis and Transplantation Registry of the Spanish Society of Nephrology (Sociedad Española de Nefrología [S.E.N.]69), and being far higher than the mortality rate observed in the general population with an equivalent age and gender distribution.
In the last few decades, this high morbidity and mortality among patients on dialysis has led to the proposal of different modalities and techniques seeking to secure the best dialysis possible. In this sense, the term “adequate dialysis”71 was introduced, representing well tolerated dialysis with the lowest morbidity-mortality possible, at an assumable cost, and well adapted to the patient expectations, as well as allowing social integration with the best quality of life possible. With this in mind, several methods have been developed to measure the dialysis dose, along with membranes that allow higher dialysis doses, more biocompatible membranes (i.e., membranes that induce a lesser inflammatory and potentially harmful response), convection techniques that improve the capacity to eliminate middle weight molecules, adsorptive techniques that improve the elimination of protein-bound toxins, and more frequent dialysis techniques. However, in recent years we have become aware that the different dialysis techniques currently available often report results that are of no relevance to the patients or their caregivers70–73, and that patient-centered factors such as autonomy, time flexibility, portability of the technique and the possibility to travel, tolerance or the effect of the technique upon nutritional status and functional capacity, must be priority issues on comparing the benefits of the different types of hemodialysis.
All this diversity of dialysis techniques and modalities means that we have not yet achieved the desired “adequate dialysis”. However, although historically there has been no clear evidence demonstrating the superiority of one type of hemodialysis over another, the publication in recent years of several controlled trials with a sufficient sample size, together with large patient registries that have analyzed this issue in terms of morbidity-mortality, have evidenced that the use of large convection volumes, more frequent hemodialysis or home hemodialysis, are strategies associated to improved patient survival and quality of life compared to standard hemodialysis74–76.
The present chapter defines the modalities of hemodialysis recognized by the S.E.N., and briefly summarizes the available evidence on their usefulness and safety for the renal replacement treatment of chronic kidney disease.
Hemodialysis modalities: definitionsDifferent modalities of hemodialysis are recognized, depending on certain characteristics of the structural elements conforming the extracorporeal dialysis system (dialyzer, dialysis fluid), the place where treatment is provided (dialysis center, home hemodialysis), the type of water and solute transport mechanism used (diffusion, convection or adsorption), and the number of weekly procedures involved. Dialysis centers may be equipped with all or only some of these modalities, but they must have an operating manual and specific protocols for each of the modalities of hemodialysis they offer.
The choice of the modality of hemodialysis should be based on the characteristics of the patient (age, body surface, comorbidity conditions, vascular access, clinical course, situation with respect to transplantation) and the structural specifications of the center. It is advisable to keep a registry of all the patients, documenting the modality of hemodialysis and the reason for the indication of treatment.
A definition is provided below of the different modalities of hemodialysis according to different parameters.
Hemodialysis modalities according to the characteristics of the dialyzer, blood flow and dialysateThe following characteristics of the dialyzer should be considered:
- •
Biocompatibility of the membrane
- ˆ
Hemodialysis with cellulose or modified cellulose membranes. Lesser biocompatibility.
- ˆ
Hemodialysis with synthetic membranes. Greater biocompatibility.
- ˆ
- •
Ultrafiltration capacity (convective permeability) defined by the ultrafiltration coefficient (Kuf):
- •
Efficiency (diffusive permeability) defined by the mass transfer-area coefficient (KoA):
- ˆ
Low efficiency: KoA < 600 ml/min
- ˆ
High efficiency: KoA > 600 ml/min
- ˆ
Based on these characteristics, the following modalities of hemodialysis can be described:
Low-flux hemodialysis (LF-HD)
This modality has been the most widely used during the last 20 years. The technique employs a low hydraulic permeability dialyzer, and the pore size is small (from cuprophane in the past, to other modified cellulose or synthetic membranes currently used). Bicarbonate is used as buffer (although until a few years ago, acetate was the predominantly used buffer). Filtration is carried out by a diffusive mechanism. Large molecules are not filtered, and middle weight molecules are insufficiently filtered. A distinction can be made between low efficacy (dialyzers of low efficiency, KoA < 600 ml/min, Kuf < 10 ml/h/mmHg, blood flow between 200 and 300 ml/min and dialysis fluid flow rate of 500 ml/min) and high efficacy (dialyzers of high efficiency, KoA > 600 ml/min, Kuf 10-20 ml/h/mmHg, blood flow between 300 and 500 ml/min and dialysate flow between 500-1000 ml/min). This type of dialysis was initially intended to reduce the dialysis time, improving performance of the diffusion processes, and thereby allowing an adequate dialysis dose to be maintained. Posteriorly, the aim was no longer to shorten the time but to afford a greater global dialysis dose.
High-flux hemodialysis (HF-HD)
This technique seeks to improve the quality of dialysis by means of a high permeability dialyzer with a larger pore size. It is made of synthetic membranes with great biocompatibility and high flux (Kuf > 20 ml/h/mmHg/m2, normally > 40). It improves the clearance of molecules of middle molecular weight. Transport remains diffusive, though there is more convective transport than in the previous modalities. Pure sterile dialysis fluid is required, since there is almost always some degree of retrofiltration77. High blood flows are advised in order to reach maximal efficiency.
Extended hemodialysis (HDx)
This technique also uses a dialyzer of high permeability, but the pore size is even greater, with a medium cut-off (MCO) point with respect to the native kidney (65 kDa). This makes it possible to eliminate molecules such as the light chains of immunoglobulins, though minimizing the albumin losses. With these dialyzers we can achieve greater filtration of middle weight molecules compared with high-flow dialyzers, due to the design involved - with a smaller internal diameter of the capillaries - backfiltration is optimized, and greater internal convective transport is added to diffusion – a condition referred as internal hemodiafiltration78.
Hemodiafiltration (HDF)
These are considered to be the most efficient modalities for optimizing the filtration of small and middle weight molecules. In addition to diffusive transport, such techniques use convective transport. Hemodiafiltration (HDF) requires membranes of high biocompatibility, efficiency and flux, as well as complex monitors with strict control of ultrafiltration and high purity of the dialysis fluid. A high ultrafiltration rate is used (4-30 liters/session), and most of the ultrafiltered volume must be replaced in a synchronized manner by a substitution fluid that requires an adequate electrolyte composition, and which must be both sterile and pyrogen-free. Substitution can be made before (pre-dilutional) or after (post-dilutional) or before and after (mild or mixed-dilutional) entry of the blood in the dialyzer. This type of technique is very efficient in filtering small and middle weight molecules, with no backfiltration.
There are many variants of hemodiafiltration according to the substitution volume, including techniques with a low reinfusion volume (under 15 liters) and hemodiafiltration methods with high reinfusion volumes (over 15 liters). The EUDIAL working group of the European Renal Association redefined HDF as a technique combining diffusion with convection, by an effective convection volume of at least 20% of the total blood volume processed79.
Likewise, all the randomized studies, in their secondary analyses, have evidenced the superiority of online hemodiafiltration (OL-HDF) with a high substitution volume. For these reasons, the current recommendation is to ensure that patients receive a substitution volume of over 21 liters or a total convection volume (sum of the substitution volumes plus weight losses achieved during treatment) of over 23 liters.
HDF with a reinfusion volume of under 15 liters:
- •
Biofiltration or conventional hemodiafiltration: Substitution volume is less than 2 liters/hour.
- •
Acetate-free biofiltration (AFB): The dialysis fluid has no buffer solution, ultrafiltration is small (about 2-3 liters/hour), and substitution is made with a bicarbonate solution ranged from 6 to 12 liters per session. This technique allows great control of the acid-base balance, since the provision of bicarbonate can be individualized.
- •
Paired filtration dialysis (PFD): Two filters connected in series are used, in which convective transport is separated from diffusive transport, and these mechanisms are put to maximum use. The first filter of high flux and efficiency, receives no dialysis fluid, and only convective transport occurs. The second filter, following the previous filter, is low-flux and is where diffusive transport with the dialysis fluid takes place. Consequently, there is no backfiltration. Reinfusion is usually made between the two filters.
- •
Paired filtration dialysis with regeneration of the ultrafiltrate (PFD – activated charcoal): This technique is identical to that described above, though the reinfusion fluid used is the patient ultrafiltrate itself, after passing through an adsorption cartridge (activated charcoal or hydrophobic resins), thus allowing elimination of the protein-bound molecules through adsorption.
HDF with a reinfusion volume of over 15 liters:
- •
Online hemodiafiltration (OL-HDF): This is currently considered to be the most efficient technique. The dialysis monitor itself generates the substitution fluid continuously from the dialysis fluid. This technique avoids storage of the substitution fluid, though the dialysis fluid must offer concrete purity characteristics (ultrapure fluid), with a high substitution volume of between 5-10 liters/hour, with the purpose of reaching over 21 liters per session. There are different OL-HDF modalities, depending on where the substitution volume is administered in the extracorporeal circuit: pre-dilutional (before the dialyzer), post-dilutional (after the dialyzer) or mixed or pre-post dilutional (before and after the dialyzer). In turn, OL-HDF with intermediate dilution (mid-dilution), is an alternative to mixed OL-HDF that uses a dialyzer specifically designed to cause the blood to enter through a series of central fibers and return in the opposite direction through peripheral fibers. The reinfusion fluid is incorporated in the middle of the two circuit portions of the dialyzer: post-dilutional hemodiafiltration takes place in the first portion and pre-dilutional hemodiafiltration in the second portion.
- •
Paired filter hemodiafiltration with regeneration of the ultrafiltrate (Hemo-Filtrate-Reinfusion [HFR]): This technique uses a dual-chamber dialyzer with a resin cartridge in which the patient ultrafiltrate is reinfused following its regeneration in this resin cartridge, adding adsorption to the diffusion and convection mechanisms. In a first phase, the blood passes through a high permeability dialyzer where - through exclusively convective transport - an ultrafiltrate is produced which in turn passes through a hydrophobic resin (adsorptive phase) to retain protein-bound toxins. The ultrafiltrate is then returned to the blood, which passes through a third low-flux filter (diffusive phase) that ensures the elimination of small molecules together with the ultrafiltration required to secure an adequate negative water balance80.
- •
Hemofiltration: In this case there is no dialysis fluid, and so there is no diffusion – only convective transport. The technique requires large volumes of ultrafiltrate that are replaced with substitution fluid (over 20 liters per session). High permeability membranes are required. Small molecules are not adequately eliminated; this technique is therefore increasingly less often used in application to chronic kidney disease, at least in Spain - though it is still used in Intensive Care Units (ICUs) as a continuous and slow technique, due to its good hemodynamic tolerance.
The following options have been described, depending on the number of weekly procedures, applying any of the above modalities:
Incremental hemodialysis
Start with one or two weekly procedures and then increase to three when residual kidney function declines.
Conventional hemodialysis
Three weekly procedures. Arbitrary reasons, and particularly the dialysis unit management strategy, cause this modality of dialysis to be the most widely used option.
Four weekly sessions or every-other-day hemodialysis
This is an interesting option, used in Lecce (Italy), with four weekly sessions or dialysis on alternate days. This protocol seeks to avoid the long weekend period, and thus ensure that the inter-dialysis period is always less than 48 hours.
Daily hemodialysis
Five or more weekly procedures. This modality of hemodialysis has been used since 1967, though it has gained relevance in recent years. The reasons why this technique is increasingly used are the notion that it is more similar to what is done by the native kidney, which performs 14-hour a day dialysis; the observation of no improvement in morbidity-mortality among patients on dialysis three days a week despite the evident improvements in dialysis techniques; and the good results currently obtained with daily hemodialysis in its two modalities:
- •
Short daily hemodialysis: 1.5-2.5 hours, 6-7 days a week.
- •
Long nocturnal hemodialysis: 6-8 hours, preferably at home.
Hemodialysis in the center
Treatment is provided in a dialysis center, which may be a satellite center or a hospital, with medical and nursing staff assisting the process. This is the most common modality, and in our setting represents over 99% of all procedures.
Home hemodialysis
Treatment in this case takes place in the home of the patient after the necessary training of the patient or relative in charge of administering dialysis care. The patient must be clinically stable and with an adequate vascular access. Although in our setting this technique historically represents less than 1% of all prevalent patients on hemodialysis, in the last decade there has been a considerable increase in the number of subjects that receive this type of therapy. This is due in part to the clinical benefits associated with more intensive hemodialysis regimens, as well as the development of hemodialysis monitors specifically designed for home use81. Home hemodialysis historically has been carried out employing a standard hemodialysis monitor with a dialysate flow of 500-700 ml/min. This inevitably required the installation of a water plant in the home of the patient – a fact that no doubt has limited the implementation of this type of treatment. However, portable monitors have been available for the last years that use a low dialysate flow (150-200 ml/h), and which allow effective daily dialysis with very low dialysate volumes (25-30 liters/session) by maintaining a very low filtration fraction (dialysate flow divided by the blood flow). This permits efficient use of the dialysate and minimizes the space requirements referred to water storage and consumption. It is even possible to obviate the water plant installation and use preloaded 5-liter bags as dialysis fluid, in a way similar to the dialysis bath employed in peritoneal dialysis82.
In-center self-care hemodialysis
In this case, although the hemodialysis sessions take place in a satellite center, the patient is personally in charge of administering his or her dialysis treatment, after receiving adequate training. The patient must be clinically stable and with an adequate vascular access. The nursing staff in the center is minimal, and no medical staff members are needed to be present during the treatment – with the consequent cost savings that this implies. The number of patients that use this technique in our setting is small and little known, though countries with more disperse populations, such as Australia or Canada, have more experience with this technique. In this context, improved quality of life has been reported in patients under this dialysis treatment, as compared to patients who receive dialysis in a center with full care provided by the nursing staff 83.
Hemodialysis modalities according to the characteristics of the patientHemodialysis in acute care
This refers to patients with acute renal failure or with advanced chronic renal failure who need urgent dialysis. The membranes used afford high flow, diffusive permeability and biocompatibility.
Hemodialysis in chronic patients
Patients with advanced chronic renal failure requiring dialysis on a continuous basis and who are included in a chronic hemodialysis program.
Results of the different hemodialysis modalitiesIn 1996, the Medical Technologies Evaluation Agency (Agencia de Evaluación de Tecnologías Sanitarias) published a report evaluating the different types of hemodialysis membranes. The report highlighted the lack of relevant scientific data from randomized prospective trials, though it was estimated that the following groups of patients could derive added benefit from treatment using dialyzers with synthetic membranes - without addressing the modality of dialysis involved(84):
- •
Patients with any of the following comorbidities:
- ˆ
Severe chronic obstructive pulmonary disease
- ˆ
Severe dilated cardiomyopathy
- ˆ
Progressive malnutrition
- ˆ
Recurrent infections
- ˆ
Polyneuropathy
- ˆ
Amyloidosis
- ˆ
Patients on hemodialysis not included on the transplantation waiting list due to definitive contraindications, and in which a need for long-term dialysis is expected:
- ˆ
Patients with acute renal failure
- ˆ
Two decades later, as a result of the different studies published in recent years – many with a high level of evidence – the aforementioned Agency published a new report on HDF-OL, evaluating the safety, effectiveness, costs and indications of the technique85. The report evidenced that HDF-OL not only does not pose additional safety or tolerance problems versus HF-HD, but also significantly reduces the risk of all-cause mortality as compared to HF-HD. At the time of publication of the mentioned report (2016), the available evidence was still inconclusive regarding the effects of HDF-OL related to hospitalization rates, variations in erythropoietin requirements, blood pressure, growth rate in children, or amyloidosis associated to dialysis. Furthermore, no differences had been demonstrated in relation to quality of life or nutritional status with respect to HF-HD.
Since the publication of this report, a number of studies have been made on the different modalities of hemodialysis, including meta-analyses of randomized clinical trials. The data obtained are summarized below in relation to some of the most widely used and most promising modalities of dialysis.
High-flux hemodialysis
In comparison with LF-HD, the HF-HD technique affords better performance in clearing middle and large weight molecules. Although many studies have described significant advantages associated to HF-HD in relation to different clinical parameters such as a lesser risk of amyloidosis due to beta-2-microglobulin, improvement of dyslipidemia associated to the kidney disease, or a lesser activation of neutrophils and monocytes86–88, to date, only three randomized trials have analyzed survival as the primary endpoint: the HEMO study and the EGE trial in prevalent patients, and the MPO study in incident cases. In the HEMO study, 1846 prevalent patients dialyzed three days a week were randomized in a 2 × 2 design to either a usual dialysis dose (eKt/V: 1.05) or a high dose (eKt/V: 1.45), and to either low- or high-flux hemodialysis. The overall findings showed global mortality to be 8% lower in the high-flux group, though the difference was not statistically significant86. A lower incidence of mortality and hospitalization due to cardiac problems (RR: 0.80 and 0.87, respectively) was documented in the high-flux group. Likewise, mortality was seen to be lower in the patients in the high-flux group that had been longer on dialysis before randomization. This was confirmed in a secondary analysis89, evidencing that among the patients treated with dialysis for a long time (8.6 years) before randomization, treatment with HF-HD reduced both overall mortality (RR 0.68) and mortality of cardiac origin (RR 0.63), versus low-flux dialysis. This beneficial effect was not observed in the group that had been on dialysis for only an average of 1.5 years before randomization.
The HEMO study did not confirm the expectations regarding the presumed beneficial effect of a higher dialysis dose. On the other hand, the effect upon morbidity-mortality associated to high flow membranes was far lower than that reported in retrospective series in which the utilization of these membranes in non-diabetic patients was associated to a decrease in mortality rate of 66-76 %90,91. These findings were confirmed in a prospective study carried out in France on the influence of the dialysis membrane upon survival in 650 patients (46% with high flux membranes), where a 38% decrease in mortality was associated with the use of high flux membranes92. The HEMO study was much criticized due to several factors, including the selected population (younger age and with a greater percentage of black patients than in the American population on hemodialysis), the fact that these patients were prevalent, the fact that over 60% had been previously subjected to high-flux dialysis, the reutilization of dialyzers, and the limitation of the duration of the dialysis session. On the other hand, the proportion of convective transport in the high-flux group was small – a fact that could have masked the theoretical beneficial effect attributed to this transport93.
The MPO study in turn included 738 European incident cases on hemodialysis, without the reutilization of dialyzers, and which were randomized to hemodialysis with low or high flux membranes. After a follow-up period of 3-7.5 years, the analysis of survival showed no significant differences between the two groups. However, in the subgroup of patients with albumin < 4 g/dl and in the diabetic patients, the survival rates were significantly higher in the high-flux group than in the low-flux group94.
The EGE study randomized 704 patients on hemodialysis three times a week to high or low flux dialyzers and ultrapure or standard dialysis in the context of a 2 × 2 factorial design. The primary outcome was a combination of fatal or non-fatal cardiovascular events over a minimum follow-up period of three years. Here again, no significant differences were observed in the primary outcome between high-flux and low-flux, or between the ultrapure and standard dialysate. However, the secondary analyses suggested that the cardiovascular event-free survival rate was significantly better in the high-flux group than in the low-flux group for the subgroup of patients with arteriovenous fistulas and the diabetic patients, while the use of ultrapure water had a positive effect upon the cardiovascular event-free survival rate among those patients who had been on dialysis for over three years95. In the subgroup of patients with an internal arteriovenous fistula (AVF), the highest overall survival rate corresponded to patients treated with HF-HD and ultrapure dialysis fluid – this suggesting a synergic effect of both interventions.
Overall, the results of the three studies suggest that high permeability membranes have a beneficial effect on survival in the subgroups of patients at risk, such as diabetics or individuals with long dialysis vintage, and those with an adequate vascular access to allow the optimized use of HF-HD96,97. In 2010, a Cochrane systematic review and meta-analysis found that HF-HD did not modify mortality due to all causes (10 studies, 2915 participants), though it reduced cardiovascular mortality (5 studies, 2612 participants, RR 0.83; 95%CI: 0.70 to 0.99)98. Two later meta-analyses including 7 and 8 studies, with 4412 and 4967 patients, respectively, did evidence a decrease in the risk of mortality due to all causes and of cardiovascular origin with the use of HF-HD versus LF-HD99,100. In the light of these findings, since 2015 the KDOQI guidelines recommend the use of membranes of high permeability and biocompatibility. Furthermore, in the case of cost restrictions, the guides advise that such membranes at least should be indicated in diabetic patients, in individuals with hypoalbuminemia or in those with a long length on dialysis101.
Expanded hemodialysis
In an attempt to improve the clinical outcomes with respect to high-flux dialyzers through an increase in the clearance of larger medium size molecules (> 20 kDa), dialyzers with a medium cut-off (MCO) point allowing the elimination of molecules of up to 45 kDa, such as immunoglobulin light chains, while minimizing the albumin losses, have been introduced very recently102,103. The clearance of medium and large molecules is clearly superior to that afforded by HF-HD, with results that are similar, better or inferior to those of online HDF102,104–107. Although this technique offers a unique opportunity to improve the filtration of medium size molecules without the need to use a substitution fluid as in the case of HDF-OL, its clinical benefits are uncertain and must be warranted by future clinical trials108. There have only been reports of improvement of some uremic symptoms in short case series, including pruritus and restless legs syndrome, and of the post-dialysis recovery time109, and a small randomized clinical trial has evidenced a better inflammatory profile with this technique compared with HF-HD110.
Hemodiafiltration
Convective transport plays a very important role in the transport of solutes of medium molecular weight such as beta2-microglobulin, leptin and vitamin B12, and of intermediate substances such as advanced glycosylation end-products, dimethylarginine and homocysteine. These substances have been implicated in the pathophysiology of amyloidosis, malnutrition, infectious complications and cardiovascular disease, which are so prevalent among patients subjected to chronic hemodialysis111. For this reason, and without abandoning diffusive transport, a number of hemodialysis modalities have been designed seeking to take maximum advantage of convective transport. These hemodialysis modalities include AFB, PFD, PFD with ultrafiltrate regeneration and OL-HDF, which require biocompatible membranes of high-flux and permeability, as well as monitors of great precision for controlling ultrafiltration and infusion rates and, in the case of OL-HDF, the use of “ultrapure” dialysis water. The impact of these hemodiafiltration techniques upon the global costs of renal replacement therapy is considerable. It is therefore necessary to determine exactly what real benefits can be obtained with these techniques and what patients stand to benefit most from their use85.
Until the publication starting in the year 2012 of the results of the four large, randomized trials that have evaluated the effect of OL-HDF upon survival as primary endpoint, the evidence for recommending this type of technique over HF-HD was limited and based on large cohort studies and small interventional trials. These studies evidenced benefits in relation to indirect morbidity-mortality parameters, including improved clearance of medium size molecules and phosphorus, a lesser risk of carpal tunnel syndrome, lesser inflammation with a lower consumption of erythropoietin, increased intra-dialysis hemodynamic stability and better preservation of muscle mass112–121. During this period some meta-analyses and systematic reviews were published including currently little-used techniques under the concept of “hemodiafiltration”, such as AFB or PFD, which did not consider the magnitude of convection volume as a confounding factor – a situation that led to conflictive results on comparing conventional hemodialysis with the convective therapies122–125. The DOPPS study was the first study to describe improved survival in patients on hemodiafiltration, provided that a minimum convective volume was reached (15-25 liters / session)126. This led to conduction of the four large randomized multicenter trials that have compared standard hemodialysis versus HDF-OL: the CONTRAST study (n=714), the Turkish trial (n=782), the ESHOL study (n=906) and the FRENCHIE trial (n=381)127–130.
Although the primary analysis of the first two studies revealed no improved survival among the patients treated with OL-HDF, the post hoc analysis of both trials did detect a decrease in mortality risk in the subgroup of patients that reached high convective volumes (> 22 liters / session)127,128. In contrast to the two previous studies, the ESHOL trial did reach high convective volume (> 22.9 liters / session) in the OL-HDF group, demonstrating in the primary analysis a 30% decrease in all-cause and cardiovascular mortality risk in the OL-HDF group versus the group treated with HF-HD129. The fourth study (FRENCHIE) was carried out in patients over 65 years of age and focused on dialysis tolerance, recording a mean convective volume in the group of patients with post-dilution OL-HDF of between 19.9 liters / session at baseline and 22.5 liters / session in month 24130. Although the proportion of patients that experienced at least one adverse event associated to the treatment, and survival, did not differ between OL-HDF and HF-HD, a significantly lesser incidence of symptomatic intra-dialysis hypotension and muscle cramps was recorded in the group treated with OL-HDF.
A posterior meta-analysis with the individual data of the patients of the four trials has attempted to define the minimum convective dose needed to improve the survival of patients on OL-HDF, analyzing a total of 2793 subjects divided into tertiles according to the convective volume. After a median follow-up of 2.5 years, OL-HDF reduced the risk of mortality due to all causes by 14% (95%CI: 1 - 25) and cardiovascular mortality by 23% (95%CI: 3-39). The greatest survival benefit corresponded to the patients that received the greatest convective volume (> 23 liters / session through post-dilution HDF-OL) in terms of mortality due to all causes [HR: 0.78 (95%CI: 0.62 - 0.98)] and of cardiovascular origin [HR: 0.69 (95%CI: 0.47-1.00)]131. This meta-analysis also showed that certain patient subgroups (elderly subjects, patients with heart disease or individuals with a long time on dialysis) would be those that could benefit most from a greater convective volume132. Having demonstrated improved survival with OL-HDF versus HF-HD, provided that the target of 23 liters / session of convective volume (equivalent to an infusion volume of 21 liters / session in patients with a mean ultrafiltration of 2 liters / session) is reached, it remains to be determined whether such treatment is also able to improve quality of life from the patient perspective, and whether it is cost-effective. The CONVINCE trial has been started with the aim of resolving these issues. This is a study financed by the European Union that will include 1800 prevalent patients on hemodialysis and will randomize them to HF-HD or HDF-OL of high convective volume (> 23 liters / session) during a follow-up period of three years133.
Hemodiafiltration with adsorptive capacity
The elimination of protein-bound uremic toxins is an issue that has not been adequately resolved by HD-HF, extended hemodialysis or OL-HDF101,102. Such toxins have been regarded as the main cause underlying the cardiovascular disease in patients with chronic kidney disease134. Adsorptive techniques such as HFR have been developed in recent years. By passing the ultrafiltrate through a resin cartridge, these methods combine adsorption, convection and diffusion. Although a small cross-over clinical trial has demonstrated improvement in the elimination of protein-bound toxins, and improvement in terms of inflammation, endothelial condition and oxidative stress with respect to OL-HDF135, at this point it is difficult to interpret the clinical relevance of adsorption as an independent morbidity-mortality factor. Future clinical trials are needed to clarify these aspects. Another technique that may allow the combination of adsorption with hemodiafiltration is the use of high-flux polymethylmethacrylate (PMMA) membranes136. Although the clinical outcomes must be evaluated by interventional studies, a recent analysis of the Japanese registry described improved survival associated to the use of PMMA membranes137.
Daily hemodialysis
The conventional protocol comprising dialysis three days a week continues to present an annual mortality rate of 15%, fundamentally associated to cardiovascular causes according to the Dialysis and Transplantation Report of the S.E.N. in 201369. The degree of malnutrition and hyperphosphatemia remain very high, and the degree of rehabilitation and quality of life of the patient on dialysis are still far from optimum.
The most possible physiological hemodialysis would be that tending to reproduce the function of the native kidney, which effectively dialyzes 24 hours every day. For this reason, in recent years the frequency of dialysis has been reconsidered, and an increasing number of centers are incorporating daily hemodialysis programs for selected cases. Such hemodialysis allows a much more regular solutes concentration profile, with lower pre-hemodialysis concentrations of urea, creatinine, potassium and hydrogen ions, among other components. In addition, ultrafiltration is much lighter and more gradual, with the cardiovascular stability advantage that this represents. On the other hand, the technique favors the provision of a greater dialysis dose than that attainable with hemodialysis three times a week138.
Between 1982 and 1997, the experiences of approximately 20 centers throughout the world had been published. In the year 2000 this number had increased to over 200 centers. The S.E.N. has established a daily hemodialysis registry to know the degree of implementation of this dialysis modality in Spain.
The two most widely used daily dialysis modalities are the following:
- a)
High-efficiency short daily hemodialysis: 1.5-2.5 hours, 6-7 days a week. Use is typically made of hemodiafiltration with high blood and dialysate flows and employing large-surface dialyzers.
- b)
Long nocturnal hemodialysis: 6-8 hours, preferably on a home basis. Use is made of conventional hemodialysis with biocompatible membranes, and low blood 200300 ml/min and dialysate flows (100-300 ml/min).
In comparison with conventional hemodialysis, high-efficiency short daily hemodialysis affords improved filtration of uremic toxins and greater hemodynamic stability, since it reduces the accumulation of interdialysis fluid and thus avoids high ultrafiltration rates during the dialysis session. Several hundreds of observational studies and a single randomized trial139 [Frequent Hemodialysis Network daily (FHNd) trial] have demonstrated the clinical benefits of short daily hemodialysis. The FHNd trial was a multicenter study including 245 patients randomized to either frequent hemodialysis 6 days a week or conventional hemodialysis. The two primary endpoints were mortality combined with changes in ventricular mass during the first year, or with changes in physical health during the first year. The outcomes were significantly better in the frequent hemodialysis group [HR 0.61 (95%CI 0.46-0.82) for mortality or change in left ventricular mass, and HR 0.70 (95%CI 0.53-0.92) for mortality or change in physical health]138. The benefit in terms of survival in the short daily hemodialysis group persisted after a median follow-up of 3.6 years after the close of the FHNd study75, which also evidenced improved control of blood pressure and a better phosphorus balance in the frequent hemodialysis group75,139–142. According to many observational studies, short daily hemodialysis may also be associated to improved patient quality of life143, improved appetite144, a lesser need for antihypertensive drugs and phosphorus binding agents108, and a lower hospital admissions rate145. As negative aspects, short daily hemodialysis could increase complications of the vascular access, including thrombosis108, although canulation of the fistula using the buttonhole technique could reduce these complications146. The cost analyses also appear to favor short daily hemodialysis versus standard hemodialysis, since the lower hospital admissions rate and medications use would compensate the greater expenditure in consumables147.
Long nocturnal hemodialysis is also associated to important clinical benefits versus conventional hemodialysis, including improved filtration of uremic toxins, better blood pressure and phosphorus control, a lesser need for drugs, reduction of left ventricular hypertrophy, and improvements in some of the aspects related to patient quality of life and fertility. These data have been corroborated by different observational studies and three clinical trials148–153. The most important trial was the Frequent Hemodialysis Network nocturnal (FHNn) trial, which randomized 87 patients to nocturnal hemodialysis 6 nights a week or conventional hemodialysis three days a week, being the primary endpoints similar to those of the FHNd trial. In contrast to the latter, and probably because of its smaller sample size, the FHNn study was unable to demonstrate benefits in terms of survival148. A number of observational studies, some involving a large sample size, have indeed reported improved survival associated to nocturnal hemodialysis versus standard hemodialysis, possibly even being comparable to that of deceased donor transplant patients154–157. Another study evidenced no decrease in mortality but did identify a lesser risk of hospital admissions158. The global costs and hospitalizations also appear to be more favorable to nocturnal hemodialysis versus standard hemodialysis147,159. As negative aspects, frequent hemodialysis may increase complications of the vascular access, as well as accelerate the loss of residual renal function160,161.
Home hemodialysis
Home hemodialysis (HHD) allows more flexible and physiological treatment schemes than standard hemodialysis, such as short daily HD or nocturnal HD. As a result, with these techniques the same benefits described above can be obtained in patients who undergo dialysis at home. Although the quality of the evidence is limited, since most of the studies are of observational in nature, the described benefits include improved hydration and blood pressure control, with a lesser ultrafiltration rate and need for antihypertensive medication162,163; greater control of the phosphorus levels, with a lesser need for phosphate-binders164; and a decrease in inflammatory status as well as less diet restrictions, with optimization of the patient nutritional status166 and improved quality of life166–169. In addition, HHD offers further clinical advantages versus daily hemodialysis in the center, such as the absence of adverse effects of the vascular access170, a better cost-efficacy ratio171,172, and greater chances for patient rehabilitation and return to work173.
The survival observed in different patient cohorts is far higher among the patients treated with HHD versus those who are dialyzed in the center, with figures of up to 89% and 50% at 5 and 15 years, respectively76,173–179. The reasons why HHD is associated to increased patient survival are not precisely clear. In addition to the aforementioned clinical benefits and the potential existence of selection bias inherent to observational studies in general (patients on HHD are usually younger and tend to have lesser comorbidity compared with patients treated in the center)180,181, psychological factors such as the fact that the patients retain their autonomy and independency and avoid so-called learned helplessness syndrome (a psychological state in which a person feels unable to modify an aversive or painful situation) may contribute to the better outcomes observed with HHD182,183. Being aware of all these potential benefits, in recent years the S.E.N. has created a Working Group for the development of HHD in Spain184.
Hemodialysis in patients with acute renal failureAcute renal failure is a frequent hospital complication and is associated to a high mortality rate (50-70%) and an important risk of developing chronic kidney disease. Up to 10% of the survivors will require chronic hemodialysis185. The main aspects in recent decades regarding acute renal replacement therapy are referred to the type of technique and the type of membrane to be used. The many studies made in this respect offer a reasonable answer to these issues:
- a)
Type of technique: There has been great controversy in the literature regarding the treatment of acute renal failure with intermittent hemodialysis or with slow continuous hemofiltration or hemodiafiltration techniques, usually in patients requiring intensive care. The slow techniques have been considered to be more advantageous due to their greater ultrafiltration capacity, which does not limit parenteral nutrition, and their improved hemodynamic tolerance. However, the randomized and controlled studies designed to detect differences in mortality and in the recovery of kidney function have yielded conflicting results in favor of one technique or other. As a result, the available evidence does not confirm the superiority of one type of renal replacement therapy over another186–190. Likewise, the published meta-analyses have not demonstrated differences in terms of mortality123,191–190. In this regard, the current KDIGO guidelines suggest the use of both continuous and intermittent techniques as complementary treatment for acute renal failure, without expressing any preference for either option197. Accordingly, the decision to use one technique or other largely depends on the patient characteristics and the possibilities for using one or both techniques in each particular dialysis center. On the other hand, the cost of the continuous hemodiafiltration techniques is far higher than that of intermittent hemodialysis198.
- b)
Type of membrane: Biocompatibility and permeability are the two characteristics of the dialysis membrane that have been postulated to affect mortality in patients with acute renal failure and the recovery of renal function. In a meta-analysis involving 867 patients, Subramanian et al. observed greater mortality in patients treated with non-modified cellulose membranes. This effect was not observed on comparing synthetic membranes versus modified cellulose membranes (more biocompatible)199. Another meta-analysis likewise recorded no significant differences in morbidity-mortality between patients dialyzed with modified cellulose membranes and those treated with synthetic membranes200. Although there are discrepancies as to whether the use of biocompatible membranes is associated to greater renal function recovery rates201–203, in the clinical setting the treatment of acute renal failure is based on the use of biocompatible membranes, not on cellulose-based membranes, in line with the current recommendations of the KDIGO guide-lines197. On the other hand, very few studies have compared the effects of membrane permeability upon the prognosis of acute renal failure patients, and no significant differences were observed between high and low permeability membranes in terms of the recovery of renal function and the survival of patients with acute renal failure201–204. However, the possibility of affording greater dialysis efficacy may justify the use of high flux membranes, despite their greater cost.
The dialysis centers may offer all or only some of the different modalities, but there must be an operating manual and specific protocols for each of the modalities of hemodialysis they offer.
The choice of the modality of hemodialysis should be based on the characteristics of the patient (age, body surface, comorbidity conditions, vascular access, clinical course, situation with respect to transplantation waiting list) and the structural specifications of the center. It is advisable to keep a registry of all the patients, documenting the modality of hemodialysis and the reason for the indication of treatment.
Given the diversity of available options and the need for personalized adjustment of dialysis therapy, the opinion of the nephrologist is crucial for establishing the technical requirements of the different options related to dialysis, with active participation in assessing the different proposals (Level of evidence C).
Water treatment and dialysateThe treatment of water, the purity characteristics of the water for dialysis and the final dialysis solution should follow the recommendations of the guidelines of the S.E.N. on dialysis fluids in their latest version published in 201637. The characteristics of the dialysate are largely dependent upon the dialysis techniques used in each center - though the tendency is to ensure that all centers use ultrapure water, since the greater the purity of the water for dialysis, the lesser the risk of inflammatory phenomena secondary to exposure to the dialysate (Level of evidence C).
Hemodialysis dose. dosing and adjustment of dialysis treatmentIntroductionHemodialysis, like any other treatment, requires a dosing and administration protocol. In this regard, quantitative methods have been developed to guarantee that the patient receives an adequate minimum dose.
Different clinical practice guides (CPGs) have addressed this problem1,101,205–207, recommending measurement based on the urea kinetic model (UKM), and establishing a recommended minimum dosage. The previous CPG of the S.E.N.208 incorporates measurement through ionic dialysance or Kt among the recommended methods.
Minimum treatment dose- •
As a general rule, the recommended minimum dose for hemodialysis in three weekly sessions is Kt/V > 1.3 and/or percentage reduction of urea (PRU) >70% and/or eKt/V >1.1 and/or Kt greater than the desired value adjusted to body surface (Level of evidence A).
- •
Increasing the dialysis dose measured by Kt can improve the survival of patients on hemodialysis and reduce the risk of hospitalization (Strong level of evidence).
- •
The dialysis dose determined by a method related to the UKM should be measured at least once a month (Non-established level of evidence).
- •
If monitors with ionic dialysance biosensors are available, it is advisable to perform monitoring of the dialysis dose based on the Kt (Strong level of evidence).
The current recommendations have been based on observational studies assessing the relationship between dialysis dose and mortality. The National Cooperative Dialysis Study (NCDS) was the first trial to relate urea kinetics to the patient clinical course, determining a series of minimum toxicity or dialysis dosage levels. This prospective study involving 160 patients found that the group of individuals with the lowest urea concentrations had lesser morbidity-mortality. A posterior reanalysis of the results by Gotch and Sargent in 1985 led to expression of the dialysis dose as Kt/V, with the observation that Kt/V > 0.8 was associated to an improved clinical course. Based on the results of the NCDS, the urea kinetic model (UKM) became widely accepted and used.
Posteriorly, a number of studies have evidenced the relationship between dialysis dose and mortality. In 1993, Owen et al., in a cross-sectional study of 13,473 patients, found the mortality relative risk (RR) to progressively decrease with an increase in PRU from 45% to 70%. In 1994, Collins et al., in a cross-sectional study of 1773 patients, found the mortality RR to progressively decrease with an increase in Kt/V from under 1 to 1.4. In 1996, Held et al., in a multicenter American study of 2311 patients, found the quintile with Kt/V < 0.9 to be associated to a 20% greater probability of death versus the reference quintile with Kt/V 1.06-1.16, while in the case of the quintile with Kt/V > 1.33, the risk decreased 29%. For every 0.1 unit of Kt/V, mortality decreased 7%. In turn, Bloembergen et al.215 found that a lower dialysis dose increased the risk of mortality due to all causes and suggested the idea that a low dialysis dose favors atherosclerosis, infection and malnutrition. Hakim et al.216, in a four-year observational study, increased Kt/V from 0.82 in 1988 to 1.33 in 1991, resulting in a decrease in annual mortality rate from 22% to 9%. Parker et al.217 in turn increased Kt/V from 1.18 to 1.46, with a decrease in annual mortality rate from 23% to 18%. Yang et al.218, in an observational study of 337 patients, found the annual crude mortality rate to decrease from 16% to 13% and to 8% on increasing Kt/V from 1.3 to 1.5 and 1.7, respectively. The best survival outcomes were published by the group of Tassin, in France, involving 445 patients subjected to dialysis with a duration of 8 hours and Kt/V 1.7 (Daugirdas monocompartmental, second generation) with acetate, cuprophane (1 m2), blood flow (QB) 200-250 ml/min and dialysate flow (QD) 350-500 ml/min219.
The HEMO study220,221, a randomized prospective trial, compared a group with a recommended minimum dose, Kt/V 1.25 or eKt/V 1.05, versus a high efficacy group with Kt/V 1.65 or eKt/V 1.45. Although this study has been widely commented from the methodological point of view, the final outcome has not been conclusive in demonstrating that the high dose group presented lower mortality than the conventional dose group86.
The current recommendations regarding the dialysis dose, according to the American multicenter trial, are Kt/V ≥ 1.3 and/or PRU 70%214. The hemodialysis practice guide (KDOQI) of the National Kidney Foundation recommends a minimum Kt/V of 1.2 and/or a PRU of 65%101, though Kt/V 1.3 and PRU 70% are advised in order to ensure these minimum values. With the aim of avoiding the rebound effect and inter-compartmental imbalances, it is advisable to use eKt/V. The recommendations of Kt/V 1.3 or PRU 70% would be equivalent to eKt/V 1.1 or ePRU 64%, respectively222.
Having commented the importance of the dialysis dose, it should be remembered that on an annual basis, only monthly, two-monthly or three-monthly determinations are made to calculate the dialysis dose - extrapolating the result of these 4, 6 or 12 measurements to all what is seen in the 156 annual sessions. The KDOQI clinical guides recommend calculation of the dose at least on a monthly basis101.
In each hemodialysis process, a number of factors can condition dialysis efficacy. Control systems therefore have been developed that quantify the dose received by the patient in each session and in real time. In this respect, at present different monitors have incorporated biosensors that on a noninvasive basis, using the conductivity probes of the machines, measure the effective ionic dialysance that is equivalent to urea clearance (K), and therefore allows calculation of the dialysis dose223–225 without work overload, laboratory tests or added costs. The systematic measurement of K over the period of dialysis allows us to obtain Kt – a real way of measuring the dialysis dose, expressed in liters.
A number of authors that have worked with ionic dialysance in hemodialysis and expressed the value as Kt/V have found that although the correlation to analytical Kt/V is good, the results differ significantly, thus evidencing variability between methods226,227. Introducing a V based on anthropometric values or electrical impedance228 generally underestimates Kt/V in relation to the value determined analytically226,227,229,230.
Some authors have proposed a method of rescaling Kt/V according to body surface area (BSA)231,232, or the use of alternative methods for measuring the dialysis dose in place of V233, though without widespread acceptance among the Nephrology community.
With the incorporation of ionic dialysance, Kt was proposed instead of Kt/V as a method for monitoring the dialysis dose234, because it allows us to avoid the J-shaped survival curve that is observed when the patients are distributed according to the urea reduction ratio (URR) or Kt/V235. In a previous study, the minimum Kt dose was individualized in terms of body surface236 and was validated in 59,644 North American patients in a cross-sectional study (March 2004) as a predictive measure during a period of one year237:
In the Spanish population, monitoring of the dialysis dose with Kt instead of Kt/V identifies 25-40% of the patients that did not reach the minimum Kt at the same time that they reached Kt/V238–240, particularly in women, patients with central venous catheters (CVCs) and patients with low body weight238.
This possibility of monitoring the dialysis dose in real time is reflected in the growing interest in quality policies leading to improvements in dialysis dosage. Thus, the study in the American population in 2006237 reached a mean Kt of 51 liters (0.3 liters more than desired on average), while in the Spanish population in 2013238 the mean Kt was 52.6 liters (3.3 liters more than the target). More recently241, and likewise in the Spanish population, the mean Kt reached was 55.1 – 6.5 liters more than the target. These data imply that in the last 10 years, adjustment of the dose measured by Kt has improved from 56% of cases reaching a minimum dose, to 67% and 81%, respectively.
Recently, Maduell et al.241 published a prospective observational study of 6129 patients on hemodialysis in 63 centers in Spain, in which the mortality risk increased gradually when the prescribed Kt adjusted for body surface area was not reached. Furthermore, this risk decreased on reaching 1-3 liters or more over the minimum target. Likewise, on reaching 9 liters or more over the minimum target Kt adjusted for body surface area, the risk of hospitalization decreased. The observational design of the study did not allow the definition of a causal relationship between mortality and the dialysis dose. The only randomized trial in this respect proved inconclusive, as previously commented86; it is therefore advisable to be particularly cautious in this respect in nonrandomized designs242. The statistical application of Propensity Score Matching to the study sample showed that those who received a greater dialysis dose according to Kt had a decreased mortality risk – though a randomized trial would be needed for definitive confirmation of this. It was seen that mortality among the patients that reached a minimum Kt/V and a minimum Kt was 12.5%, while the patients that reached a minimum Kt/V but not the minimum Kt showed a mortality rate of 23.5%. The new formula recommended for obtaining the minimum Kt, based on the results obtained in this study, would be a modification of the previous formula:
Minimum Kt dose in liters = (1 / [0.0069 + (0.0237/BSA)]) + 2)
Time and frequency of dialysisDialysis is to be performed at least three times a week, with a duration of at least 12 hours weekly, except if the patient presents significant residual renal function.
An increase in the duration of dialysis or in the weekly frequency may be considered in situations of cardiovascular instability, refractory arterial hypertension despite ultrafiltration, or hyperphosphoremia.
In incident patients with preserved residual renal function, treatment individualization on an incremental basis can be decided, though controlled studies are needed for due standardization to be established.
No studies can be found in the current literature clearly demonstrating the possible benefit of dialysis time independently of the dose. Although some authors suggest that dialysis time may be an independent factor in the evolution of the patient244,244, others conclude that it is very difficult to separate time from dosage245.
As indicated in the European guides on dialysis strategies246, and in concordance with the Japanese dialysis registry, among others247, there is no evidence to indicate that the duration of the session can be reduced to under four hours without compromising the outcome; in the present guide we thus consider this indication to be reasonable.
The relationship between the increase in time and/or frequency and survival remains subject to controversy. Although it is true that improved outcomes have been suggested with long dialysis244, frequent dialysis139 and frequent long dialysis148, and a relationship has even been postulated between mortality and the weekend inter-dialysis period248, it is no less true that good survival outcomes have been recorded with shorter dialysis using a conventional protocol. It is complicated to carry out randomized trials with a large sample size in order to draw firm conclusions in this respect; moreover, therapeutic protocols based on greater frequency of dialysis may pose organizational issues, elevate the costs and increase problems related to the vascular access139, as well as result in the burnout phenomenon described in up to 41% of the patients in a meta-analysis published in 2006249. Therefore, although it cannot be established as a generalized recommendation, in certain situations an increase in dialysis time and/or frequency might be indicated.
Long dialysis improves blood pressure control in patients on hemodialysis244, in the same way that an increase in dialysis time reduces the hourly ultrafiltration rate and improves hemodynamic stability243. Similar data on blood pressure139 and volume control have been reported with an increase in frequency, even in patients with important comorbidity145,250.
An increase in time251 or frequency139,149 may improve phosphorus control in patients with persistent hyperphosphoremia.
Recently, some authors252–254 have advocated progressive or incremental hemodialysis in incident populations with the purpose of preserving residual renal function, which may improve the elimination of molecules of different molecular weights, facilitate normohydration and improve survival255,256. At present there is not enough information to establish which patients may benefit, or to standardize the progression of hemodialysis – though some studies are ongoing or in the planning stage257.
Calculation of the hemodialysis dose and its recommendations are referred to a protocol characterized by three weekly sessions. However, if the frequency of dialysis changes, the dosage proves more difficult to compare. The weekly Kt/V, which would be a simple approach, is of little use, since we know that more frequent dialysis is more effective. The recommendations of the KDOQI regarding conventional hemodialysis are a weekly Kt/V of 3.6, while this figure decreases to 2.0 when a continuous technique is used, such as peritoneal dialysis, based on the clinical experience of nephrologists in treating thousands of patients for over 20 years. The change from Kt/V 3.6 corresponding to three weekly sessions to Kt/V 2.0 for a continuous technique such as peritoneal dialysis includes variations in frequency of 4, 5, 6 and 7 sessions a week.
A number of authors have made proposals for quantifying the dialysis dose when there are variations in frequency: equivalent renal urea clearance (EKR), published by Casino and Lopez236; the standard Kt/V (Kt/Vstd) proposed by Gotch237; and weekly percentage urea reduction238:
Casino and Lopez238: according to the authors, three formulas can be used for calculation:
Gotch237:
Since predialysis urea is not determined in each session, it can be calculated from the figure published by the author with Kt/V and frequency.
Maduell238:
Vascular accessesCorrect functioning and maintenance of the vascular access is essential for the normal performance of hemodialysis. We thus refer to the S.E.N. guides on vascular accesses12.
Monitoring of hemodialysisObjectives- a)
To ensure that all patients on hemodialysis, independently of the center where dialysis is provided, undergo a minimum series of laboratory tests and clinical controls to allow adequate monitoring and treatment. However, these minimum requirements must not neglect the first requirement of dialysis: individualization. There are patients in which the minimum criteria are insufficient, for different reasons, and it must be made sure that these individuals have access to adequate care.
- b)
To ensure that clinical evaluation, compilation of the case history and monitoring of the patients on hemodialysis are carried out with adequate periodicity by nephrologists.
- c)
To ensure access to these services for all patients on hemodialysis, independently of the type and geographical location of the dialysis center.
Objective
This guide describes the minimum case history to be compiled on patients subjected to hemodialysis. The quality of the case history will facilitate adequate treatment for the patient on dialysis and good care continuity on the part of the different areas.
General recommendations
- a)
Initial reception of a patient on dialysis should contemplate the compilation of a detailed case history at the start of the program, addressing the aspects described below.
- b)
Since the case history of a patient on hemodialysis may extend for years, and is constantly evolving, adequate compilation in time and form is crucial. This means:
- •
Time: Recording of the most important events (new disease conditions, admissions, etc.) should be made on a timely basis in the moment when the information is received, in order to keep the case history up to date at all times. Updating of the case history should be a constant task of the nephrologist in the hemodialysis unit.
- •
Form: The recording of this information should be made in such a way that despite the passing of time, the data are always available and easy to access by the nephrologists of the center, primary care physicians and the reference hospital. Accordingly, this information should be recorded with the date (day/month/year) in the global case history of the patient within the section corresponding to his or her disease condition (CARDIOLOGICAL, PNEUMOLOGICAL) under REVIEW BY SYSTEMS, and with updating of the DIAGNOSES.
- •
- c)
The electronic case history of the patient of the dialysis center must be shared in real time with the reference hospital, in case the patient needs to report to the emergency room, and to allow due evaluation by other specialists.
- d)
The section CLINICAL EVOLUTION of the software systems should be used to record the most important events during follow-up of the patient but should not impede compilation of the case history by sections and the diagnoses, which facilitates access to the information.
- e)
It must be ensured that the case history is up to date when the patient is referred to another hemodialysis center or to the reference hospital.
- f)
All patients should receive an updated medical report at least every 6 months, and every time they experience an important event.
A description is provided below of the minimum information to be collected at the start of the dialysis program and which must be updated over time.
- a)
Personal information: Full name / Address and telephone numbers (personal and of close relatives) / City and postcode / Date and place of birth / ID number / Social security number / SIP number.
- b)
History of the current nephrological-urological disease. A brief and chronological description should be made of the renal disorder that has led to dialysis, as well as the evolution and follow-up time by the nephrologist.
- c)
Family history. History of diabetes mellitus (DM) / arterial hypertension (AHT) / ischemic heart disease / early cardiovascular mortality. History of kidney disease, familial deafness, ocular problems. Oncological history.
- d)
Personal history prior to inclusion in hemodialisis: Arterial hypertension / diabetes mellitus / dyslipidemia / hyperuricemia / previous admissions / surgeries / gynecological history.
- e)
Allergies. Specify the type of allergy and whether allergological studies have been made.
- f)
Toxic habits: Smoking / alcohol / other drugs.
- g)
Social particulars: Level of education / occupational status.
- h)
Review by systems:
- •
Hematological disease and blood transfusions: anemia, polyglobulia, administration of intravenous iron, use of erythropoietin, presence of resistance to erythropoietin, coagulation disorders, previous blood transfusions (number and dates), antiplatelet / anticoagulation treatment and cause.
- •
Cardiovascular disease: arterial hypertension, arteriosclerosis, cardiomyopathy, ischemic heart disease (type), pericarditis, arterial calcification, valve disease, acute lung edema episodes, arrhythmias, pericardial effusion.
- •
Peripheral vascular disease: intermittent claudication, cramps, venous insufficiency, history of peripheral venous thrombosis, amputations.
- •
Gastrointestinal and liver disease: heartburn, dysphagia, bowel habit (constipation / diarrhea), nausea, vomiting, history of gastrointestinal bleeding (melenas / hematemesis), food intolerance, bleeding hemorrhoids, gallbladder problems, eventration / abdominal hernia. Viral markers (HBV, HCV and HIV) at start of hemodialysis. In case of treated liver disease: treatment received, duration, ending date, viral load negativization or not.
- •
Pneumological disease: pleural effusion, lung calcifications, pulmonary hypertension, sleep apnea syndrome, chronic obstructive pulmonary disease (COPD) / respiratory failure, use of bronchodilators, home oxygen or nocturnal continuous positive airway pressure (CPAP), bronchial asthma, bronchial hyperresponsiveness, history of pneumonia, hemoptysis, tuberculosis. Domestic pets.
- •
Neurological disease: encephalopathy, cognitive disorders, peripheral neuropathy (sensory or motor), autonomic nervous system dysfunction disease cerebrovascular, headache.
- •
Infectious disease: related to the vascular access, viral (HBV, HCV, HIV) urogenital, intraabdominal, etc.
- •
Dermatological disease: pruritus, skin eczema, dry skin, calciphylaxis.
- •
Vascular access: FISTULAS: date of creation of fistula, location, evolution, complications (steal syndrome, high flow, aneurysms), thrombosis (date), infection (date). CATHETERS: location, dates, thrombosis, malfunction.
- •
Mineral and bone metabolism: renal osteodystrophy, osteoporosis, adynamic bone, fractures, treatments and dates of start and suspension, use of phosphorus binders, tolerance and adherence.
- •
Vaccinations: vaccination scheme: HBV, pneumococcus, influenza. Tetanus.
- •
Endocrinological disease: type I or type II diabetes mellitus, thyroid disorders.
- •
Neurological / Psychiatric disease: history of anxiety or depression.
- •
Ophthalmological disease: glaucoma, cataracts, corneal calcifications, diabetic retinopathy.
- •
Gynecological conditions: pregnancies, menopause, vaginal bleeding, latest mammography, latest bone densitometry test, gynecological ultrasound, vaginal cytology.
- •
Musculoskeletal disease: joint pain, muscle pain.
- •
Urinary disease: prostate problems (males).
- •
Nutrition problems: nutritional parameters, use of appetite stimulants, dietetic supplements, chewing or swallowing difficulties, obesity, potassemia.
- •
Others: sensory function, psychomotricity and intellect. Mood and adaptation to dialysis, hearing or vision problems, psychomotricity and altered gait, need for crutches or wheelchair. Corrective lenses / hearing aids / dentures. Sphincter continence / need for diapers / permanent bladder catheter / colostomy. Autonomous / dependent / requires help with activities of daily living. Adequate adherence to diet / regular transgressor. Adherence to drug treatment / non-adherence to drug treatment.
- •
Lives with ……/. Lives alone / in home for the elderly, with adequate family support / social isolation. Comes to first session alone /accompanied by … Goes out from home daily. Active lifestyle for age. Life limited to bed-chair-bathroom.
- •
Renal transplantation waiting list status
- •
- i)
Physical examination: full physical examination at the start of the program. Initial vital signs are to be recorded, including blood pressure (right and left arm) and heart rate in supine decubitus and in the standing position. Height, weight and body mass index (BMI) are to be determined. Level of consciousness and orientation. Skin and mucous membrane color and hydration. Adenopathies (supraclavicular, axillary or neck). Jugular vein ingurgitation at 45 degrees. Goiter. Palpable and symmetrical carotid pulses, presence of murmurs. Cardiac auscultation: rhythm, murmurs, added sounds, friction sounds. Pulmonary auscultation: vesicular murmur, crepitants, rhonchus or wheezing. Abdomen: soft, depressible, pain or no pain in response to palpation. Abdominal murmurs. Hepatomegaly or splenomegaly. Abdominal masses. Blumberg, Murphy. Ascites. Lower extremities: edemas, femoral, tibial, popliteal and pedal pulses. Neurological exploration: cranial nerves, motor or sensory defects.
- •
Other information of interest
- ˆ
Charlson comorbidity index.
- ˆ
Patient satisfaction survey.
- ˆ
- •
- j)
Complementary tests: of the reference hospital. The following are initially required: chest X-rays, ECG, echocardiography and abdominal ultrasound.
- k)
Initial laboratory tests (with viral markers: HCV, HBV, HIV)
- •
Initial hemodialysis regimen.
- •
Initial treatment.
- •
The following information should be incorporated progressively and in a timely manner to the patient case history, along with the corresponding dates:
- •
Adequacy of dialysis.
- •
Changes in prescription of the patient.
- •
Complications of dialysis.
- •
Hemodialysis access and its complications.
- •
Hematological and blood transfusion status.
- •
Cardiovascular condition and volume overload.
- •
Infectious complications.
- •
Bone and mineral metabolism.
- •
Electrolyte alterations.
- •
Nutritional status.
- •
Endocrinological status.
- •
Hospitalizations (consider checking of hepatitis markers according to time and cause of admission).
- •
Psychological and social condition of the patient.
- •
Situation referred to renal transplantation.
- •
Complementary tests made.
- •
Evolution of blood test parameters.
- •
Vaccination status.
The hemodialysis regimen is to be modified according to the needs and clinical condition of the patient. A registry is advisable to document the changes in the hemodialysis regimen over time.
A report should be produced on an annual basis, including a full physical examination, review by body systems, and the most relevant aspects of the evolution of the patient during that period of time.
Monitoring during the hemodialisis sessionSpecial emphasis should be placed on the following aspects:
- •
Monitoring and adjustment of dry weight: this is to be done in each hemodialysis session, assessing the presence of symptoms or evidence of hyper- or hypovolemia (arterial hypertension of recent onset or with poor control using the habitual drugs, dyspnea in the period prior to hemodialysis, orthopnea, hypotension, cramps, edemas, jugular vein ingurgitation, crepitants upon auscultation, etc.). The use of bioimpedance as a tool for the adjustment of dry weight should be considered. In case of doubt, chest X-rays could be considered.
- •
The monitoring of body weight in the period prior to patient incorporation to the hemodialysis program and the interdialysis weight gains can provide information on the nutritional status of the patients.
- •
Monitoring of the function of the vascular access258: consideration is required of those data allowing the early detection of dysfunction before any diagnostic test is made. For this purpose, we must keep a registry of the vascular access with the following data:
- ˆ
Physical examination (edemas, aneurysms and signs of infection).
- ˆ
Pressures of the vascular access: dynamic venous pressure, intra-access or static pressure.
- ˆ
Recirculation of the vascular access.
- ˆ
Alteration of the adequacy of dialysis.
- ˆ
Assessment of the puncture sites.
- ˆ
Hemostasis.
- ˆ
Dialysis registries
Individual for each patient.
The data to be recorded in the hemodialysis sessions are:
- a)
Name of the patient, allergies, date, identification of the monitor, dialysis technique, session starting and ending time, and identification of the nurse or nurses in charge of the session.
- b)
Dialysis material: dialyzer, needles, priming fluid, substitution fluid, type of heparin, acid and bicarbonate concentrate.
- c)
Fluid balance: dry weight, pre-HD weight, post-HD weight, interdialysis weight gain, intradialysis loss of weight and fluid perfused during the session.
- d)
Vascular access: type of access, location, condition, functionality, date of creation, date of first puncture and special cares.
- e)
Special cares during the session: maximum tolerated ultrafiltration, intake, etc.
- f)
Session program:
- •
Heparin flush, continuous heparin or in initial bolus dose and then hourly, heparin ending time.
- •
Dialysis time, programmed ultrafiltration (UF).
- •
Ultrafiltration profile.
- •
Conductivity profile.
- •
- g)
Control of vital signs: blood pressure and heart rate are to be recorded at the start, on an hourly basis and at the end of the session, and whenever required by the condition of the patient. Body temperature is to be recorded. Hourly blood pressure is to be monitored.
- h)
Control of parameters of the monitor: real blood flow (Qb), arterial and venous pressure, transmembrane pressure (TMP), temperature of the bath, conductivity, bath flow and hourly and total UF.
- i)
End condition of the dialyzer and lines.
- j)
Control of glycemia in diabetic patients.
- k)
Medication administrated.
- l)
Extractions for laboratory tests.
- m)
Medical comments.
- n)
Nursing comments.
The batch number of the dialyzers, blood lines and dialysis fluids should be recorded on the treatment sheet / plot. A registry of the drugs used in the rooms by weeks should be kept, recording the batch number and expiry date. This is important in order to ensure the traceability of the drugs and products used in the dialysis sessions254.
Laboratory test controls and complementary tests of the patient on hemodialisis
Objective
This guide describes the main parameters related to the control of patients on hemodialysis, as well as the periodicity of the determinations.
General recommendations
Stable patients on hemodialysis are to undergo the minimum laboratory and complementary test controls defined in this guide, and with the indicated frequency. Apart from these minimum requirements, however, we also must perform all additional controls considered necessary on an individualized basis for each patient, according to the criterion of the supervising nephrologist.
Justification
Accordingly, in addition to the scheduled tests, we must perform all those laboratory procedures considered opportune, with variable periodicity, according to the possible clinical needs and changes in the stability of the process requiring therapeutic intervention.
Even in out-hospital centers, it is considered to be important to be able to expand the routine tests according to the needs of the patient (requests from other specialists, extension of studies in concrete cases), in order to avoid unnecessary patient visits to the hospital and additional punctures.
- •
The laboratory tests are to be planned on an annual basis with the reference laboratory, and the centers will be in charge of controlling that these tests are performed in all the patients.
- •
The centers must know the measurement methods used in their reference laboratory, as well as the best way to handle the samples in order to ensure correct interpretation of the results (1B).
Justification
This recommendation is particularly relevant for the measurement of PTH259, calcidiol and other hormones, as well as for the measurement of albumin (with important differences between methods that use bromocresol purple or blue).
- •
Sample extraction in patients on hemodialysis should always be performed in the same short period, i.e., pre-dialysis in the middle of the week.
Justification
This recommendation is particularly relevant for the measurement of phosphorus, which is characterized by variations in the measure between the short and the long period. Measurement in the short period has been found to be more correlated to mortality than measurement in the long period260.
Laboratory test controls and periodicity of the measurementsIn coordination with the reference laboratory, and on an annual basis, it is advisable to plan the laboratory tests required during the current year.
Anemia and iron metabolism Recommendation
| Measurement parameter | Level of requirement | Recommended periodicity | Indications |
|---|---|---|---|
| Blood count (Hb, hematocrit, MCV, MCHC) | Required | Monthly and upon demand | Scheduled |
| Platelets | Required | Monthly and upon demand | Scheduled |
| Leukocytes and formula | Required | Monthly and upon demand | Scheduled |
| Ferritin, TSI | Required | Every 2 months | Scheduled |
| Reticulocytes | Recommendable | Every 2 months | Scheduled |
| % hypochromic erythrocytes | Recommendable | Every 2 months | Scheduled |
| Folic acid | Recommendable | Annually and upon demand | Scheduled In patients with resistance to erythropoietic stimulants |
| Vitamin B12 | Required | Six-monthly and upon demand | Scheduled In patients with resistance to erythropoietic stimulants |
| C-reactive protein (CRP) | Required | Monthly and upon demand | Scheduled |
Justification
The erythropoietic stimulants are to be adjusted according to the hemoglobin levels (Hb). In this respect, the determination of hemoglobin should be made with the frequency needed to allow these dose adjustments in the right moment. During the anemia correction phase, the hemoglobin levels should be monitored every 2-4 weeks. During the maintenance phase, once hemoglobin has been stabilized, the hemoglobin levels are to be measured every 1-3 months (in the opinion of the group of experts, monitoring is indicated on a monthly basis)(Level of evidence C)261–266.
The physiological hemoglobin value in adults depends on age, gender and race. Criteria regarding anemia therefore should be established taking these factors into account.
Iron metabolism status should be monitored regularly in patients on dialysis. The different guides recommend regular monitoring of iron status (every 1–3 months) during the start or adjustment (titration) of the erythropoietic stimulant doses, in patients receiving intravenous iron treatment, in order to avoid toxicity (2B) - serum ferritin systematically above 800 μg/L in the absence of evidence of inflammation (normal CRP levels) may be suggestive of iron overload (1B) - and in patients with stable hemoglobin levels265,266.
In our setting, the two determinations that assess iron metabolism best and which are probably accessible to all centers are ferritin and the transferrin saturation index (TSI). The levels of ferritin are used to measure iron storage, while TSI reports on iron availability for erythropoiesis. The data obtained from these measurements are to be interpreted with caution in patients with chronic kidney disease (CKD), since ferritin is an acute phase reactant that is influenced by inflammation, especially in patients on dialysis with subclinical inflammation267,268.
The periodic determination of C-reactive protein can help identify these patients and allow correct interpretation of the parameters referred to anemia and iron metabolism; it is therefore advisable to measure CRP monthly together with hemoglobin269,270.
The levels of ferritin and TSI should not be measured until at least one week has gone by since the last intravenous iron dose, since otherwise falsely elevated values may be recorded. We recommend coordination of the weekly, two-weekly or monthly administration of iron coinciding with the day of laboratory testing263–266.
In place of, or in addition to the determination of ferritin and the transferrin saturation index, other iron status tests can be used if available, such as the percentage of hypochromic erythrocytes, which expresses functional iron deficit, and reticulocyte hemoglobin content. The measurement of hepcidin has not been shown to be clinically useful or superior to other standard iron status tests in patients with CKD; its routine use is therefore not recommended271,272.
Patients on hemodialysis are often supplemented with vitamin B12 and folic acid. A recent study has shown higher concentrations of vitamin B12 and lower folic acid levels to be associated to greater mortality due to all causes in patients on hemodialysis, independently of the sociode-mographic and laboratory test variables273.
Further studies are needed to determine the optimum target levels referred to vitamin B12 and folate in this population, though it appears to be prudent to monitor the levels periodically, especially in the case of supplementing. On the other hand, in selected cases of resistance to the action of erythropoietic agents, monitoring of the serum vitamin B12 and folic acid concentrations is required274.
Vitamin B12 deficiency is increased in elderly patients with a low intake of proteins and/or the use of antacids (protein pump inhibitors [PPIs]), and this situation may increase resistance to erythropoietin.
Bone and mineral metabolism Recommendation
| Measurement parameter | Level of requirement | Recommended periodicity | Indications |
|---|---|---|---|
| Calcium and phosphorus | Required | Monthly | Scheduled |
| Recommendable | With greater frequency under special conditions | Modification of dose of vitamin D/calcimimetic agents | |
| Upon demand | Hypercalcemia/hypocalcemia Hyperphosphatemia/hypophosphatemia | ||
| Parathyroidectomy (greater frequency required in post-parathyroidectomy) | |||
| Antiresorptive treatment (especially denosumab if considered according to risk/benefit) | |||
| PTH | Required | Two-/three-monthly | Scheduled |
| Required | Monthly | Modification of dose of vitamin D and/or calcimimetics agents | |
| Alkaline phosphatase | Required | Every 3-6 months | Scheduled |
| Serum aluminum | Required | Annual | Scheduled |
| Water for dialysis aluminum content | Required | Six-monthly | Scheduled |
| 25-(OH)-Vitamin D (calcidiol) | Required | At start and every 6 months (summer and winter) | Scheduled In treatment with native vitamin D (cholecalciferol, ergocalciferol or calcidiol-calcifediol |
Justification
The levels of calcium, phosphorus and parathyroid hormone (PTH) must be measured in all patients on hemodialysis. The KDIGO guides advise the control of Ca and P every 1-3 months and of PTH every 3-6 months – though more frequent controls are recommended in patients subjected to treatment275,276.
In our opinion, the minimum periodicity should be the scheduled determination of PTH every two months and of calcium and phosphorus every month. This frequency is justified by the fact that treatment decisions in chronic kidney disease – mineral and bone disorder (CKD-MBD) complex should be based on the trends in values, with global interpretation of the latter. In this regard, a greater frequency of determination will afford a more realistic view of the patient. On the other hand, the frequent measurement of parameters such as phosphorus, which are dependent upon patient adherence, will contribute to therapeutic compliance.
In patients under treatment, especially during the dose adjustment phase, a greater frequency of measurement is needed to analyze efficacy and side effects.
It is advisable to know the measurement method used by the laboratory for determining PTH.
The mathematical calcium-phosphorus product (Ca × P) does not contribute information independent of the separate values corresponding to calcium and phosphorus evaluated globally; we therefore do not consider its measurement to be necessary(277).
The determination of total alkaline phosphatase may be questionable.
The KDIGO recommends its measurement every 12 months, or more often if PTH is elevated. The group of experts consulted for the development of these guides considers that there is still not much evidence on this matter; its determination may be useful together with that of PTH, as a predictor of bone turnover in patients without liver disease, and at present its measurement may be particularly interesting in patients in which the start of treatment for osteoporosis is contemplated. The presence of relatively low levels of PTH and alkaline phosphatase may cause us to suspect the existence of adynamic bone disease, and in these cases, the use of antiresorptive agents would be contraindicated. We thus consider that determination should be made with a periodicity of 6 months. Probably, in the near future, with the treatment of fracture risk, we will have to start requesting the measurement of bone alkaline phosphatase (AP)278,279.
Recently, dialysate aluminum content has come under improved control, with dual osmosis systems and the obtainment of ultrapure water in most Units, together with the fact that phosphate binders containing aluminum have largely been replaced by other binders. Nevertheless, the accumulation of aluminum has not disappeared entirely, and may be due to accident280.
Aluminum is added to the water as an organic material flocculant in amounts that vary according to the time of year; its levels thus may become very high. In these situations, the only way to ensure optimum levels in the dialysis fluid is to operate in series with two reverse osmosis units. Although some dialysis centers do not advise its routine determination281, we consider it to be a good safety and control measure to determine aluminum in water every 6 months and serum aluminum annually. It also should be determined on an extraordinary basis in those situations where aluminum toxicity is suspected in the Unit (microcytic anemia, dementia or osteomalacia)37.
It is advisable to measure the levels of vitamin D (calcidiol) in order to prevent and treat the frequent insufficiency or deficiency of this prohormone. We should monitor the levels of vitamin D every 6 months in supplemented patients in order to avoid toxicity and adjust the dose282–285.
The measurement of post-hemodialysis calcium has been questioned, with most of the consulted experts considering that it is not necessary. The present guide therefore does not recommend its routine determination. If measurement is made in concrete patients, we should assess their habitual profile with their pre- and post-dialysis calcium levels, and their usual ultrafiltration with stable medication, in the middle of the week, two or three times, in order to define the individual calcium gain profile of the patient, and preferably using ionic calcium. The individualization of dialysate calcium according to the predialysis levels of calcium in serum can prevent or reduce undesired excursions of both serum calcium and PTH286.
Viral serology and liver enzymes Recommendation
| Measurement parameter | Level of requirement | Recommended periodicity | Indications |
|---|---|---|---|
| GPT, GGT | Required | Monthly | Upon entry to dialysis and scheduled |
| HCV Ab | Required Recommendable | Six-monthly Three-monthly | Upon entry to dialysis and scheduled |
| HCV-PCR | Required Recommendable | Six-monthly Three-monthly | Upon entry to dialysis and scheduled |
| HBsAg and HBcAb | Required | Annually/Three-monthly in patients with negative HBsAb | Upon entry to dialysis and scheduled |
| HBV viral DNA | Recommendable | At the start and every three months if HBcAb and/or HBsAb are positive | When HBcAb is positive and HBsAb is negative or positive |
| HBsAb | Required | Six-monthly | Upon entry to dialysis and scheduled |
| HIV | Required | Annually | Upon entry to dialysis and scheduled |
Justification
All patients starting treatment with hemodialysis should undergo testing for human immunodeficiency virus (HIV), hepatitis C virus (HCV) (preferably using qualitative HCV-PCR and HCV-Ab) and hepatitis B virus (at least comprising HBsAg, HBsAb and HBcAb), in order to screen for these viruses and consider treatment, isolation or vaccination against HBV in candidate subjects42,287,288.
Enzymatic and serological monitoringHIV: Although subsequent studies are not needed, annual testing would be advisable42.
HCV: The study of HCV infection is crucial to identify transmission of the disease in hemodialysis units. The United States Centers for Disease Control (CDC) and the KDIGO (2018) guides recommend that all patients on maintenance hemodialysis should undergo evaluation of the levels of anti-HCV and alanine aminotransferase (ALT) at the time of admission42.
Hepatitis C virus antibodies are to be determined 6-monthly and ALT on a monthly basis in patients that might be infected.
At least every 6 months, all patients on hemodialysis should be tested for HCV antibodies using a third-generation ELISA test and/or qualitative PCR (the latter technique being preferable). The frequency of determination should be every three months in Units at an increased risk (prevalence > 20% of the total; nurse / patient ratio of over 1/4 in Units without isolation per room and shift; Units with three or more shifts; Units in which HCV seroconversion has been detected), and monthly in patients presenting liver enzyme elevation.
In patients with positive qualitative PCR testing for HCV, we should determine the viral load and HCV genotype to complete the study of the infection289.
HBV: Determination of HBsAb and HBcAb at least once a year is required in all patients of the Unit42.
The patients should be vaccinated prior to the start of hemodialysis (predialysis stage). If HBV vaccination has not been started or completed at the start of hemodialysis, it should be done as soon as possible. The efficacy of vaccination must be evaluated in all patients289.
The viral load is to be determined in all HBcAb-positive cases, independently of the HBsAb titers, at the start of hemodialysis and then every three months, due to the risk in this particular population of HBV reactivation and of possible patients with occult HBV290.
Liver enzymes: The liver enzymes GPT and GGT are to be determined in all patients on hemodialysis at least once every two months. It is advisable for such testing to be made on a monthly basis, particularly in Units at increased risk42, 289.
Special situations:
- •
In all cases of HCV seroconversion, liver enzymes are to be determined every month, with testing for antibodies and PCR among all the patients of the Unit monthly during the window period (6 months).
- •
In patients with resolved HCV infection that have been treated with the latest antiretroviral drugs, HCV-PCR testing should be performed every 6 months291,292.
Dialysis dose
See Dialysis dose.
Ions and acid-base balance Recommendation
| Measurement parameter | Level of requirement | Recommended periodicity | Indications |
|---|---|---|---|
| Potassium | Required | Monthly | Scheduled |
| Sodium | Required | Every 2 months | Scheduled |
| Magnesium | Required | Every 2 months | Scheduled |
| Bicarbonate or total CO2 | Required | Every 2 months | Scheduled |
| Post-hemodialysis potassium | Recommendable | Every 2 months | Scheduled |
| Post-hemodialysis bicarbonate | Recommendable | Every 2 months | Scheduled |
Justification
The composition of the dialysate is a crucial issue in the prescription of hemodialysis. Nevertheless, there is an almost complete lack of data from clinical trials capable of offering guidance regarding optimum dialysate composition. The concentrations of the key components are often chosen on an intuitive basis, and the dialysate composition may also be predetermined according to the specifications of the manufacturer of the dialysate or the practices in force in the hemodialysis unit. Recently, emphasis has been placed on the importance of selecting the composition of the dialysate referred to bicarbonate, calcium, magnesium and potassium293.
Both high and low serum bicarbonate levels are associated to an increased risk of mortality in patients subjected to hemodialysis. Concern has recently arisen regarding dialysates with a high bicarbonate content and the alkalemia induced by dialysis, but further studies are needed to define the optimum targets. In this respect, optimum treatment may require knowledge of the acid-base parameters both before and after dialysis, with individualized treatment decisions weighing the risks of predialysis acidosis, rapid intradialytic alkalinization and alkalosis after dialysis, and also assessing the patient comorbidities. In clinical practice it is not fully clear when to intervene, since variability within a given patient can complicate clinical decision making. A recent article has proposed a novel solution to minimize intradialytic alkalosis while still adequately treating the acidosis. The underlying premise is that expansion of the plasma bicarbonate reserve in the first part of dialysis treatment generates a gradient that suffices to saturate the intracellular buffers. The additional administration of alkali causes alkalosis but has no major effect upon the buffer reserves. Therefore, high bicarbonate in the dialysate is initially useful, but subsequently proves harmful, and the bicarbonate of the dialysate must be gradually reduced during the rest of dialysis treatment. Further studies are needed to determine the usefulness of this maneuver, as well as define the specific dialysate modeling approach – though the proposal is interesting294–296.
A growing number of observational studies have evidenced the relationship between lowered serum magnesium levels and poorer survival among patients on hemodialysis. Magnesium modulates the pathogenesis of bone and mineral disorders and could offer a new therapeutic approach to vascular calcification. Further interventional studies are needed to clarify whether magnesium supplementation and/or an increase in the concentration of magnesium in the dialysate improves the prognosis of patients undergoing hemodialysis. It has been demonstrated that most patients on hemodialysis exhibit a negative magnesium balance during dialysis297–300.
One of the fundamental objectives of the prescription of hemodialysis is to maintain the serum potassium levels within a narrow normal range during the intradialytic and interdialytic intervals. While the normal serum potassium range is typically considered to be between 3.5 and 5.0 mEq/L in the general population, the optimum range in patients on dialysis is greater, and different studies have identified the lowest mortality to be associated to values between 4 and 5.5 mEq/L.
Taking into account the extraordinarily high cardiovascular mortality rate in the population on hemodialysis, it is crucial to optimize potassium management in patients on hemodialysis. This implies the reduction of large intradialytic potassium changes and adequate potassium elimination in order to minimize hyperpotassemia. The potassium profile in the dialysate offers the possibility of reaching both objectives, maintaining a constant dialysate-serum potassium gradient in which the dialysate provides potassium separate from other components of the dialysate, with gradual reduction of the concentration of potassium in the dialysate as the serum potassium levels decrease.
Since there is evidence that the highest risk associated to the dialysate potassium corresponds to patients with an apparent lack of coincidence between serum and dialysate (i.e., the use of dialysate potassium < 2 mEq/L for patients with potassium ≤ 5 or the use of dialysate potassium > 3 for patients with K ≥ 5), it is essential for the prescription of potassium in the dialysate to be revised and adjusted regularly in response to the predialysis serum potassium concentrations, particularly during the vulnerable periods when the serum potassium levels may experience acute changes, as following hospitalization267,301,302.
Serum sodium (natremia) in patients on hemodialysis does not exhibit constant values, though the variation of the levels is low, and correction for glycemia is required. The coefficient of variation of sodium is greater in diabetics, in whom natremia is lower. It has been shown that there is a lack of association between natremia and the volume status, which supports the need for individualization of the sodium and salt provision during the hemodialysis session. In order to secure an isonatremic hemodialysis session, the concentration of sodium in the dialysate should approximately coincide with the serum sodium concentration of the patient, and particularly the sodium obtained in the interdialytic period must be eliminated by convection. The pre-hemodialysis serum sodium concentration can be used as reference for the prescription of sodium in the dialysate in chronic hemodialysis303–305.
We consider it advisable to include the periodic determination of these pre- and post-hemodialysis values in clinical practice, in order to be able to individualize the treatments; furthermore, due to the variability of some of the determinations, such as bicarbonate, frequent measurements are required. In this regard, we advise determinations every two months.
Observations: In order for the venous blood gas results to be valid, we recommend sampling to be performed anaerobically, avoiding the formation of bubbles, at a temperature of 2-8°C, i.e., transported in ice, or performing the measurement 15 minutes after extraction286–299.
Cardiovascular risk Recommendation
| Measurement parameter | Level of requirement | Recommended periodicity | Indications |
|---|---|---|---|
| Total cholesterol, LDL-C, HDL-C, triglycerides | Required | At start and annually. In patients treated with statins or other lipidlowering drugs, whenever the dose is modified. | Scheduled |
| Glycemia | Required | Every 2 months | Scheduled |
| Glycosylated hemoglobin | Required | Every 6 months | In diabetic patients |
| Troponin T | Recommendable | Annually | Scheduled |
Justification
Based on the new evidence regarding lipid management in kidney patients, the KDIGO guides on lipid management in CKD published in 2013 recommend evaluation of the lipid profile (total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides) in all patients subjected to hemodialysis (1C), though the routine determination of these parameters in most patients is not advised (Grade of recommendation not determined)306–309.
Different studies have shown that the prevalence of cardiovascular disease in patients on dialysis is very high, and the mortality rate due to this cause is 10-30 times greater than in the general population. In the latter there is a clear relationship between LDL-cholesterol and the main atherosclerotic events. However, in patients with CKD, the LDL-cholesterol levels show a negative association to these outcomes at LDL-cholesterol concentrations below average, and a neutral or weakly positive association to mortality at higher LDL-cholesterol levels. In general, the available information suggests that a reduction of LDL-cholesterol is beneficial for preventing major atherosclerotic events in patients with CKD and in kidney transplant recipients but is not beneficial in patients requiring dialysis310, 311.
Accordingly, in adults on hemodialysis, the KDIGO recommends that the start of statins or combinations of statins / ezetimibe should be avoided. However, it is not advisable to suspend the treatment in those patients on dialysis who already receive statins or combinations of statins / ezetimibe312.
These recommendations are based on a series of clinical trials including 4D (Die Deutsche Diabetes Dialyse)313, AURORA (a study on the use of rosuvastatin in subjects on hemodialysis) and the SHARP analysis of subgroups314.
A number of epidemiological studies suggest that this increased cardiovascular risk is not explained only by the high prevalence of risk factors for cardiovascular disease, but that other emergent risk markers or factors with a high prevalence in the population on hemodialysis may also be involved (lipoprotein-a, hyperhomocysteinemia, hypofibrinogenemia, inflammation, etc.). We consider that their routine determination in dialysis patients cannot yet be recommended on a general basis, due to the lack of evidence in patients on hemodialysis, the paradoxical effects in this population, and the lack of effective specific treatments315–317.
Diabetic persons on hemodialysis are highly vulnerable, with complex comorbidities, and have a high risk of adverse cardiovascular outcomes. In particular, patients with deficient glycemia control are susceptible to different influences associated with greater fluctuation of plasma glucose and a greater risk of hyperglycemia and hypoglycemia. Since hypoglycemia may imply a poorer prognosis and quality of life, it is necessary to control the plasma glucose levels in order to improve the prognosis and avoid hypoglycemia. On the other hand, prolonged hyperglycemia leads to the non-enzymatic glycosylation of proteins and produces Amadori products, such as glycated albumin (GA) and glycosylated hemoglobin (HbA1c). The usefulness of HbA1c in the context of chronic kidney disease (CKD) may be problematic due to the alteration of erythrocyte half-life, the use of iron and / or erythropoietin therapy, uremia, etc. In addition, glycemia control may be underestimated. Thus, as an alternative marker, GA has been proposed as a more reliable and sensitive glycemic index in patients with CKD. In addition to the mean concentration of glucose in plasma, GA also reflects postprandial plasma glucose and glycemic excursion. Likewise, with a half-life of approximately 2-3 weeks, GA may reflect blood glucose status faster than HbA1c. Glycated albumin is also an early precursor of advanced glycation end-products (AGE), which cause alterations of different cell proteins and organelles. It seems reasonable to determine glycosylated hemoglobin or preferably GA in diabetic patients at least twice a year318–320.
Troponin, at high concentrations, is associated to a poorer long-term cardiovascular prognosis in patients with severe renal failure. Elevated troponin T levels have afforded better results than troponin I, probably because it more specifically identifies lesser myocardial damage and silent coronary disease. On the other hand, troponin T has been found to be independently associated to the severity of coronary artery calcification in asymptomatic patients on hemodialysis. Therefore, in addition to the classical cardiovascular risk markers, we should include elevated C-reactive protein (CRP) and troponin T as valid new markers. On the other hand, patients on dialysis have troponin T levels abnormally elevated with respect to those found in the general population; the availability of an annual determination of this parameter therefore could help to interpret the results in the case of an acute event321–323.
Nutrition Recommendation
| Measurement parameter | Level of requirement | Recommended periodicity | Indications |
|---|---|---|---|
| Total proteins and albumin | Required | Two-monthly | Scheduled |
| Prealbumin | Recommendable | Two-monthly | Scheduled |
| Cholesterol | Recommendable | Six-monthly | Scheduled |
| Creatinine | Required | Two-monthly | Scheduled |
| nPCR | Required | Two-monthly | Scheduled |
Justification
Malnutrition is common among patients on dialysis and is closely related to morbidity and mortality. The assessment of nutritional status and its management in patients on dialysis is a fundamental element of routine clinical practice. The nutritional status of patients on hemodialysis should be monitored regularly in order to ensure early identification of malnutrition – inflammation, and to adopt the opportune corrective measures324–326.
There is no single marker of nutritional status, and its interpretation is complex and is influenced by many dialysis-related factors. The evaluation of nutritional status therefore must be based on the combination of various biochemical and physical parameters, taking into account the limitations of each of them. Among the laboratory test parameters most commonly used to assess nutritional status, mention must be made of the concentrations of albumin, prealbumin, transferrin and cholesterol. In turn, C-reactive protein (CRP) allows us to identify inflammatory comorbidities and helps to interpret the serum levels of albumin and prealbumin, while the normalized protein catabolic rate (nPCR) is an indirect marker of protein intake327–329.
Albumin is the biochemical parameter most often used a nutritional marker, since its determination can be made by any laboratory. Hypoalbuminemia has been defined as a predictor of mortality in patients with renal failure. However, it has also drawn criticism, and some authors such as Steinman (in Seminars in Dialysis, 2000) have recommended the suppression of albumin concentration as an indicator of malnutrition in renal patients. The problem is that the albumin levels may decrease due to other reasons (as an acute phase reactant, secondary to plasma volume expansion, through redistribution, exogenous losses and a decrease in albumin synthesis), and its value moreover varies from one laboratory to another depending on the measurement technique used330,331.
Another possible marker for assessing nutritional status is prealbumin, which has a shorter half-life than albumin, is closely correlated to nutritional status, and constitutes a good prognostic indicator. As an inconvenience, important overlap is observed between nurtured and malnourished patients. However, in the future, prealbumin might prove to be an additional routine marker in patients on dialysis332.
Other biochemical parameters where low concentrations indicate malnutrition and a poor prognosis include creatinine and total cholesterol. Low predialysis creatinine levels are usually associated to reduced muscle mass and are indicative of a poor patient prognosis. Serum total cholesterol is less sensitive as a nutritional marker330.
On the other hand, nPCR may be underestimated due to the influence of the permeability of the dialyzer, the amount of blood, dialysate flow, and the distribution of urea in obese, malnourished or edematous patients. In contrast, nPCR may be overestimated by the posterior urea rebound following dialysis333–335.
Others
In hemodialysis units there are patients who in addition to renal failure suffer other concomitant disease conditions requiring follow-up and special monitoring. The most common of these conditions are described below.
Recommendation
| Measurement parameter | Level of requirement | Recommended periodicity | Indications |
|---|---|---|---|
| Thyroid hormones (TSH) | Required | Six-monthly | In patients receiving treatment with amiodarone or Levothyroxine |
| Recommendable | At start and annually | ||
| Scheduled | |||
| Beta-2-microglobulin | Optional | Annually or on changing to another hemodialysis technique (switch to HDF-OL) or modifying the technique (change of dialyzer, increase in time, etc.) | Scheduled |
| Coagulation | Optional | Annually | Scheduled |
| Required | Upon demand (at least once every month) | In anticoagulated patients |
Justification
The prevalence of thyroid dysfunction is high among patients receiving amiodarone as antiarrhythmic drug, and the thyroid hormone levels should be monitored regularly in these subjects. On the other hand, an association has been described between euthyroid disease syndrome and mortality among patients on hemodialysis.
Regular evaluation of thyroid function has been suggested as a marker of cardiac risk in these patients. Amiodarone increases free T4 levels, lowers free T3 and transiently increases TSH concentration – though in some cases the levels prove undetectable. Amiodarone is able to induce: a) hypothyroidism, diagnosed from low free T4 levels, and which requires treatment with levothyroxine; and b) thyrotoxicosis, diagnosed from elevated total or free T3 levels. These alterations are excluded in the presence of normal TSH concentrations, assessed twice a year336–340.
- •
Beta-2-microglobulin is elevated in most patients on hemodialysis. At present there appears to be no effective treatment - other than kidney transplantation - capable of slowing the progression of amyloidosis secondary to beta-2-microglobulin or afford symptoms relief. The KDOQI guides do not recommend measurement of the serum levels of beta-2-microglobulin (Level of evidence C). Nevertheless, a decrease in beta-2-microglobulin has been demonstrated in patients dialyzed with high flow techniques; monitoring of this parameter therefore would be desirable341,342.
- •
Patients with vasculitis: Most forms of vasculitis (except vasculitis induced by drug hypersensitivity) may relapse. In these patients, prolonged monitoring is indicated, and different laboratory tests can be used for this purpose – though most of them are relatively nonspecific (such as the erythrocyte sedimentation rate [ESR] or C-reactive protein) – a fact that limits their usefulness. Once remission has been induced, it has been suggested that an increase in plasma ANCA antibody titers is strongly predictive of relapse in patients with Wegener's granulomatosis. However, the lack of uniformity in this respect makes it more important to monitor clinical signs of active disease343,344.
In order to avoid test duplications and ensure that the patients undergo their tests when these are effectively required, a control is advised towards the end of the current year (September) of the complementary tests that have been made during that year, with a request for those tests that are still pending.
| Measurement parameter | Periodicity | Indications | Level of requirement |
|---|---|---|---|
| Electrocardiogram (ECG) | Annually | On starting HD Scheduled Upon demand | Required |
| Chest X-ray | Annually | Scheduled Upon demand | Required |
| Fundoscopy | Annually | In diabetics Upon demand | Recommendable |
| Abdominal ultrasound | On starting HD | Required | |
| Annually | Patients with < 5 years on HD | Recommendable | |
| Every 2 years | Patients with > 5 years on HD | Required | |
| Densitometry | Every 2 years | On starting HD Scheduled | Recommendable |
| Recommendable | |||
| Echocardiography | On starting HD | Required | |
| Annually | Scheduled in patient without disease | Recommendable | |
| Upon demand | Required | ||
| Scheduled in patient with heart disease | |||
| Spiral CAT or magnetic resonance angiography | In patients on kidney transplant waiting list (basal) Scheduled | Required | |
| Annually | Recommendable according to transplant waiting list | ||
| Gynecological control | Annually | Women between 50-65 years of age | Required |
| Bioimpedance | Every 6 months | On starting HD Scheduled | Required |
| Lateral abdominal X-ray | Every 2 years | On starting HD Scheduled | Required Recommendable |
Electrocardiogram (ECG)
Electrocardiography is routinely used for the detection of left ventricular hypertrophy, ischemic heart disease and rhythm disorders.
Recommendation
Electrocardiographic evaluation is advised at least once a year.
When
Electrocardiography should be performed on a routine basis before the hemodialysis session in order to control for other parameters that might alter the recording. There is controversy regarding the influence of hemodialysis upon the ECG tracing: some authors have reported improvement of the ECG parameters after hemodialysis, while others consider that hemodialysis results in alterations of the ECG tracing.
In this respect, Girgis et al345. have found hemodialysis to exert a positive effect upon the electrical parameters in most patients, possibly as a consequence of the improvement in electrolyte levels and fluid loss, which reduces ventricular overload. In contrast, other investigators have found hemodialysis to prolong the QT interval independently of the correction of electrolytes – with the consequent arrhythmogenic effect this produces. However, it seems to be more prudent to perform ECG before hemodialysis, except if we intend to perform other studies advising the contrary.
A pre- and post-hemodialysis study should be made if we intend to determine electrical differences indicative of risk cardiovascular.
How
A 12-lead ECG study is indicated to precisely determine the amplitude of the QRS complexes.
Justification
The purpose of securing an annual ECG study is to have a reference ECG tracing of each patient under basal conditions that can be used for comparison purposes. The tracings therefore should be kept in an easily accessible place in the care area of the patient on hemodialysis.
The ECG tracing should be used to assess the following:
- •
Left ventricular hypertrophy (LVH): The ECG tracing is less sensitive but more specific than in most populations. It is useful for detecting LVH, however. Left ventricular hypertrophy measured by ECG is an independent marker of cardiovascular mortality risk.
- •
Ischemia: Nakamura et al345. found intra-hemodialysis ECG to be useful in detecting ischemia. Patients with an ST-segment descent of over 1 mV versus baseline are at a high risk of suffering a cardiac event within 21 months. Likewise, the presence of arrhythmias during the hemodialysis session is related to silent myocardial ischemia; a specific study would therefore be indicated.
- •
QT and QRS alterations: Hemodialysis prolongs the QT interval, which favors the development of ventricular arrhythmias at the end of hemodialysis or in the period immediately after the session. Prolongation of the QRS complex is observed after the hemodialysis session that is correlated to weight loss during dialysis. These and other parameters may be used as markers of cardiac mortality risk. Many authors use variation of the QT interval before and after hemodialysis as a good marker of cardiovascular mortality. This parameter is referred to as QT dispersion (QTd)7,18, and is influenced by low bath calcium concentration (1.25 mmol/L). The QTc (QT corrected for heart rate) is usually defined as > 460 ms in women and > 450 ms in men. The QT interval reflects alteration of cardiac conduction and repolarization, myocardial ischemia and structural heart disease, and is associated to an increased risk of tachyarrhythmia. The QTc value in turn is a good morbidity-mortality indicator.
- •
Arrhythmia and variations of heart rate: The ECG tracing can be used to detect paroxysmal or established atrial fibrillation or supraventricular arrhythmias, which are related to increased morbidity-mortality.
Recently, variations in cardiac frequency have become relevant as a marker of cardiovascular risk 345–370.
Chest X-ray Recommendation
It is advisable to perform a posteroanterior and lateral chest X-ray study once a year. A chest X-ray study should always be made when the patient enters the hemodialysis program, as an initial reference and for subsequent follow-up comparison purposes.
Justification
Dialysis treatment is associated to frequent thoracic complications, particularly pleuritis, pericarditis, pneumonia, osteodystrophy, infections, metastatic calcifications and primary or metastatic neoplasms. This list does not include specific acute problems such as fluid overload, which need concrete and timely intervention, and which also require a chest X-ray study.
The chest X-ray study allows us to detect lung, heart, vascular and bone lesions on a regular basis, with a view to controlling the situation of the patient. In most cases, the radiological findings afford rapid and noninvasive diagnostic assessment with a high benefit / cost ratio.
It is also useful for checking the position of central catheters and for assessing venous alterations of the arteriovenous fistulas371–375.
Funduscopy Recommendation
Routine funduscopy is not required. The technique is only advised when indicated due to specific concomitant disease.
Justification
Uremic patients suffer multiple ophthalmological complications (papillary edema, vascular alterations, ischemic neuropathy, etc.). However, these disorders – with the exception of hypertensive retinopathy – are infrequent and do not require periodic follow-up376.
Abdominal ultrasound
Abdominal ultrasound is of interest for the early detection of any possible malignization of acquired renal cysts. In addition, it can be used to discard other disorders such as neoplasms, vascular disease, ascites or liver conditions. The technique is noninvasive and provides much information.
Recommendation
Abdominal ultrasound on an annual basis is optional during the first 5 years on hemodialysis. However, it becomes mandatory every two years in patients who have been on dialysis for more than 5 years, and in those patients, who are on the transplantation waiting list. Also, the technique should always be performed in the presence of hematuria or pain in the kidney zone. If the ultrasound findings prove negative for cysts and masses, the risk of malignancy is low, and ultrasound only needs to be repeated every 3-5 years, unless symptoms appear (pain or hematuria).
Justification
Twenty-two percent of all patients on hemodialysis have acquired kidney cysts. Acquired renal cyst disease develops after several years on dialysis. According to Narasimhan et al378., the presence of this disease is observed in patients who have been an average of 49 months on dialysis. After 10 years, between 50-80% of the patients suffer this problem. The most common symptoms are hematuria, flank pain, infection and lithiasis. Screening of patients with ultrasound has been shown to improve survival, with a 1.6-year increase in life expectancy. However, the population on dialysis has a much shorter life expectancy, and the benefit is consequently measured in days, and is not sufficiently large to justify screening of the entire dialysis population – though it is indeed indicated in those patients who have been on dialysis for a long period of time.
The probability of developing renal carcinoma secondary to acquired renal cyst disease varies according to the different studies between 2% at 7 years and 7% at 10 years. A review published by Truong et al379. estimated the probability of malignization of these cysts to be 0.18% per year. In an analysis carried out by Denton et al380., on examining the nephrectomies performed during renal transplantation, 4.2% of the patients were found to present renal carcinoma in different stages. Most of the malignancies developed between 8-10 years after the start of replacement therapy.
In order to allow the early identification of these malignant conditions, annual ultrasound or CAT explorations should be made of those patients who have been on dialysis for more than 5 years. The most effective technique for the detection of renal tumors has not been established. Computed axial tomography with contrast injection is more sensitive than ultrasound in identifying these complicated cysts but is not superior to ultrasound in detecting solid tumors. At present, PET-CAT with radiolabeled choline is considered to be the most accurate diagnostic technique for tumors of this kind.
If ultrasound proves negative for cysts and masses, the risk of malignancy is low, and the technique only needs to be repeated in 3-5 years - unless symptoms develop (pain or hematuria)377–394.
Densitometry Recommendation
It is advisable to perform bone densitometry upon starting hemodialysis treatment, and again later on depending on the needs – though a minimum periodicity of once every two years is recommended.
Justification
In patients on dialysis with evidence of CKD-MBD and / or risk factors for osteoporosis, we suggest densitometry to assess the risk of fracture; the results obtained may have an impact upon the treatment. There is growing evidence that the mineral content of bone as measured by densitometry predicts fractures in patients on dialysis.
Simplification of the classification of bone density has been proposed by dividing patients into two groups: those with “low bone density” and those with “normal / high bone density”. Accordingly, a patient may have high turnover bone disease with normal / high bone density or low turnover bone disease with low or normal / high bone density. Standardization of the bone mineral density (BMD) measurements has also been proposed, based on the Z-score (bone mass adjusted for race, age and gender) in contraposition to the T-score (adjusted to the maximum mass of a young adult corrected for gender and race). A low BMD would be defined as a Z-score of –1 or less, although these latter recommendations proved controversial and did not receive generalized acceptance396–400.
Echocardiography Recommendation
A basal echocardiographic study is required on starting hemodialysis treatment. Unless more frequent explorations are needed due to the presence of heart disease, echocardiography should be repeated once a year in order to assess the evolution of the myocardium, valves and pericardium. In patients with heart disease, the technique should be performed with variable periodicity depending on the degree of dysfunction and the severity of the condition, based on the criterion of the cardiologist. Echocardiography can identify heart diseases that require a specific type of dialysis.
Justification
Fifty percent of all patients on hemodialysis die due to cardiovascular causes. The cardiovascular mortality rate among patients on dialysis is 10-20 times higher than in the healthy population.
The usefulness of echocardiography focuses on three aspects: the control of myocardial dysfunction, the assessment of heart valve function, and the monitoring of pericardial status.
Myocardial dysfunction is common before the patient starts dialysis treatment. In effect, 75% of all patients that enter a hemodialysis program present left ventricular hypertrophy. Uremic cardiomyopathy is characterized by cardiac hypertrophy, cardiac fibrosis, microvascular alterations and diastolic dysfunction. It is specific of the patient with kidney disease401,402. The diagnosis is established by echocardiography. In these patients with left ventricular hypertrophy, the diagnosis is defined by a ventricular mass of > 134 g/m2 body surface in men and > 110 g/m2 in women403.
The poor prognosis associated to left ventricular hypertrophy can be improved by reducing left ventricle mass towards normal levels. The correction of arterial hypertension (AHT), over-hydration or anemia may reduce the LV mass partially or completely, improving the patient's prognosis.
In patients with chronic kidney disease, it is common to observe pericardial alterations, including pericarditis, effusion and chronic constrictive pericarditis. Chronic renal failure may give rise to important pericardial effusion in 20% of the cases. In the context of hemodialysis, pericarditis associated to dialysis has been reported in 13% of the patients. These problems are attributable to two main factors: inadequate dialysis and fluid overload. The diagnosis is established by ultrasound, and it is not advisable to wait until clinical manifestations develop, since the condition often goes unnoticed. In fact, among patients with intradialysis hypotension, pericardial effusion is present in 50% of the cases, with no other discomfort404–407.
Heart valve lesions are common in patients on dialysis408–415.
The most frequent anomalies include valve and ring thickening, the calcification of leaflets and signs of insufficiency and stenosis. Doppler ultrasound is the diagnostic technique of choice for detecting the presence of valve disease. Two-dimensional (2D) ultrasound allows us to visualize the valve anatomy, subvalvular structure, valve ring and other heart structures. Doppler ultrasound can evaluate the degree of regurgitation / stenosis and dynamic flow. A recent meta-analysis has evidenced that vascular calcification in patients on dialysis is associated to increased mortality due to all causes, and to increased cardiovascular mortality in particular416. The number of calcified heart valves is correlated to mortality risk. The detection of valve calcification is useful for the risk stratification of patients on dialysis417.
In patients with known valve disease, serial echocardiography is advised in order to monitor progression of the disease418.
Spiral CAT or magnetic resonance angiography Recommendation
Patients entered on the kidney transplantation list should undergo a spiral CAT or magnetic resonance angiography exploration of the iliac territory upon admission to the list. The different existing protocols are then to be followed, recommending another exploration every 6 months or every year.
Justification
Arterial calcifications are frequent in patients on hemodialysis and are associated to a greater incidence of cardiovascular disease. The most commonly used techniques for evaluating such calcifications are spiral CAT419,420 and magnetic resonance angiography421. These imaging techniques are able to identify iliac, carotid and coronary calcifications, and are also useful for determining the possibility of performing a graft in one of the iliac arteries – transplantation being contraindicated in those cases where this is not possible. This procedure allows us to determine where the vascular anastomosis can be made before transplantation is carried out422.
The early detection of these calcifications using noninvasive techniques allows us to modify the medical interventions with a view to reducing their progression and thus lower the cardiovascular risk423.
Gynecological control Recommendation
In women between 50-65 years of age, an annual gynecological control should be made according to the gynecological recommendations referred to the general population. The nephrologist must ensure that the breast, cervical and uterine cancer detection programs are duly followed.
Justification
These patients have a high incidence of gynecological tumor disease, especially at cervical and endometrial level424–427.
Bioimpedance Recommendation
It is advisable to perform a bioimpedance study at the start of hemodialysis treatment, and then with a periodicity conditioned to the needs, but always at least once every 6 months.
When
Bioimpedance should be made on a routine basis before the hemodialysis (HD) session, in order to avoid interferences from other parameters that may alter the results. Hemodialysis exerts an influence upon bioimpedance, and the latter always should be performed after a resting period of half an hour.
An evolutive bioimpedance study over time is indicated.
How
A resting period of half an hour is required, with correct placement of the electrodes.
Justification
Bioimpedance is useful for calculating dry weight428 and for assessing the nutritional status of the patient429.
Dry weight is a parameter that is difficult to determine, particularly in the first sessions of dialysis, and it moreover experiences changes over time. The dry weight of the patient must be re-evaluated on a regular basis due both to malnutrition and increased nutritional status. Bioimpedance is able to differentiate over-hydration and volume contraction, taking into account the nutritional status of the patient. The exploration can be used to adjust hydration in order to maintain residual renal function430. The aim is to avoid hypotension in hemodialysis and heart failure episodes. Such monitoring can be of help in controlling blood pressure.
In the analysis of nutritional status, bioimpedance determines the fat mass and lean or muscle mass content. Patients on dialysis may present a normal body mass index (BMI) and be malnourished and obese, due to a predominance of fat mass431.
This information allows us to guide nutritional treatment and is related to morbidity-mortality. The result obtained from the bioimpedance studies should not be used as an independent objective value for determining the dry weight of the patient but should be used in combination with clinical assessment of the patient hydration status432,433.
Lateral abdominal X-ray Recommendation
It is advisable to obtain a lateral abdominal X-ray at the start of hemodialysis treatment, and then with a periodicity conditioned to the needs, but always at least once every two years.
Justification
A plain lateral abdominal X-ray is a simple way to determine the presence of vascular calcification. It is based on the Kauppila index434, which has been established as a mortality marker435.
Lateral abdominal radiography serves to orientate different mineral metabolic treatments, but it also constitutes a prognostic indicator. The protocolized periodic application of the technique allows us to assess the progression of vascular calcification436,437. Such progression in turn should cause the different dialysis and therapeutic protocols to undergo modifications with the aim of controlling progression, since it is moreover also correlated to calcification of the coronary arteries.
Transient patient careDialysis Units must have a protocol for patient admission and referral defining coordination with other levels or organizations; the procedures for referral to other levels or centers; definition of the characteristics of patients that are acceptable and not acceptable for the organization; and the admission suitability criteria. This information should be public and accessible on the internet. In order to request dialysis care, on a temporary basis, a written application should be completed and forwarded to the destination Dialysis Center (DC), accompanied by the pertinent clinical reports. This does not exclude possible prior telephone or e-mail contacts to determine whether the capacities of the DC meet the requirements, and to ascertain its availability on the requested dates. Annex 3 contains an application model including all the data necessary for evaluation of the proposal. This application is to be signed by the patient and by one of his or her supervising physicians. The medical and nursing case history models that are to accompany the application are established (Annexes 4 and 5). The following is described: procedure for issuing the application and information; type of reply; special circumstances of patients on the transplantation waiting list; elements to be presented at the time of start of care; elements to be received at the time of start of care; report on the care received and its incidents. Annex 6 contains a model of reply to the application.
IntroductionOne of the fundamental objectives of a healthcare system is to improve the quality of life of the patients. The concept of quality of life includes the possibility of travelling and of receiving safe healthcare at the point of destination. This principle also applies to patients on hemodialysis. The care offered to patients must be considered as part of an integrated system of places, services, healthcare professionals and care levels guaranteeing the continuity of medical care under conditions equivalent to those of the center of origin, i.e., adequate adjustment is required between the needs of the patient and the type of integral service offered. The organization must guarantee patient access to the required type of care, based on the results of the case evaluation procedures that take into account the needs of the patient, the appropriate place of care, and the capacities of the center. The patient reporting to the facility in seek of medical care must be evaluated physically, psychologically and socially on an individualized basis. Based on this evaluation, a first diagnosis is established, and along with it the corresponding care plan. The care facility will determine the actions in accordance with the plan, considering its possibilities and limitations, in order to be able to resort to other alternative solutions - in all cases guaranteeing adequate compliance with the care objectives. The established organization must have a procedure for the admission and referral of patients, establishing the following: coordination with other levels or organizations; procedures for referral to other levels or centers; definition of the characteristics of those patients that are acceptable and not acceptable for the organization, and admission suitability criteria.
In order to facilitate and improve dialysis care for transient patients, it is advisable over the short term to establish a range of services and admission criteria for transient patients in all the Hemodialysis Units throughout Spain. This information should be public and accessible on the internet, and will facilitate the application or request for a dialysis station for a specific patient. Another aspect that requires improvement is the unification of care criteria, such as for example viral markers, special dialysis techniques, etc. These two aspects should be included in the technical specifications of the dialysis service contracts and agreements.
Form of applicationIn order to apply for temporary (transient) dialysis care, a written request must be submitted to the destination Dialysis Center (DC), along with the pertinent clinical reports, via fax or e-mail. Apart from this, prior contacts may be made to learn about the availability of dialysis stations on the requested dates, as well as the capacity of the DC to accept patients with certain concrete characteristics such as positive serology tests, four weekly sessions, need for transport, etc.
Annex 3 presents an application model that includes all the data necessary for evaluation of the proposal. This application must be signed by the patient and the requesting physician.
In parallel, if dialysis care is required in another Spanish region (Autonomous Community), the request should be made through the Cohesion Fund Information System (Sistema de Información del Fondo de Cohesión [SIFCO]).
Required clinical informationStandardized medical and nursing clinical report.
The temporary (transient) dialysis care application (Annex 3) is to be accompanied by a medical clinical report and a nursing clinical report. Annexes 4 and 5 include models of these two types of report. Information must be supplied corresponding to all the indicated sections. With regard to the dates of the reports, these must be no more than one month old, and in the case of viral serological tests, the data supplied must be no more than three months old.
Processing of the application and informationThe Dialysis Centers are to have a list of the DCs in Spain, and should have access to information about the DCs in other countries, in order to be able to address the requests directly. The nursing associations and healthcare authorities should also collaborate in this task as intermediaries. The existence of a services catalog corresponding to each DC is advisable.
Type of replyThe Dialysis Centers should reply in writing (via fax or e-mail) and as soon as possible to the formal applications made. In this regard, the optimum reply time is considered to be within 15 working days. The DC will inform about the administrative requirements, the possibilities for care related transport and the required steps, as well as the mechanisms for the continuation of treatment with hospital-dispensed medications. If the DC in question does not meet some of the patient needs or requirements, this must be duly stated, with the indication of care alternatives, if any. Annex 6 contains a reply model.
Patients on the renal transplantation waiting listPatients on the renal transplantation list will have some immediate localization mechanism in force during their displacements, in case a donor organ becomes available in their area of origin. They moreover also must have detailed information on the fastest transport means available to return to their reference hospital. If this is not possible, or if the patient is unwilling, this must be commented before leaving his or her point of origin, in order to receive a “temporary contraindication” status classification. These aspects will be explained to the patient and will be his or her responsibility.
Elements to be presented at first start of care- •
Clinical information (recent medical and nursing reports: no older than one month).
- •
Administrative requirements (according to the specifications of the point of destination).
- •
Transport requirements (according to the specifications of the point of destination).
- •
Medication (according to the specifications of the point of destination).
- •
A photocopy of a recent ECG tracing is recommended.
Urgent and conventional means of communication with the DC: the destination DC will inform the patient about the way and who to contact for care in the event of an emergency (reference hospital or center).
Report on the care provided and its incidentsAt the end of the temporary care period, the destination DC will issue a report reflecting the incidents, emergency care (where applicable), tolerance of dialysis, pertinent laboratory test results, changes of treatment, and all other information considered by the physician and/or nurse of the DC to be of interest for the patient and healthcare staff of the DC of origin. Medical and nursing clinical report.
Inclusion on the renal transplantation waiting listRelationship between the nephrology unit without transplantation and the hemodialysis center and the renal transplantation unitJustification
The maximum effectiveness of the renal transplantation (RT) program can be achieved provided there is good coordination among the different levels of renal patient care. It is very important for each care level to know and comply with its care responsibilities in the setting of good coordination and collaboration. The Nephrology Units without Transplantation, the Hemodialysis Centers and the Renal Transplantation Units (RTUs) will identify in each case the specific level of participation in the preparation and follow-up of patients for RT.
Preparation for RT before starting dialysis is important, since the outcomes are better if transplantation is performed in the predialysis stage or with the patient already on dialysis for the shortest time possible438. In addition, preparation for RT in the predialysis stage may allow greater chances for live donation, which must be the priority option when this possibility exists.
Recommendations
- a)
The Nephrology Units without Transplantation and the Hemodialysis Centers must ensure collaboration and coordination with the Renal Transplantation Unit (RTU) in order to facilitate the inclusion and follow-up of patients presenting progressive and irreversible deterioration of renal function, with an estimated glomerular filtration rate (eGFR) < 15 ml/min/1.73 m2 who are on the RT waiting list. Each Nephrology Unit without Transplantation and each Hemodialysis Center must have one or more reference RTUs with which to collaborate and refer the patients.
- b)
The RTU is in charge of including the patient on the deceased donor RT waiting list. Likewise, the RTU will periodically (at least once every 6 months) inform the Nephrology Units without Transplantation and the Hemodialysis Centers of the status of their patients in relation to the RT waiting list. Fluid communication between both care levels, focused on the needs of the patients, is essential.
Justification
The survival and quality of life of patients following renal transplantation is better than when on dialysis for an equivalent age and with similar comorbidities, over both the short and the long term439. Transplantation therefore should be actively promoted.
The case history of transplant candidates must be carefully evaluated before definitively excluding them for transplantation. In this regard, a patient history of chronic infections, cancer, gastrointestinal diseases, viral hepatitis, myocardial infarction or peripheral arthropathy does not always constitute an absolute contraindication to transplantation. If the patient is initially discarded as a candidate, due consideration must be made of the difficulties of posterior re-evaluation, since a long time on dialysis is an independent predictor of a poor renal graft outcome. Nevertheless, such posterior reconsideration is always an option that must remain open.
The annual mortality rate of patients on the waiting list is 5-10%. Studies of registries have identified risk factors for this high mortality (age > 50 years, catheter as access for hemodialysis, Charlson index > 3, physical inactivity, peripheral vascular disease or retransplantation)440–443.
In some cases, it is important to perform a psychological evaluation of patients in whom therapeutic compliance may be doubtful, since failure to adhere to treatment after renal transplantation implies failure of the graft. A history of attempted suicide, poor adherence to medication, psychosis, cognitive dysfunction or alcohol or drug abuse are relative contraindications for RT.
Recommendations
- a)
All patients on dialysis or with eGFR < 15 ml/min/1.73 m2 should be regarded as potential candidates for RT provided there are no absolute contraindications, since RT offers greater quality of life and life expectancy than dialysis.
- b)
The conditions that increase the risk of morbidity-mortality in the post-transplant period should be carefully evaluated before being regarded as absolute contraindications.
- c)
The identification of patients with a high mortality risk during their stay on the waiting list may contribute to implement transplant prioritization strategies.
- d)
The psychological evaluation of candidates for RT may be useful to assess their capacity to comply with the necessary future immunosuppressor treatment, since poor compliance is associated to poorer graft survival.
Justification
Informed consent is a patient right and an obligation for healthcare professionals that is contemplated by current Spanish legislation on healthcare. Effective communication is required in order to obtain informed consent to RT. It is the responsibility of the Nephrology Units and the Hemodialysis Centers to obtain the informed consent from the patients with the purpose of including them on the waiting list, though final informed consent to perform kidney transplantation must be obtained in the RTU. All the centers should have a program referred to the informative activity applied to patients, as well as a registry of the informed consents.
Recommendations
- a)
In the Nephrology Units without Transplantation and in the Hemodialysis Centers, comprehensible information on RT is to be given to all potential transplant candidates, including morbidity, mortality, outcomes versus dialysis and information concerning the different sources of the grafts (live and deceased donors). b) The evaluation process and subsequent referral to the RTU are only carried out if the patient provides informed consent. The information given to the patients for obtaining informed consent in the Nephrology Units without Transplantation and in the Hemodialysis Centers is to be coordinated with the respective RTUs. Such coordination will establish the degree of study to be made at each of the healthcare levels, and depends on the agreements established among those care levels.
Justification
The principal objective of RT is to improve patient quality of life and offer a life expectancy at least equal to that afforded by dialysis. Renal transplantation might offer no advantages for patients with a short life expectancy related to the existence of comorbidities444.
Recommendations
- a)
The following are regarded as contraindications for RT: active malignant disease, severe generalized arteriosclerosis, severe organ failure without options for correction, active infections, uncontrolled psychiatric disease, and the active consumption of toxic substances. In general, contraindication applies when the patient suffers an active process that can worsen significantly with the RT process (surgery and immunosuppressor treatment); when there are no reasonable guarantees that the patient will take the immunosuppressor treatment as instructed; and when the patient life expectancy is under two years.
Patient age Justification
There has been gradual improvement in the outcomes of RT in the elderly population (> 70 years of age). This improvement in outcomes warrants the expansion of RT445. On the other hand, on comparing the survival of elderly patients subjected to transplantation versus those remaining on dialysis, the outcomes are better among the former446.
An evaluation of comorbidity and perioperative mortality risk can contribute to prevent serious post-transplantation complications.
Candidates for RT – particularly the more elderly individuals – must undergo careful screening for possible occult neoplasms. In this regard it is advisable to perform an occult blood in stools test, mammography in women over 40 years of age or with a family history of breast cancer, prostate-specific antigen (PSA) testing and prostate ultrasound exploration in men over 50 years of age, and a renal ultrasound study in patients with acquired renal cyst disease447.
Recommendations
- a)
Advanced patient age is not a contraindication for RT in itself.
- b)
In the elderly population, RT can be offered because it increases the possibility of survival compared with dialysis.
- c)
In transplantation candidates of older age, particularly beyond 50 years, careful screening for possible occult neoplasms is indicated, following the recommendations applicable to the general population.
Previous neoplastic disease Justification
It is difficult to decide the appropriate timing for transplantation in patients who have suffered malignant disease and are cancer-free. In this regard, the individualization and analysis of each case with the oncologist may facilitate the decision448.
Patients with in situ skin or cervical cancer and renal cancer incidentally diagnosed and successfully treated can be entered on the transplantation waiting list immediately.
Patients with localized cancer and a good prognosis (thyroid, uterus, cervix, larynx, bladder) should wait 1-3 years before being included on the transplantation waiting list.
In cancer patients with a poor prognosis and greater disease spread (lung, esophagus, stomach, brain, infiltrating bladder tumor), renal transplantation is not advised during a minimum period of 5 years449. There are sporadic cases of low-grade tumors that allow RT even if not eradicated 450.
Recommendations
- a)
In patients with previous malignant disease, RT should only be considered in the absence of evidence of persistent cancer. It is advisable for the waiting period between treatment of the tumor and RT to be based on the type, stage and grade of the tumor, the age of the patient and the existence of comorbidities.
Infection due to hepatitis C virus (HCV) is the main cause of liver disease following RT451. In addition, kidney graft failure can also occur in patients with hepatitis secondary to glomerulonephritis caused by cryoglobulinemia. An increased risk of cirrhosis and hepatocellular carcinoma has been observed during the long-term follow-up of transplanted patients with hepatitis C that have not been treated with the new direct acting antiviral drugs. Treatment with direct acting antiviral drugs for both hepatitis B and hepatitis C can be provided before or after transplantation, with high success rates, though treatment before transplantation is preferred452–454.
Renal transplantation in treated patients with a negative viral load is not associated to greater morbidity-mortality after transplantation, at least over the short to middle term455.
In the case of infection due to hepatitis C virus (HCV) or hepatitis B virus (HBV) with liver cirrhosis, combined liver-kidney transplantation should be postponed due to the high risk of post-transplantation liver failure456.
Recommendations
Hepatitis C
- a)
Screening for hepatitis C virus is recommended in all patients. Carriers of hepatitis C virus may be regarded as candidates for RT, since post-transplantation survival is greater than when remaining on dialysis. The candidates are to be thoroughly evaluated for the liver disease, since there is a risk of accelerated severe liver disease following RT.
- b)
Patients with liver cirrhosis are not regarded as candidates for isolated RT, though combined kidney-liver transplantation may be considered.
- c)
Carriers of hepatitis C virus (positive serology or viral load) or with active chronic hepatitis are to be treated with direct action antiviral drugs before RT and for three months, until negativization of the viral load is achieved.
- d)
In those patients treated with direct acting antiviral drugs and with negativization of the viral load, RT does not entail increased risks over the short to middle term.
Hepatitis B
- a)
Candidates for RT infected with hepatitis B virus (HBV) are to be thoroughly evaluated for the liver disease – including a liver biopsy if the liver transaminase concentrations are elevated – because there is an increased risk of acceleration of the liver disease after RT.
- b)
Patients with liver cirrhosis are not regarded as candidates for isolated RT, though combined kidney-liver transplantation may be considered.
- c)
Patients with active chronic hepatitis can be treated with specific drugs.
Infection due to human immunodeficiency virus (HIV)
Justification
Multiple antiretroviral therapy has caused HIV infection to become a chronic disorder, with a prolongation of patient life expectancy. Positive outcomes with RT have been reported by a number of studies in patients with controlled HIV infection457. For these reasons, HIV infection should not be regarded as an absolute contraindication for RT458,459.
Recommendations
- a)
Human immunodeficiency virus carriers with good adherence to antiretroviral therapy, with an undetectable viral load, a stable CD4+ T cell count > 200 cells/mL and the absence of opportunistic infections in the last 6 months, progressive multifocal leukoencephalopathy, chronic intestinal cryptococcosis or lymphoma can be evaluated for inclusion on the RT waiting list (European guides).
Considerations according to the primary kidney disease Justification
The degree of recurrence of the original kidney disease after RT is variable460 and has been underestimated for a number of reasons: the duration of the studies is variable, the criteria used to program the protocolized biopsies differ among the different teams, and the diagnostic modalities used for the early detection of the recurrent original kidney disease also differ. It is important to take into account that recent data indicate that, in patients with certain primary renal disorders (membranoproliferative glomerulonephritis, mesangial IgA deposits glomerulonephritis, focal segmental glomerulonephritis and membranous kidney disease), the predominant cause (60%) of graft failure is recurrence of the original kidney disease. Furthermore, the graft survival rate at 5 years in patients with recurrent original kidney disease is 45%.
Patients with segmental and focal glomerulosclerosis are at a high risk of recurrence of the primary kidney disease, with figures ranging from 15-50%, depending on the series(461). Membranous glomerulonephritis has a recurrence rate of 20-30% in adults(462). Mesangial IgA deposits glomerulonephritis in turn presents a histological relapse rate of 20-60%, with a far lower clinical relapse rate463.
Relapsing lupus kidney disease is rare. Relapse in the case of IgA vasculitis manifesting as microscopic hematuria and proteinuria has been documented in 18% of the cases, with loss of the graft in 11% of the patients at 5 years464. The recurrence rate of uremic-hemolytic syndrome after renal transplantation has been estimated to be 10-45%, with recurrence being more frequent in children and conditioned to the type of genetic mutation identified. The recurrence rate of vasculitis associated to ANCA is close to 20%.
Both primary and secondary amyloidosis can relapse in the transplanted graft with a frequency of 10-40%465. Recurrence usually manifests in the first three years. In the case of secondary amyloidosis, the risk of relapse depends on the activity of the causal disease condition. Thorough evaluation of cardiac involvement is needed in candidates for transplantation. The recurrence of multiple myeloma and of light chain disease after RT is common, and is generally associated to a poor prognosis. The patients therefore must be disease-free before considering renal transplantation. Nevertheless, in patients with light chain amyloidosis that reach remission of their pre-RT hematological disease thanks to the new treatments (melphalan plus autologous bone marrow transplantation) and who do not have amyloid cardiomyopathy, acceptable survival rates have been recorded equivalent to those of patients with important comorbidity, such as the diabetic population466.
Relapse of incipient diabetic kidney disease lesions is seen in 100% of the cases at four years, though loss of the graft due to diabetic kidney disease is very rare467. Functioning dual kidney-pancreas transplantation may prevent the development of diabetic kidney disease468 and can contribute to treat diabetes.
The outcomes of RT in cystinosis and Fabry disease are comparable to those of RT with other diseases, and such conditions do not represent a contraindication. In patients with primary hyperoxaluria, the treatment of choice is combined kidney-liver transplantation in all cases.
Recommendations
- a)
There is no contraindication to evaluation for RT in candidates with kidney disease in the form of primary glomerulonephritis, though there is a variable risk of relapse depending on the type of primary glomerulonephritis involved. In cases of glomerulonephritis mediated by circulating antibodies, it is advisable to wait until the latter have been cleared from blood before performing RT.
- b)
There is no contraindication to RT in candidates with kidney disease in the form of systemic disorders such as lupus erythematosus, IgA vasculitis, uremic-hemolytic syndrome or vasculitis associated to ANCA, though clinical quiescence of the disease is advisable before transplantation.
- c)
Candidates with primary kidney disease in the form of amyloidosis require highly individualized evaluation to assess the relapse prevention capacity and the degree of involvement of vital organs such as the heart, before being placed on the RT waiting list.
- d)
Patients with light chain amyloidosis (AL amyloidosis) without amyloid cardiomyopathy and with pre-transplantation hematological therapeutic remission can be considered for inclusion on the RT waiting list.
- e)
Patients with primary kidney disease in the form of diabetes mellitus can be included on the RT waiting list provided the severe comorbidities inherent to diabetes do not contraindicate inclusion (severe generalized atheromatosis, non-revascularizable ischemic heart disease, etc.). In those patients presenting type I diabetes mellitus without contraindication for RT, possible simultaneous or delayed transplantation of the pancreas should also be considered.
- f)
Renal transplantation is not contraindicated in patients with cystinosis or Fabry disease.
- g)
In patients with primary hyperoxaluria, combined kidney and liver transplantation should be contemplated in order to avoid rapid relapse of the disease.
Cardiovascular disease Justification
Cardiovascular disease is the leading cause of death after RT; careful cardiovascular evaluation prior to RT is therefore needed(469,470). Coronary angiography is required in cases of suspected ischemic heart disease, with the recommendation of pre-RT surgery or angioplasty, where indicated, in order to improve the prognosis of the transplant recipients471–473. It is important to evaluate the pelvic arteries due to the surgical implications and the risk of amputations. Special attention should focus on the presence of signs of occlusive peripheral vascular disease442,473. Likewise, possible occlusive carotid artery disease should be assessed. In young patients without comorbidity, standard pre-transplantation evaluation may be sufficient474.
Recommendations
- a)
Cardiac evaluation is important in order to detect and treat symptomatic coronary disease, heart failure secondary to valve disease, myocardiopathy and pericarditis. The correction of these disorders should be carried out prior to patient inclusion on the RT waiting list.
- b)
The evaluation of severe arteriosclerosis should be made to detect involvement of the aorta, pelvic arteries and cerebral arteries, and the presence of peripheral arterial disease. Severe involvement requires prior treatment or exclusion of the patient from the RT waiting list.
Justification
The initial clinical evaluation of the patient for possible inclusion on the RT waiting list should include an exhaustive evaluation of the possible risk factors and comorbidities that can affect the prognosis of RT. The detected problems should be corrected if possible, with the assessment of potential absolute contraindications447.
Recommendations
The initial documentation for possible inclusion on the RT waiting list comprises the following:
- a)
Complete clinical report.
- b)
Cardiovascular evaluation: Chest X-rays, ECG, echocardiogram, and aortoiliac CT angiography. In asymptomatic high-risk patients, it is advisable to perform a standard exercise test or echocardiography with dobutamine. In the case of inconclusive or positive test results, noninvasive exercise testing or coronary angiography is recommended.
- c)
Evaluation of infectious risks: chronic bacterial infections (dentures, sinusitis, gallbladder lithiasis, hemodialysis prosthesis, urinary tract anomalies) and chronic viral infections (HCV, HBV, HIV, cytomegalovirus [CMV], Epstein-Barr virus [EBV]), with the performance of: retrograde cystography (serial voiding cystourethrography [VCUG]) in the case of suspected urinary tract anomalies, HBsAg, HBsAb, HBcAb, HCV antibodies (in case of HCV RNA positivity), cytomegalovirus, Epstein-Barr virus, toxoplasma.
- d)
Evaluation of occult neoplastic disease: abdominal ultrasound, PSA in men over 50 years of age, gynecological examination in women. The family history or history of prior immunosuppression should be taken into account.
- e)
Hematological – immunological study: blood group (ABO), HLA typing and HLA antibodies (in the RTU).
Justification
A large percentage of patients on the RT waiting list present HLA antibodies – in most cases as a consequence of transfusions, pregnancies or failed previous RT. A minority of them are highly sensitized (> 80% reaction with the cells of the test panel) and account for 1220% of the patients on the waiting list. Highly sensitized patients require special organizational care, because it is difficult to find donors with a negative cross-match. Transplantation with a direct live or cross-match donor may be a therapeutic alternative in these cases.
Recommendations
- a)
A blood sample should be obtained from the RT candidates for the determination of lymphocytotoxic HLA antibodies. Testing should be made regularly (every 3-4 months) and also two weeks after a potentially sensitizing episode (transfusion, major surgery, pregnancy). The results are to be incorporated to the information on typing in the RTU.
Justification
Regular follow-up of the patients on the RT waiting list is important when the waiting time exceeds 1-2 years. The accelerated heart disease observed in renal failure and especially in the context of dialysis469,471 makes regular re-evaluation necessary to confirm the adequacy of inclusion of the patient on the RT waiting list. The same applies in relation to the risk of occult neoplastic diseases448,449.
Recommendations
- a)
Patients on dialysis are to be referred annually to the RTU with the corresponding clinical report in order to update continuity on the RT waiting list.
- b)
The following should be analyzed on an annual basis: ECG and echocardiogram, with the latest general laboratory test results.
- c)
The patients on the RT waiting list are to be re-evaluated periodically while on the list. The interval varies depending on the case: every 6-12 months in diabetic patients with high comorbidity or individuals with a high mortality risk on the waiting list (previous RT, peripheral vascular disease, elderly patients, etc.) or every 12-24 months in the rest of the cases.
- d)
When the patients on the waiting list present intercurrent clinical complications constituting a contraindication for transplantation, the reference RTU should be duly informed of the fact in order to temporarily or permanently remove the patient from the list, as applicable. The patient also must be duly informed. Likewise, once the clinical problem has been resolved, patient re-inclusion on the list should be considered. In the presence of clinical factors that entail an increased risk for transplantation or for clinical management of the patient, the RTU is to be informed accordingly and, if applicable, a clinical re-evaluation should be made.
The management of chronic kidney disease (CKD) is too “fragmented”, essentially because of a healthcare model based on the “disease”, resulting from a care design intended to deal with “acute episodes” and not adapted to respond to chronic disorders - which requires a model more centered on the “patient”475. On the other hand, we are witnessing a profound change in the care model, as a consequence of the following:
- 1.°
The patient has changed: We now have a more active patient with multiple disease conditions, who wishes to participate in “decision making”, expects good management of his or her disease, uses the internet, and habitually reports to the clinic with knowledge about the disease - expecting continuity and safety of care throughout the healthcare system476. But we are also experiencing a “culture of risk” situation (“how we should live in the world, where everything that used to be natural or traditional now must be the subject of decision or choice”)477, and the response to this situation is more “autonomy” of the individual478. Unfortunately, however, people are not autonomous, particularly when they are most vulnerable, i.e., when they are ill. This context of uncertainty and “limited autonomy” in the case of CKD is most patent at the time of deciding treatment, since currently there is no systematized educational process to guarantee autonomous “free choice of treatment” or preserve the “quality of the decision process”479.
- 2.°
The epidemiological pattern of diseases has changed: At present, 70% of overall healthcare expenditure is related to chronic diseases and to aging of the population. Based on the evolution of the demographic data, it is estimated that the prevalence of patients aged 65 years or older on renal replacement therapy (RRT) will increase greatly over the next two decades: a 39% rise is expected by the year 2026, with a 41% increase by 2036480. The coincidence of this demographic evolution with the “adjustment” of healthcare expenditure creates the scenario for a “perfect storm”481, and in order for the system to survive, we must focus on three crucial aspects: patient-centered care, hospital efficiency, and the performance of interventions in an optimum setting481.
- 3.°
The technologies have changed: The incorporation of remote monitoring systems, telemedicine and clinical decision-making support systems is allowing for improved management of chronic diseases476.
- 4.°
The healthcare system is not changing: In the face of this avalanche of changes, the healthcare system, due to its organizational, funding and services structure, is not adequately adapted for the control of chronic diseases482.
Considering this changing environment, modern healthcare systems must evolve towards an integrated care process (ICP) management model in order to guarantee the quality of the services provided and the continuity of patient care.
Thus, the management of CKD-RRT must be based on a “patient-centered healthcare model” and an “integrated services network”483,484, and should possess the following elements18:
- •
A defined geographical and populational setting (CKD care area).
- •
Definition of the human and physical resources conforming the “network”.
- •
Definition of the instruments supporting the network (protocols, clinical routes, integrated care processes, online communication network, etc.) and which guarantee the continuity of the process and of support for all the participating professionals483.
The integrated care of patients with CKD forms part of the strategies of the S.E.N. and of other organizations, compiled in the Document of the MSC 201118, and essentially implies a change in care model, evolving from a model based on “episodes” towards a model characterized by “processes” (Figure 1). As a consequence of this change, the “isolation” of hospitals is overcome and the latter are integrated within a “network structured around the care process, guaranteeing the continuum of care”18.
Structure and management of the integrated care processes (ICPs)
The structural characteristics of the different units are defined in detail in the Extrarenal filtration unit: standards and recommendations MSC 201118. A summarized account is provided below:
- a)
Hospital Nephrology Department or Unit / Area Central Renal Unit (CRU). This is the reference center of the healthcare area, designed with strategic planning criteria, and it addresses both its own demands and those derived from the healthcare resources network of its area of coverage485. This network is operationally composed of the hospital and out-hospital dialysis units484. The functional characteristics of all of them have been defined, and the geographical setting, structure, population and human and physical resources are described in (Table 1).
Table 1.Care structure of the Department of Nephrology
Care level Activity/Observations Home of the patient PD/HD Support from CRU Primary care Primary/secondary prevention Criteria for referral to Nephrology clinic Criteria for joint follow-up with CRU Local/district hospital <100,000 inhabitants ACKD clinic Satellite extrarenal filtration unit (ERFU) Support from CRU Healthcare area hospital >250000 inhabitants CRU Support of “local” hospital and coordination of the “network” Regional hospital >1,000,000 inhabitants CRU Adult transplantation unit Support of “local” hospital and coordination of the “network” Supra-regional hospital Pediatric transplantation unit - b)
Advanced chronic kidney disease (ACKD) - Renal palliative care (RPC) clinic. In accordance with its particularities, the Department of Nephrology must establish the care structure of this Unit. The population amenable to care in an ACKD clinic comprises patients with CKD grade E3b or more (in any case GFR < 30 ml/min) and those who opt for conservative management, either in the form of first treatment or referred from other therapeutic modalities due to suspension of the technique. The aim is to provide integral care of the patient in this situation, with specific management and patient information objectives.
- c)
Home Dialysis Unit (Home peritoneal dialysis / Hemodialysis). In its conventional HD or daily HD modality, this Unit addresses the demands of those patients who undergo dialysis at home either as a personal choice or by medical prescription. Home hemodialysis (HHD) is a consolidated therapeutic option486,487, in the same way as peritoneal dialysis.
- d)
Renal Transplantation Unit (RTU). The prolongation of time on dialysis is one of the indicators of poorest survival referred to both the graft and the patient. Pre-emptive renal transplantation avoids the inconveniences derived from the dialysis techniques, reducing the costs generated by the latter18. Consequently, the extrarenal filtration unit (ERFU) and the renal transplantation unit must establish due coordination with each other in order to facilitate inclusion of the dialyzed patient on the renal transplantation waiting list488,489.
Structure and management of the network
A care network must be developed, with a common specific structure, independently of the fact that each care unit maintains its own entity.
- a)
The civil structure of the different units (ACKD-RPC, Hemodialysis, Peritoneal dialysis) must abide with the standards recommended in the guide “Extrarenal filtration units. Standards and recommendations MSC 2011”18.
- b)
The management structure of the network is to comprise a Director – Coordinator, as well as a nursing supervisor and a collegiate organ with representation of all the units forming part of the network. A periodic meeting schedule of the network is to be established.
- c)
The organization must have a single common case history (CH) in the network and with online access. The CH must serve to guarantee concrete functions covering care, teaching, research, clinical management and the planning of care resources, legal aspects, and care quality control. There must be a single CH for each patient, containing all his or her clinical data, and it must be integrated with all the information of all contacts and episodes of the patient490.
Likewise, care protocols and process indicators must be developed to guarantee quality of care and patient safety (Figure 2).
- a)
The care network must ensure effective and continuous communication based on modern information and communications technology (ICT), allowing not only the communication of professionals, but also the avoidance of unnecessary patient displacements.
- b)
The performance of complementary tests from the out-hospital dialysis centers will be made according to the established “agreements” with the different health services of the Spanish Autonomous Communities.
- c)
Teaching and R&D&I activities are to be developed.
- d)
The transfer of patients between the different therapeutic modalities should be established according to the current protocols and clinical guides.
Concept of the electronic case history180,488
A process indicator (ID) is a “variable with quality, quantity and time characteristics, used to directly or indirectly measure the changes in a situation and assess the progress made in dealing with it. It also provides a basis for the development of adequate plans for improvement”492.
Process indicators are more sensitive than outcome indicators in determining real differences in the quality of the care afforded and are easy to interpret – though they lack intrinsic value and are only valid if associated to an outcome.
The process indicators that help to assess the quality of the ICP are:
- 1.°
Process indicators of transfer-therapeutic choice from ACKD-RPC (Table 2).
Table 2.Process indicators of transfer-therapeutic choice from ACKD-RPC
Denomination Process for exit from ACKD-Processing Summary ACKD Formula No. of people meeting criteria for RRT/conservative management established by Guide × 100/total no. of people exiting from ACKD Definition People meeting criteria of therapeutic choice – conservative management Type of indicator Process Dimension Efficacy-effectiveness Rationale Need for choice of treatment RRT – conservative management according to criterion of clinical guide Population of the indicator Population in ACKD Recommended data source Clinical guide Periodicity Six-monthly Standard 100% RPC: renal palliative care; ACKD: advanced chronic kidney disease; RRT: renal replacement therapy.
- 2.°
Process indicators of transfer between RRT techniques: HD-RT-HoD (PD-HHD) (Table 3).
Table 3.Process indicators of transfer between RRT techniques: HD-RT-HoD (PD-HHD)
Denomination Process of transfer between RRT techniques Formula No. of people meeting criteria for transfer established by Guide × 100/total no. people transferred Definition People meeting criteria of transfer of technique Type indicator Process Dimension Efficacy-effectiveness Rationale Need for transfer according to criterion of clinical guide Population of the indicator Population in RRT Recommended data source Clinical guide Periodicity Six-monthly Standard 100% RRT: renal replacement therapy.
- 3.°
Process indicators of transfer between ACKD and conservative management (CM) (Table 4).
Table 4.Process indicators of transfer between ACKD and conservative management (CM)
Denomination Process of transfer ACKD to CM Formula No. of people meeting criteria for transfer established by Guide × 100/total no. people transferred Definition People meeting criteria of transfer of technique Type indicator Process Dimension Efficacy-effectiveness Rationale Need for transfer according to criterion of clinical guide Population of the indicator Population in ACKD-RRT Recommended data source Clinical guide Periodicity Six-monthly Standard 100% ACKD: advanced chronic kidney disease; RRT: renal replacement therapy; CM: conservative management.
- 4.°
Process indicators of communication in the network (Table 5).
Table 5.Process indicators of communication in the network
Denomination Meeting of the collegiate organ of network Formula No. collegiate organ meetings established in protocol × 100/of total meetings Definition Collegiate organ Type indicator Process Dimension Efficacy-effectiveness Rationale Need for revision of ICP Population of the indicator Collegiate organ Recommended data source ICP protocol Periodicity Six-monthly Standard 100% ICP: integrated care process
The quality indicators are those defined in the Hemodialysis Centers Guide of the S.E.N. of the year 2005 1.
Management of patient safety
The World Health Organization (WHO) defines patient safety as the reduction of unnecessary risks of harm for the patient to a minimally acceptable level. Harm related to healthcare is damage derived from the plans or actions of a healthcare professional during the provision of healthcare or associated to them, and not related to an underlying disease or lesion1.
The Quality Plan of the Spanish Ministry of Health, Social Services and Equality (Ministerio de Sanidad, Servicios Sociales e Igualdad [MSSSI]) includes as strategy no. 8 the “Improvement of the safety of the patients admitted to the healthcare centers of the National Health system”. One of the established objectives for achieving such improvement is the “Design and implementation of a system for the reporting of incidents related to patient safety”. This objective was materialized through the design, development and implantation of the Reporting and Learning for Patient Safety System (Sistema de Notificación y Aprendizaje para la Seguridad del Paciente [SiNASP]) – a protocol developed by the MSSSI for the National Health System493. Implantation of the SiNASP is therefore mandatory throughout the network.
Relation of the extrarenal filtration unit (ERFU) with other renal replacement therapy (RRT) modalitiesAccording to the characteristics defined in ICP, the relations of the ERFU with the other functional units are:
Relation with the ACKD clinic
Optimum patient care in the ACKD phase, prior to the start of RRT, should contemplate the early detection of progressive kidney disease, interventions to slow its progression, the prevention of uremic complications, attenuation of the associated comorbidities, the indication or contraindication of RRT, and information for the patient about the existing treatment options18.
In the transition between the advanced phases of chronic kidney disease, i.e., stages 4 and 5, and the first months of RRT, patient mortality is extremely high, particularly in hemodialysis (HD)494,495. Accordingly, this transition period is characterized by a series of particularly relevant processes, such as:
- 1.°
Explore individualization for each patient, based on data such as: progression rate and comorbidity, which not only have important survival predictive value but can also be very important in decision making in order to individualize the transition between ACKD and RRT496.
- 2.°
Define the “OPTIMUM” start of RRT, understood as497:
- •
When the patient starts RRT on an ambulatory basis (not hospitalized).
- •
With a mature arteriovenous fistula (AVF), or any usable access (catheter due to indication or patient preference) and peritoneal catheter.
- •
The RRT modality chosen by the patient and the physician.
- •
To secure an optimum start of RRT, the ACKD clinic should establish a patient and family “educational” process, based on:
- 1.
Education of the ACKD patient
- •
Promotion of self-care.
- •
Promotion of home techniques.
- •
Dietetic management.
- •
Management of healthy habits.
- •
Preservation of vascular reserve.
- •
Management of reliable information sources on the internet.
- •
Help for managing the available psychological resources.
- •
Help for managing the available social resources.
There is ever growing evidence that education of the patients improves their quality of life and the clinical-biological outcomes, and reduces the healthcare costs. However, no detailed guidelines are yet available regarding the best way to organize these educational programs, and there is also great variability among units498. The current guides point to the need to design educational programs for patients499, of quality and free of biases500.
- •
- 2.
Eligibility testing for the techniques
The existence of indications and contraindications for the technique must be established by the physician in a structured manner.
- 3.
It is necessary to ensure the conditions allowing the patient to participate in the choice of RRT best adapted to his or her needs and lifestyle501–503.
- 4.
Reduction of general vascular risk and risk inherent to ACKD
In-depth study of the patient is required, with a basic vascular exploration including echocardiography, Doppler ultrasound of the supraaortic trunks, ankle-brachial index (ABI), and in some cases CT angiography of the lower extremities.
In patients with moderate CKD (stages 3A and 3B), the cardiovascular mortality rate is much greater than at the start of RRT504.
- 5.
Vaccination
The infections associated to greater risk for these patients include some that can be prevented through vaccination, such as hepatitis B505, pneumococcal infections506 and influenza507.
- 6.
Planning and creation of the vascular access
In patients with progressive chronic kidney disease, it is advisable to consider creating the vascular access when eGFR < 15 ml/min/1.73 m2 and/or when the start of dialysis treatment is expected to occur within 6 months12.
- 7.
Management of transplantation (live, deceased donor)
Patients eligible for transplantation are to be prepared for inclusion on the deceased donor transplant waiting list on the first day of dialysis. Despite the existence of guides for the evaluation of candidates for renal transplantation, there is great variability in access to transplantation508,509.
- 8.
Start of HD in the right moment (not before, not after)
An important cause of “non-optimum” preparation is the still limited knowledge available regarding the prediction of the trajectories of ACKD. The results of the IDEAL trial indicate that the decision as to when to start periodic hemodialysis should be made based on a patient-focused approach in which assessment of the patient symptoms and objectives are a central element – not the glomerular filtration rate. A reasonable approach in this regard is to postpone the start of dialysis in asymptomatic individuals until uremic signs and symptoms appear which reasonably would be expected to improve with dialysis.
- 9.
Programming of first dialysis
An optimum start, and thus an indicator of good practice on the part of the ACKD unit, therefore would comprise management / follow-up of the patient making it possible not to have to start dialysis on an urgent basis.
A particularly important situation is the “transition” from HD to ACKD. Readmission to this care unit should be made in the following situations:
- a)
Patients starting hemodialysis on an urgent basis should be referred to the ACKD clinic in order to start the entire training process and be able to feely choose between renal replacement techniques or conservative management.
- b)
When there is a change in patient decision regarding the modality of treatment.
- c)
Transplant patients with graft loss can be readmitted to the ACKD clinic (Table 5).
Discharge from this clinic, independently of the general case history, is to be accompanied by a full report-summary of the situation of the patient (Table 6) that is rapid and easy to read and understand.
Table 6.Summaiy of ACKD
Age Gender Charlson comorbidity index(510) Frailty (FRIED index)(511) Level of dependency (Barthel score)(512) Level of therapeutic adherence (Hermes score)(513) Nutrition (Ulibarri score)(514) Socioeconomic status(515) ABPM (SBP/DBP and pattern) SAT US Echocardiogram ABI Risk of “dying” USRDS(516) Vaccination Serology Mantoux – IGRA Renal transplantation waiting list (yes/no) Treatment choice: *HD (hospital, out-hospital, home) *PD Conservative management Informed consent: *RRT technique *Conservative management Previous instructions statement Vascular access ABPM: Ambulatory blood pressure monitoring; IGRA: interferongamma release assay; SAT US: supraaortic trunk ultrasound ABI: ankle-brachial index
- a)
Relation with the ACKD clinic – renal palliative care (RPC)
Renal palliative care (RPC) is defined as a medical practice model focused on the patient and comprising management of the symptoms associated to ACKD, respecting the patient preferences, and with the ultimate purpose of improving quality of life. It must be available over the entire trajectory of ACKD, from diagnosis to death (Figure 3), and is applicable to all the RRT modalities up to the end of life, with the intervention of a multidisciplinary team517.
This means that the patient can directly choose conservative management or be transferred to conservative renal care (CRC) in the course of the different renal replacement therapeutic modalities (Figure 4). In order for this to be done effectively and efficiently, it is advisable to establish inter-relations between the different Nephrology units and the conservative ACKD clinic, as well as due coordination with other specialities implicated in patient care.
The KDIGO guides propose a roadmap to improve the care and quality of life of palliative renal patients. These guides underscore fundamental aspects as to why and for whom it is necessary to establish conservative ACKD and RPC development programs, and what the desirable approach should be518,519.
Starting or not starting RRT, or the withdrawal of chronic dialysis, implies a prior adequate decision-making process with participation of the patient, family and physician. We must adequately explain the advantages and inconveniences of each technique and quality of life in RRT and CRC520, 521, in the awareness that there is no system guaranteeing free therapeutic choice479.
The RPC team may also inform the rest of the intervening team of the existence of patients with a previous instructions statement. This can be very useful in relation to decision-making and communication with the patient, favoring dialogue and the sensation of self-control522.
The decision-making process will be completed successfully if we have been able to convey the message that we are concerned about the patient, and that we are there to help and plan his or her care to avoid suffering, while also respecting his or her personal preferences.
Patient referral from other areas will be made based on the needs of RPC and the prognostic criterion indicating a poor course with renal replacement therapy (Table 7)523–525, where it is ethically indicated to suspend dialysis, and an advanced support and care plan (ASCP) is to be started.
Indicators of poor prognosis in ackd with RRT
| Age >75 years (age in itself is not a poor prognosis indicator except if associated to important comorbidity) |
| Comorbidity (Charlson comorbidity index) |
| Functional impairment: (Karnofsky score/Performance status < 40) |
| Congestive heart failure, type IV |
| Severe, non-revascularizable ischemic heart disease |
| Chronic lower limb ischemia (grade IV). Amputations |
| End-stage cancer disease |
| Acute stroke without rehabilitation options |
| Severe calciphylaxis |
| Persistent hemodynamic instability |
| Severe symptoms that do not improve with dialysis |
| Severe cognitive and functional impairment |
| Patient wish: tired of the technique |
RRT: renal replacement therapy.
An advanced support and care plan (ASCP) is defined as a plan implemented by an interdisciplinary renal care team (nephrologists, palliative care, psychologists, social workers, primary care, etc.) with objectives focused on the patient and the family. This must be a structured, effective, accessible and continuous process. In this phase the fundamental concerns are symptoms management, the adoption of measures of comfort for the patient, and communication skills in difficult situations. The patient and family must participate in the care plan. This will allow us to be prepared for the decisions that need to be made and to ensure effective care at the end of life and in mourning526,527.
Relation with the peritoneal dialysis unit
Renal replacement therapy is an integrated process ranging from the ACKD clinic to the different dialysis and transplantation techniques. Global planning is needed in view of the high economical costs and individual and social repercussions involved. Accordingly, the PD and HD units must be in continuous contact due to the bidirectional transfer that takes place between these two RRT modalities.
Different reasons may cause a patient enrolled in a PD program to be transferred to HD either on a transient basis or permanently. Transient or temporary transfer is a transfer for a period of less than 8 weeks. The causes of such transfer are shown in Table 8. In all these situations peritoneal rest is needed in order to replace the peritoneal access, restore the integrity of the peritoneal membrane and/or abdominal wall, or restore permeability. The causes of definitive transfer to HD are described in Table 9.
Many studies have analyzed the factors predicting PD failure. In this respect, advanced patient age, high peritoneal transport, diminished ultrafiltration, malnutrition, diabetes mellitus and obesity have been associated to poorer PD survival516.
The transition period between PD and HD is complicated. Patients who change their mode of dialysis in an unplanned manner suffer a greater number of hospitalizations and spend more days in hospital than those in which the change is made on a scheduled basis528. On the other hand, an orderly transfer to HD is considered to have a lesser impact upon the patient psychological condition and quality of life.
It is common for transfer from PD to HD to take place on an acute basis, with no time to prepare the patient for HD. When transfer is done in a scheduled or programmed manner, adequate preparation for the change is possible, based fundamentally on the creation of the vascular access on which patient survival is going to depend. It is not exactly clear when creation of the vascular access should be made in patients on PD. The creation of arteriovenous fistulas on a preventive basis in incident patients on PD is not recommended529. On the other hand, however, since in many cases it is not possible to predict when transfer between the techniques will occur, many patients will start HD without a definitive vascular access and will require central venous catheters. In this regard, incident patients on HD are known to suffer greater mortality associated to the use of these catheters530,531.
No studies to date have compared survival among patients transferred from PD to HD according to whether or not an arteriovenous fistula has been created. However, it seems logical that the lack of a definitive vascular access may have a negative impact.
The survival of patients permanently transferred from PD to HD is poorer than in incident patients on HD as a first RRT modality, at least during the first 12 months. It has been postulated that such poor outcomes are attributable to circumstances inherent to the end of the PD technique, such as malnutrition, inflammation or infradialysis532. In view of all these data, transfer is advised in a timely and adequate manner.
Transition from hemodialysis to peritoneal dialysis
The transfer of patients on HD usually occurs due to two reasons. On one hand we have patients that start dialysis in a non-scheduled manner and who were not previously able to receive information about the different RRT modalities, or therefore to choose PD. Once their situation has been stabilized, and after being duly informed, some of these patients are transferred to PD within less than a year. Other patients who do choose HD are subsequently changed to PD, generally due to vascular access failure, but also because of changes in preferences, place of residency or access to caregivers, and other clinical circumstances.
Having previously been on HD has a negative impact upon the outcome of patients on PD. In this regard, it has been seen that patients transferred to PD during the first year show poorer survival of the technique and greater mortality533. Similar data have been reported on analyzing the evolution of patients who are transferred to PD at any time of their clinical course534.
In sum, it is crucial for PD and HD units to maintain constant communication and collaboration due to the need for transient or permanent transfers between the two RRT modalities. From the data of those studies that have analyzed transition between the techniques, it can be concluded that PD and HD transfer must be done on a planned basis whenever possible, due to its impact upon survival. On the other hand, the effect of having previously undergone HD upon the evolution of patients transferred to PD underscores the importance of promoting PD as the initial RRT modality, and of the provision of adequate information on the different RRT modalities that are available to the patient. These data are warranted by the results of a recent study carried out by the Escuela Andaluza de Salud Pública535, according to which patients who start PD and then are transferred to HD after three or five years have greater survival rates than patients who undergo HD from the start. Furthermore, the “peritoneal dialysis first” model is more cost-efficient than introducing HD from the start.
Relation with the renal transplantation unit (RTU)
Preemptive renal transplantation is a scientific reality489, and the integral care network for patients with CKD must include a reference unit for preemptive renal transplantation from both live and deceased donors. The ACKD clinic and RTU should establish the necessary coordination-protocols for inclusion on the transplantation waiting list of all those patients who are candidates for this therapeutic option. Likewise, the ACKD clinic should proactively promote live donor renal transplantation.
Quality management in hemodialysisIntroductionThe evaluation and improvement of the quality of care has always been a concern among healthcare professionals. The first attempts to develop the concept of quality in the healthcare setting focused on measuring quality through the definition of a series of standards indicating that the service provided was technically adequate. Healthcare accreditation understood as external and voluntary evaluation with respect to a series of professional standards-In the United States health care accreditation was introduced in the early twentieth century and after different experiences it culminated in the development of the Joint Commission.
In parallel, in the industrial setting, the concept of quality was introduced at the end of the nineteenth century through control of the quality of the finished product. This approach did not improve quality, however, since it simply eliminated the defective products. Posteriorly, the quality models evolved towards quality assurance – the aim of which is to prevent the appearance of defects and guarantee that the entire production process takes place as planned. The first ISO standards appeared in this setting, being published for the first time in 1987.
The quality assurance models in turn have evolved towards total quality models. An organization with a total quality management system understands quality not as an attribute of the service provided, but as something that affects the functioning of the entire organization, in a quest for continuous improvement. Thus, the ISO 9001 standard, upon being updated (5 revisions in total to date), no longer referred to quality assurance but to a quality management system (QMS).
On the other hand, the EFQM (European Foundation for Quality Management) model addresses the best management practices for an organization to be considered excellent. Process management lies at the base of both models.
In our setting, a basic accreditation model is due authorization from the Administration, which is required for all dialysis centers before they can start accepting patients in the Hemodialysis Unit536. This authorization is based on compliance with current legislation and confirmation of a series of fundamental requirements aimed at guaranteeing that the center has the technical means, facilities and minimum professional staff members needed to carry out the intended activities. Apart from complying with these minimum requirements, initiatives have recently been introduced seeking to ensure quality of the hemodialysis provided, requiring abidance with a series of previously defined clinical criteria encouraging (or promoting) the achievement of external certifications537. These specifications are contemplated in the contract-program (in public centers) or through a tender process in the case of public private partnership) n subsidized agreement (in subsidized centers).
Process Oriented Management
All quality systems are based on the premise that the organization, in order to be successful, must have its processes well organized and managed systematically. A process is defined as a series of interventions, decisions, activities and tasks that are implemented in a sequential and orderly manner to secure a result or outcome that satisfies the requirements of the intended client(534).
Process Oriented Management-implies the creation of a coherent structure of processes that represent functioning of the organization, with interaction between them, in order to ensure that the processes develop in a coordinated manner - thereby improving effectiveness and satisfaction of all the parties involved.
Process management Methodology is based on the following steps:
- a)
The development of an organization processes map through the systematic analysis of the procedures and process involved in the quality management of a dialysis unit through visual illustrations (Figure 5).
- b)
The identification of the key processes and the individuals who will supervise them, ensuring improved compliance with all the requirements.
- c)
The systematic management of the processes.
The key processes are the most important elements for an organization, since they have a direct impact in patients related outcomes and/ or client satisfaction.
Medical care processes in particular require adequate identification and management, since their inherent complexity favors the existence of inefficiencies.
Once the processes have been defined, they must be kept under control or be “stabilized”, avoiding excessive variability539. In the clinical setting, this approach has led to the implantation of clinical practice guides based on best practices analysis that define how the service should be provided, and (thus) avoiding variability.
Once the processes have been specified, we must define the quality characteristics or features of the different activities that conform the process and periodically examine whether the latter meets the expectations or needs of the client, and whether it is effective and or efficient. Indicators are used to measure the efficacy and efficiency of processes, and to control their variability.
When the indicators or other sources of information detect deviations in the process, an analysis of the underlying causes is required. The commonly used method of analysis comprises a review of the sequence of activities, in order to detect deviations and improve the process. The basic tool is the PDCA (Plan, Do, Check and Adjust) cycle or Deming cycle.
Management processes offers a number of important advantages538:
- •
It allows systematic orientation of all the activities towards the needs and expectations of the clients.
- •
It facilitates the participation of all the people that intervene in the processes.
- •
Evidence-based medicine is incorporated in the healthcare processes, through the definition of the quality characteristics of the activities
- •
The definition of indicators facilitates systematic measurement of the most relevant results or outcomes of the services.
- •
The tool is simple and easy to use.
ISO 9001:2015
The ISO 9001 standard is the reference whereby organizations establish, document and implement their quality management systems539.
This international standard focuses on all the quality management aspects which an organization must consider in order to have an effective system allowing it to improve the quality of its products or services, and to ensure that these meet the specifications made by their clients.
Those organizations that voluntarily decide to incorporate the requirements of the ISO 9001 standard are audited by an independent entity (certifying entity). If the results of the auditory process are favorable, the organization receives external recognition of compliance with the standard in the form of a certificate.
The basic principles of the ISO 9001 standard can be summarized by the following 8 points540:
- 1.
The Organization is oriented towards all of its Clients.
- 2.
Leadership: The concept of leader refers to all the people bearing some responsibility in the organization.
- 3.
Continuous improvement: This is achieved through the planning process, the meeting of objectives, evaluation and control, analysis and adjustment of the planning process (PDCA [Plan, Do, Check and Adjust)] cycle).
- 4.
Processes: These are understood as the global activities whereby the services received by the patient / client are carried out.
- 5.
Involvement and participation of the staff: Empowerment of the people conforming the organization to manage its activities and participate in the improvements through well defined communication channels.
- 6.
Management: The quality system is focused on management.
- 7.
Information-based decision making.
- 8.
Mutually beneficial relations with the supplier.
A number of major dimensions can be highlighted in the new version of the standard (ISO 9001:2015):
The Context of the organization, the Leadership, the Focus on processes APPROACH, Change Management process and risk based thinking540.
Previous versions cited the leading management requirements, but the new version makes specific mention of the term “leadership” combined with “commitment capacity” as qualities required for directing and controlling a quality management system. This causes us to understand that although leading management may delegate authority and provide resources within the organization, it must accept ultimate responsibility for the corresponding outcomes.
With regard to risk based thinking, the concept of risk refers to a deviation that may affect the efficacy of our processes, making it difficult to achieve the expected results. A practical tool for identifying the risks present in a Hemodialysis Unit is the determination of those external and internal issues that affect the Unit (context of the organization) through the application of t SWOT analysis (PLEASE change orders sin the acronym is SWOT and not WTSO identification of W: Weaknesses, T: Threats, S: Strengths and O: Opportunities) principle, particularly in the context of a processes-based approach. If the definition and planning of a process are made considering these possible situations, we will be in the best position to obtain the intended results.
Once the possible risks of each of the processes have been identified, it is advisable to perform a cause (why has this risk appeared?) and effect (what is the impact of this risk?) analysis and calculate the corresponding risk index, i.e., quantify the level of risk, e.g., according to a Severity (S) × Probability (P) index, in order to establish priority actions (Figure 6).
A useful tool for assessing risk is failure mode and effects analysis (FMEA)542.
Once the risk index has been quantified, it is advisable to decide and plan the actions that might minimize the risk, i.e., actions allowing us to lower this value and thus reduce the level of risk. A practical way to achieve control is to associate indicators to these risks in order to objectively assess the efficacy of the actions carried out. These indicators in future may be incorporated to the general indicators of the Hemodialysis Unit in question.
Certain risks cannot be minimized and must be assumed. In these cases we must ensure that their magnitude (risk index) does not increase over time, i.e., although we cannot lower the level of risk, we can monitor the situation to make sure that it remains stable and does not increase.
European Foundation for Quality Management (EFQM) model
The EFQM model, impulsed by the European Union, was introduced in 1991 and offers a management model based on Total Quality Management, which allows self-evaluation aimed at identifying critical aspects within the organization, using the continuous improvements cycle or RADAR (Results > Approaches > Deploy > Assess > Refine) matrix cycle as instrument541.
The fundamental concepts of the EFQM model can be summarized as follows: Excellent results with respect to the performance of an organization, the clients, people (employees) and society as a whole are achieved through leadership promoting and directing Policy and Strategy, the people of the organization, the alliances and resources through processes management (Figure 7).
The EFQM model and the ISO standards have a number of aspects in common: they allow organizations to identify their strong and weak points; establish generic requirements regarding a model for conducting the evaluation; afford the basis for continuous improvements; and imply involves or requires external recognition540.
In addition to the principles already mentioned in relation to the ISO standard, the EFQM model contemplates comparison criteria based on the evaluation of the results of the organization (organizations must be efficient as well as effective). Additional criteria are the implication not only of the clients but also of all the interested parties and of society as a whole, as well as the importance which the EFQM model attributes to participation and satisfaction of the employees543.
Quality indicators
Quality indicators (QI) constitute one of the instruments used by quality management models (ISO 9000, EFQM, Joint Commission, etc.) for analyzing the processes and results of their healthcare organizations.
Quality indicators are useful because they allow us to document the quality of our work, see the direction and evolution of our organization, and compare ourselves with other organizations – optimizing the decisions we need to make in order to improve the organization536.
Characteristics of an ideal quality indicator537
The ideal quality indicator would have the following characteristics: it would be defined and described with precision and would be based on scientific evidence; it would offer great sensitivity and specificity; and would be useful for establishing comparisons with ourselves and with other similar organizations.
A quality indicator is valid when it proves effective and accurate in measuring what it intends to measure, and is exact when it obtains the same results after performing the measurement repeatedly in different patients and medical organizations. A valid QI must be both reproducible and constant.
Types of quality indicators
The types of QI we use are the following:
Indicator based on ratio: This is the most frequent indicator. The Numerator indicates the number of times we have measured the event being analyzed, while the Denominator indicates the total number of opportunities we have had for this event to occur.
For example, the number of incident patients on HD during the period: Numerator = number of NEW patients in the Unit during the study period (31 December); Denominator = number of patients in the Unit at the start of the year (1 January).
Sentinel indicators: These indicators allow us to identify an unexpected event or event of special relevance. This event will require and analysis and investigation of its underlying cause, such as for example the hepatitis C seroconversion rate.
Quality systems categories
In relation to the quality of medical care, three dimensions can be distinguished which in turn are related to three categories of quality indicators.
These three categories of indicators are: indicators associated to the structure, associated to the processes, and associated to the outcomes of the medical organization538.
- a)
Structural indicators: These indicators are related to the characteristics of the healthcare environment that affect the capacity of the system to address the health needs of the patients. Examples are the conductivity of dialysis water treated water, the percentage of dialysis water cultures with bacterial growth below the range, aluminum levels in treated water, or endotoxin levels in osmotized water (LAL test).
- b)
Process indicators: These indicators are related to the hemodialysis process itself, and to management of the patients. They include for example the hemodialysis treatment duration and frequency, the type of vascular access, hepatitis B vaccination, etc.
- c)
Outcome indicators: Examples of these indicators are length of time in renal replacement therapy, the annual mortality rate, Kt/V or Kt of the patients, the percentage of patients with PTH between 150-500 pg/mL, or the percentage of patients with serum phosphorus < 5.5 mg/dL, etc.536
The quality pyramid
Nissenson(539) proposed a change in paradigm in the approach to the evaluation of quality of the care we offer our patients on hemodialysis. This paradigm is described as a QUALITY PYRAMID.
At the base of the pyramid lie the fundamental indicators, most of which are contemplated in our guides of the year 2006 (except weight gain and sodium).
Higher up in the pyramid lie the indicators that are more complex to manage, since they depend on various components such as water overload, the management of cardiovascular disease, etc.
On an even higher level we have the indicators that measure efficacy, such as mortality or the number of hospital admissions.
Lastly, the quality of life of the patient lies at the top of the pyramid.
Review of the indicatorsGlobal indicators
NOTE: No changes with respect to those of the year 2006. These indicators are useful for analyzing the general characteristics of the patients being treated in our hemodialysis programs, facilitating comparison with other centers and evaluation of the evolution of our center over time.
With the exception of the crude annual mortality rate, these are not actually indicators as such but reference terms that allow us to know certain characteristics of the patients and of the centers that exert an influence upon the outcomes.
- a)
Incidence in hemodialysis (HD)
Definition: number of new patients that have been incorporated to the HD Unit between 1 January and 31 December of that year, in relation to the number of patients there were in the Unit at the start of the year. A new patient is considered to be a patient starting dialysis treatment on an ambulatory basis.
Formula:
Numerator: 100 × no. of patients that have been incorporated to the Hemodialysis Unit between 1 January and 31 December.
Denominator: no. of patients in the Unit at the start of the year (1 January).
Units: percentage.
Periodicity: annually.
- b)
Prevalence of HD period
Definition:
This is the TOTAL number of patients that are being or have been treated in the HD Unit between 1 January and 31 December of that year.
Formula:
Sum of prevalent patients on 31 December of the study period + withdrawals from HD (withdrawals from HD: deaths + transplanted + transfers + recovery of renal function).
Units: number of patients/year.
Periodicity: annually.
- c)
Crude annual mortality rate
Definition: the percentage of patients treated in the Unit that have died between 1 January and 31 December of that year.
Formula: Numerator = 100 × no. of deaths up until 31 December.
Denominator: prevalence of HD period.
Units: percentage.
Periodicity: annually.
- d)
Median value of Charlson comorbidity Index in incident patients on HD
Rationale:
The Charlson comorbidity index (CCI) is useful for assessing comorbidity and for predicting survival among incident patients on hemodialysis. The original Charlson comorbidity index modified by Beddhu et al. is proposed (Annex 8).
Formula:
Median modified Charlson comorbidity score calculated during the first month of treatment, corresponding to all incident patients.
Units: index score (numerical).
Periodicity: annually.
Anemia
- a)
Percentage of patients with target hemoglobin
Comment
The KDIGO guide of the year 2012 recommended263 the avoidance of erythropoiesis stimulating agents (ESAs) in the presence of hemoglobin (Hb) > 11.5 g/L, though it recognized that some patients may require Hb concentrations above this level in order to improve their quality of life, with due understanding of the possible risk associated to Hb above these levels.
The guide moreover recommends that ESAs should not be used to intentionally elevate Hb > 13 g/dL, since the possibility of adverse effects outweighs the benefits in terms of possible improvement in quality of life of the patients or a decrease in the transfusion requirements.
In patients on dialysis, the KDOQI540 considers that the target Hb level usually should be within the range of 1112.0 g/dL, and should not exceed > 13.0 g/dL. This is not applicable to the pediatric population544. The anemia working group of the European Renal Best Practice (ERBP) guide establishes the same recommendations as those of the KDOQI in 2007543.
Given the important variability of Hb, it is very difficult to make sure that this parameter is kept within a very narrow concentration range. In a retrospective study, Soffritti et al.545 found that only 4.3% of the patients maintained Hb within a range of 11-12 g/dL during the study period. Portoles et al546., in a prospective multicenter study, in turn observed that 3.8% of the patients maintained Hb within a range of 11-13 g/dL during the 12 months of the study.
We therefore consider that this indicator, defined within such narrow concentration margins, is of doubtful usefulness and should be reconsidered, because its intrinsic variability results in low specificity and sensitivity.
Only patients who have been on HD for over four months are considered for establishing an anemia correction margin biases between Units with different incidences of stage 5 chronic kidney disease (CKD).
Women are more likely to be diagnosed with anemia and to receive erythropoietin in excess. The CKD clinical guides contemplate a single anemia cut-off point for both genders(536), though women physiologically have less hemoglobin than men. Consequently, women require more erythropoietin to reach the same hematocrit547.
Rationale:
In patients with CKD and subjected to HD, the target Hb level usually should be between 1112 g/dL. In the case of various measurements of this parameter, we should use the average of the Hb determinations for each patient in the period involved (one month). A once-monthly measurement is considered to be adequate.
Only patients who have been on HD for over four months are considered for establishing an anemia correction margin.
Formula:
Numerator: no. of patients in the Denominator with mean Hb > 11 g/dL and < 12 g/dL during the study period.
Denominator: no. of prevalent patients on HD (during at least 4 months) in the study period.
Units: percentage.
Periodicity: monthly.
Standard: to be defined.
- b)
Percentage of patients with ferritin in optimum range 100-500 μg/L
Rationale:
Patients with CKD and subjected to HD should have sufficient iron reserves to reach and maintain Hb > 11 g/dL and < 12 g/dL.
Serum Ferritin levels < 100 μg/L are inadequate for the regeneration of hemoglobin and are suggestive of an absolute iron deficit.
Formula:
Numerator: 100 × number of patients with ferritin 100-500 μg/L in the study period.
Denominator: number of prevalent patients in the study period.
Units: percentage.
Periodicity: two-monthly.
Standard: > 80%.
Dialysis adequacy
Percentage of prevalent patients with target Kt/V that have been on HD over 91 days and undergo dialysis three times a week
The KDOQI dialysis adequacy practice guidelines updated in the year 2015 recommend a spKt/V 1.4 per HD session in patients treated three times a week, with a minimum spKt/V of 1.2548. These recommendations remain the same as those in the previous KDOQI guidelines. The European guides on adequate dialysis205 recommend the use of equilibrated Kt/V (eKt/V) 1.2 (spKt/V 1.4) to correct the overestimation of the dialysis dose that occurs with the monocompartmental model of the second-generation Daugirdas equation549.
Formula:
Numerator: 100 × no. of patients in the Denominator with mean period *spKt/V (Daugirdas II) > 1.4 (eKt/V 1.2).
Denominator: no. of prevalent patients of the period with HD > 3 months and who are dialyzed three times a week.
Frequency: two-monthly.
Standard: > 88%.
Percentage of prevalent patients with Kt
It is preferable to use Kt, since we avoid the error produced by introducing an estimated urea volume that tends to result in overestimation of KT/V in women86, low-weight individuals or young patients238,550. In 2005, the minimum Kt dose was individualized according to the body surface area (BSA)236, and was validated in an additional study237.
In order to define and validate the recommendations on minimum Kt in the Spanish dialysis population, a recent prospective, multicenter observational study550 showed that those patients who received the adequate dialysis dose according to individualized Kt improved their survival, with fewer hospital admissions, after two years versus those who did not. Likewise, it was seen that the prescription of an additional dose of three liters or more above the recommended minimum Kt dose could potentially reduce the mortality risk, while an additional dose of 9 liters or more could reduce the risk of hospitalization.
Equation [1] minimum Kt = (1/[0.0069 + (0.0237/BSA])
Formula recommended by the new guide of the S.E.N., adapted to the article550:
Equation [2] minimum Kt = (1/[0.0069 + (0.0237/BSA])+3
Table of individualized Kt according to BSA in Annex 9.
Formula:
Numerator: 100 × no. of patients with minimum Kt.
Denominator: no. of prevalent patients in the period on HD for more of 91 days and who undergo dialysis three times a week.
Frequency: monthly.
Standard: > 88%.
Percentage of prevalent patients on HDF-OL with recommended substitution volume
Online hemodiafiltration (HDF-OL) with high substitution volume combines diffusion with convection treatment, allowing greater clearance of solutes of medium and high molecular weight, and improved intra-dialysis hemodynamic tolerance551. The ESHOL trial129 showed postdilution HDF-OL to reduce patient mortality due to all causes versus hemodialysis (HD) in prevalent patients on HD. Posteriorly, the inclusion of the main randomized clinical trials in a pooling project with more of 2700 patients131 and several meta-analyses122,125 confirmed the decrease in global and cardiovascular mortality. In a post hoc analysis, the clinical trials with mortality as the primary end-point127,128 established an association between convection volume and patient survival. On the basis of these results, a substitution volume > 21 liters per session has been recommended552.
Formula:
Numerator: 100 × no. of patients with substitution volume > 21 liters.
Denominator: no. of prevalent patients in the period subjected to online hemodiafiltration (HDF-OL) for more of 91 days and dialyzed three times a week.
Frequency: monthly.
Standard: > 88%.
Dialysis fluid indicators
The water indicators have been modified according to the recommendations of the dialysis fluid water management guides (second edition 2015)37, and the recommendations for the obtainment of ultrapure water are included.
Purified water
Osmotic Water Conductivity
Rationale:
The purified water should have a maximum conductivity of 4.3 μScm-1 at 20°C, as specified by the Spanish Pharmacopoeia and the European Guides. In places where the supplied water is very hard, conductivities below 20 μScm-1 may be temporarily be accepted.
Formula:
Numerator: 100 × no. of determinations with values < 5 μScm-1.
Denominator: total no. of conductivity determinations of the treated water in the study period.
Units: percentage.
Periodicity: monthly.
Standard: > 80%.
Percentage of purified water cultures with bacterial growth below range
Rationale:
The presence of bacterial growth levels > 50 CFU/mL is associated to a significant inflammatory response, with the production of cytokines and elevation of C-reactive protein - with the subsequent repercussions in terms of morbidity-mortality.
Formula:
Numerator: 100 × no. of treated water cultures with bacterial growth > 50 CFU/mL.
Denominator: no. of treated water cultures in the study period.
Units: percentage.
Periodicity: monthly.
Standard: to be defined.
Aluminum levels in osmotic water
Rationale:
In order to secure a negative aluminum balance, we must maintain a concentration in the dialysis fluid of < 5 μg/L. The Hemodialysis Units should take into account the characteristics of the mains water source, performing aluminum controls of the dialysis fluid with greater frequency in those mains waters in which alumina (aluminum phosphate) is frequently used as a flocculant agent. It is advisable to measure the concentration of aluminum before and after water treatment, and also after any modification of the water treatment plant.
Formula:
Numerator: 100 × no. of determinations of aluminum in the treated water < 5 μg/L.
Denominator: no. of determinations of aluminum in treated water in the study period.
Units: μg/L.
Periodicity: 6-monthly.
Standard: 100%.
Endotoxin levels in Osmotic Water
Rationale:
Bacterial contamination is the origin of endotoxins, which may enter the blood compartment of the dialyzers through retro-filtration or, in the case of those of smaller size, through retro-diffusion, inducing an inflammatory state secondary to monocyte activation. The penetration of endotoxins has been demonstrated in all dialyzers. The detection of endotoxins can be made using different methods - with the Limulus Amebocyte Lysate (LAL) test being the most widely used option.
Formula:
Numerator: 100 × number of determinations of the Denominator with values < 0.25 EU/mL.
Denominator: total number of LAL tests made during the period.
Units: percentage.
Periodicity: monthly.
Standard: 100%.
Ultrapure water
Conductivity of ultrapure water
Numerator: 100 × no. of determinations with values < 5 μScm-1.
Denominator: total no. of conductivity determinations of the treated water in the study period.
Units: percentage.
Periodicity: monthly.
Standard: > 80
Frequency: monthly.
Standard: > 80%.
Percentage of osmotic water cultures with bacterial growth below range
Numerator: 100 × no. of treated water cultures with bacterial growth < 10 CFU/100 ml.
Denominator: no. of treated water cultures in the study period.
Frequency: monthly.
Standard: 100%.
Aluminum levels in ultrapure water
Numerator: 100 × no. of aluminum determinations in treated water < 5 ug/L.
Denominator: no. of aluminum determinations in treated water in the study period.
Frequency: 6-monthly.
Standard: 100%.
Endotoxin levels in ultrapure water
Formula:
Numerator: 100 × number of determinations of the Denominator with values < 0.03 EU/mL.
Denominator: total number of LAL tests made during the period.
Units: percentage.
Periodicity: monthly.
Standard: 100%.
Dialysate
Percentage of dialysate cultures with bacterial growth below range
Rationale:
In order to minimize inflammation in patients on hemodialysis, all the Dialysis Units must have ultrapure dialysis fluid for all modalities of dialysis.
Formula: Numerator: 100 × dialy sis fluid with bacterial growth < 0.01 CFU/mL.
Denominator: no. of treated water cultures in the study period.
Units: Percentage.
Periodicity: monthly.
Standard: 100%.
Dialysate Endotoxin levels
Formula:
Numerator: 100 × no. of determinations values < 0.03 EU/mL.
Denominator: total number of LAL tests made during the period.
Units: percentage.
Periodicity: monthly.
Standard: 100%.
Vascular access
Percentage of patients with a graphic recording of puncture sites during the hemodialysis sessions
Definition of terms
Graphic recording of puncture sites. This consists of a schematic representation of the extremity of the arteriovenous fistula (AVF) with a drawing of the latter and of the puncture sites.
Rationale:
In each HD session a full and detailed examination of the AVF is required, together with a registry of the puncture sites. The existence in the patient hemodilysis history of a AVF map with the puncture sites would be very useful in this regard.
Target population:
Prevalent patients on HD with a functioning AVF.
Data sources:
Patient case history.
Formula:
Numerator: 100 × no. of patients on HD in which the puncture sites are recorded × 100.
Denominator: total no. of patients on HD in which an AVF is punctured.
Units: percentage.
Periodicity: 3-monthly.
Standard: 100%.
Percentage of incident patients with a usable vascular access
Rationale:
The number of scheduled patients that start hemodialysis with a usable vascular access - whether an autologous or a prosthetic arteriovenous fistula (AVF) - provides an indication as to whether planning of the fistula from the predialysis stage has been adequate.
Formula:
Numerator: 100 × no. of patients with a usable autologous or prosthetic arteriovenous fistula (AVF).
Denominator: no. of incident patients in the study period.
Units: percentage.
Periodicity: annually.
Standard: > 75%.
Percentage of incident patients carrying a central venous catheter, without contraindications for an arteriovenous fistula, in which the fistula is created within 6 weeks after placement of the catheter
Rationale:
This indicator evaluates the efficacy of the multidisciplinary team in reducing the duration of HD patient exposure to the central venous catheter (CVC).
Target population:
Patients with CKD starting HD through CVC.
Data sources:
Patient case history.
Formula:
Numerator: no. of incident patients without contraindications for AVF starting HD through CVC and in which an AVF is created within 6 weeks × 100.
Denominator: no. of incident patients without contraindications for AVF who start HD through CVC.
Units: percentage.
Periodicity: monthly.
Standard: > 90%.
Exceptions
Patients with disease conditions suggesting the possibility of renal functional recovery and in which the decision is made to maintain CVC, or where the patient rejects AVF.
Percentage of prevalent patients with over three months in the hemodialysis program who on 31 December of the studied year were dialyzed through a native arteriovenous fistula
Rationale:
This indicator reflects the degree of implementation of structured AVF follow-up programs in each HD unit.
Target population:
Prevalent patients dialyzed in the HD unit on 31 December of the study year.
Data sources:
Patient case history.
Software.
Formula:
Numerator: no. of patients on HD being dialyzed through a functioning native AVF (nAVF) on 31 December of the studied year × 100.
Denominator: no. of patients with more than three months in the HD program on 31 December of the current year.
Units: percentage.
Periodicity: annually.
Standard: > 75%.
Comment
The different guides published to date define as target a variable percentage of 60-85% of prevalent patients dialyzed through nAVF12. In Spain, and on an orientative basis, the results of the multicenter study of the quality management working group of the S.E.N., published in the year 2008, evidence that the median nAVF rate in the HD centers is 50% (percentile 25: 34.5%; percentile 75: 61.2%)664. In other studies, such as those carried out in the Spanish Autonomous Communities of Madrid, the Canary Islands or Catalonia, the nAVF rates among prevalent patients were found to be 58.6%, 64% and 73.3%, respectively278, 950,951. According to the DOPPS 5 trial (2013-2014), this percentage was 65% for Spain as a whole32. Considering these antecedents, the standard has been established by the Spanish Multidisciplinary Vascular Access Group (Grupo Español Multidisciplinar del Acceso Vascular [GEMAV]) as a minimum of 75%.
Percentage of prevalent patients with over three months in the hemodialysis program who on 31 December of the studied year were dialyzed through a tunneled central venous catheter
Rationale:
This indicator reports on the degree of implementation of the structured arteriovenous (AV) follow-up programs in HD Units (see section: “Monitoring and control of the arteriovenous fistula”).
Target population:
Prevalent patients dialyzed in the HD Unit on 31 December of the studied year.
Data sources:
Patient case history.
Formula:
Numerator: no. of patients on HD being dialyzed through a tunneled CVC (TVC) on 31 December of the studied year × 100.
Denominator: no. of patients with more than three months in the HD program on 31 December of the current year.
Units: percentage.
Periodicity: annually.
Standard: < 20%.
Annual thrombosis rate of native arteriovenous fistulas
Definition of terms
Thrombosis. This represents functional annulment of the nAVF, i.e., the blood flow (QA) is 0 ml/min, which results in disappearance of the fistular thrill and murmur at physical examination.
Patients/year at risk. The number of days for which each patient carries a concrete type of AVF for one year (maximum 365 days) divided by 365. Example: patients with nAVF and number of days of dialysis (patient A 365 days, patient B 200 days and patient C 165 days); the sum is 730 days. This, divided by 365, shows the number of patients/year at risk with nAVF to be 2.
Rationale:
This indicator reports on the degree of implementation of the structured arteriovenous (AV) follow-up programs in each HD Unit (see section 4: “Monitoring and control of the arteriovenous fistula”).
Target population:
Prevalent patients with nAVF dialyzed in the HD Unit during the study year.
Data sources:
Patient case history.
Formula:
Numerator: no. of thromboses of the nAVF in the study year.
Denominator: total number of patients/year at risk with nAVF in the study year.
Units: rate.
Periodicity: annually.
Standard: < 0.15 thromboses/patient/year.
Annual thrombosis rate of prosthetic arteriovenous fistulas
Rationale:
This indicator reports on the degree of implementation of the structured arteriovenous (AV) follow-up programs in each HD Unit (see section 4: “Monitoring and control of the arteriovenous fistula”).
Target population:
Prevalent patients with a prosthetic arteriovenous fistula (pAVF) dialyzed in the HD Unit during the study year.
Data sources:
Patient case history.
Formula:
Numerator: no. of thromboses of the pAVF in the study year.
Denominator: total number of patients/year at risk with pAVF in the study year.
Units: rate.
Periodicity: annually.
Standard: < 0.50 thromboses/patient/year.
Percentage of patients dialyzed through a non-tunneled central venous catheter for more than two consecutive weeks
Type of indicator:
Outcome indicator.
Definition of terms:
Non-tunneled venous catheter (NTVC). A type of CVC for HD not housed in a tunnel within the subcutaneous tissue or anchored (cuff) in it.
Rationale:
A patient should not be dialyzed for more than two weeks with NTVC, due to the increased risk of infection, venous thrombosis and central venous stenosis.
Target population:
Prevalent patients dialyzed in the HD Unit through NTVC.
Data sources:
Patient case history.
Formula:
Numerator: no. of patients on HD being dialyzed through NTVC during more than two weeks × 100.
Denominator: no. of patients on HD being dialyzed through NTVC.
Units: percentage.
Periodicity: monthly.
Standard: < 5%.
Comment
In view of the lack of evidence, the standard has been established by the GEMAV on a consensus basis.
Percentage of surgically repaired thrombotic native arteriovenous fistulas remaining patent after one year
Definition of terms:
Thrombosis. Similar to the previous indicators.
Rationale:
This indicator assesses the efficacy of surgery in the treatment of thrombotic nAVF, in the context of the multidisciplinary management.
Target population:
Prevalent patients with nAVF that are dialyzed in the HD Unit or are in the ACKD clinic during the study year and require rescue surgery following thrombosis.
Data sources:
Patient case history.
Formula:
Numerator: no. of patients with nAVF remaining patent after rescue surgery due to thrombosis after one year of follow-up × 100.
Denominator: no. of patients with nAVF and surgically rescued thrombosis that have been followed-up on for one year.
Units: percentage.
Periodicity: annually.
Standard: > 50% at one year.
Comments:
The KDOQI-2006 guides suggest a patency standard > 50% at one year, while the Canadian guides (2006) recommend 40% at one year, and the European guides (2007) recommend 80% at one year87,547,550.
The GEMAV consensus has established patency > 50% at one year. However, there are studies272,273, that indicate that the elective treatment of dysfunctional AVF increases the duration of its patency versus repair carried out once thrombosis has occurred. Therefore, although the GEMAV has decided to establish a patency rate similar to that of other guides (> 50% al year), this indicator is probably not well established.
Percentage of endovascularly repaired thrombotic native arteriovenous fistulas remaining patent after 6 months
Definition of terms:
Thrombosis. Similar to the previous indicators.
Rationale:
This indicator assesses the efficacy of endovascular management in the treatment of thrombotic nAVF, in the context of the multidisciplinary management.
Target population:
Prevalent patients with nAVF that are dialyzed in the HD Unit or are in the ACKD clinic during the study year and require interventional radiological rescue following thrombosis.
Data sources:
Patient case history.
Formula:
Numerator: no. of patients with nAVF remaining patent after endovascular rescue due to thrombosis after 6 months of follow-up × 100.
Denominator: no. of patients with nAVF and thrombosis rescued using interventional radiological techniques that have been followed-up on for 6 months.
Units: percentage.
Periodicity: annually.
Standard: > 50% at 6 months.
Comments:
The KDOQI-2006 guides suggest a patency standard > 50% at 6 months, while the Canadian guides (2006) recommend 40% at three months, and the European guides recommend 50% at one year86,547,550.
The GEMAV* consensus establishes patency > 50% at 6 months.
Viral diseases
Hepatitis C virus seroconversion rate
Rationale:
Horizontal nosocomial transmission is currently the main HCV transmission route in HD units. Provided the recommended universal measures for the control of infections are applied, no seroconversion phenomena should be expected42.
Formula:
Numerator: 100 × no. of patients with HCV positive conversion in the studied period (HCV-Ab positive and/or PCR positive).
Denominator: no. of patients at risk (with negative HCV Ab and/or HCV-PCR dialyzed in the unit).
Units: percentage.
Periodicity: at least 6-monthly.
Standard: 0%.
Transplantation
Percentage of patients referred for evaluation to the Transplantation Center after 6 months of periodic hemodialysis
Rationale:
The transplantation centers, due to their experience and specialization, should assess all appropriate patients for possible inclusion on the renal transplantation waiting list. An appropriate patient is considered to be an individual without absolute contraindications (see renal transplantation guide) and who wishes to be evaluated for transplantation. Inclusion on the waiting list should be made as soon as possible, once the periodic hemodialysis program has started.
Formula:
Numerator: 100 × no. of patients that have been referred to the transplantation center within 6 months after the start of periodic hemodialysis.
Denominator: all patients except those with contraindications or who reject inclusion on the transplantation waiting list.
Units: percentage.
Standard: to be defined.
Percentage of patients on the waiting list that have been transplanted
Rationale:
This is an indirect indicator of the transplantation activity of the reference centers, and is useful for monitoring the evolution of the number of transplants in relation to patients on the waiting list in the Hemodialysis Units.
Formula:
Numerator: 100 × no. of patients transplanted.
Denominator: no. of patients on waiting list.
Units: percentage.
Periodicity: annually.
Standard: to be defined.
Patient satisfaction
In the United States, in addition to the use of quality indicators, a patient satisfaction survey is carried out called ICH-CAHPS (In-Center Hemodialysis Consumer Assessment of Healthcare Providers and Systems)553.
The aim of this tool is to assess the experience of hemodialysis treatment from the patient perspective. Since the year 2014, its use has gradually increased, since the conduction of this patient satisfaction survey is awarded with 10 points554. Mainly two patient satisfaction surveys have been validated in Spain (modified SERVQHOS for hemodialysis).
KDQOL-SF
SF-36 or SF-12 Health Questionnaire
Criterion:
Health-related quality of life (HRQoL) is a chronic hemodialysis outcome or treatment measure. It should be assessed using a duly validated questionnaire.
Formula:
Numerator: 100 × no. of patients of the Denominator that have answered an HRQoL questionnaire (SF-36 or SF-12).
Denominator: no. of prevalent patients on HD at the time of the assessment and who are able to answer the questionnaire.
Units: percentage.
Periodicity: annually.
Standard: > 90%.
Reviewer: Fernando Álvarez-Ude
Comment
This is an indicator proposed by Dr. Alvarez-Ude, who is a pioneer in Spain in this field and therefore a key reference for assessing the use of patient satisfaction questionnaires such as the SF-36 / SF-12 or its possible alternatives.
SERVQUAL
SERVQUAL is a quality survey comprising 44 items or questions that assess quality as the difference between the client expectations and perceptions.
The SERVQHOS is an adaptation of this instrument, developed to assess patient perceived satisfaction with the care received. It has been validated for hospital use in Spanish555 and is employed to measure the degree of quality perceived by the patients on hemodialysis556–561.
Risk preventionSafety of healthcare is the absence or reduction to a minimally acceptable level of the risk of unnecessary patient harm in the course of medical care (source: World Alliance for Patient Safety/WHO).
The safety of patients is a component of quality care, and in this regard, it is the obligation of the centers to offer safe, effective and efficient hemodialysis. Undesired effects derived from healthcare represent a non-negligible cause of morbidity-mortality and are associated to important healthcare costs562,563.
Both the type of patient treated in Hemodialysis Centers (population at high risk, with important comorbidity and frailty) and the increasing complexity of the working environment (leading technology and great human factor interactions) make it necessary to establish systems to guarantee safety. The hemodialysis technique is associated with a broad range of possible errors that need to be specifically identified. Complications are frequent in hemodialysis, though many of them could be avoided or at least minimized provided there is awareness of the possible adverse events and adequate preventive measures are adopted564 (Annexe 10).
Dialysis centers are associated with important safety risks; patients report more anxiety regarding the safety practices than the staff is able to predict, and the staff members in turn believe the Unit to be safer than actually demonstrated by the available data565.
Recommendations
- •
We consider it necessary for the centers to have a safety plan, i.e., a protocol defining and implementing a series of actions to reduce the unnecessary risks inherent to medical care566.
- •
Any strategy used to implement safety plans in healthcare is useful. One of these strategies is failure mode and effects analysis (FMEA) - a prevention technique involving process-correcting actions especially selected to reduce the effect of failure from the perspective of the end user. This is done by either reducing or eliminating the probability of failure, or by improving the detection system in order to prevent the effect of failure from reaching the patient567, 568.
- •
Increased safety among hemodialysis patients requires knowledge of those complications that meet the requirements for being regarded as potential or real adverse events, and which consequently their causes should be analysis for prevention implementation should be. On the other hand, safe practice in healthcare must be implemented (risk priority number [RPN]), using for example the FMEA technique, which allows us to stratify the possible errors and adverse events in the Dialysis Units; depending on the severity or importance of the RPN in the process, we should act timely to develop and implement the necessary improvement measures. After a certain period of time, the failure mode will be re-evaluated and a new RPN will be assigned. This same procedure is likewise carried out with each of the failure modes developed in the FMEA569, 570.
- •
There must be a team specifically dedicated to safety. This team should include not only healthcare professionals but also patients and healthy subjects (relatives). The healthcare professionals and the patients should be encouraged to express their concerns in a guilt-free environment that should seek to create a culture of safety. The Dialysis Unit supervisor must establish this culture of safety and direct the process of quality assessment and performance improvement571, 572.
- •
Each center should have its own safety plans. As an example, in relation to some of the main adverse events, the preventive measures described below should be established570.
Indicators to be considered for exclusion from the guides 2019
Nutrition
Rationale:
Percentage of patients with mean albumin > 3.5 g/dL
Formula:
Numerator: 100 × no. of patients of the Denominator with mean serum albumin in the study period > 3.5 g/dL.
Denominator: no. of relevant patients in those cut-off points.
Periodicity: Two-monthly.
Standard: to be defined.
Comment
Albumin concentrations > 3.5 mg/dL are associated to improved survival of patients on hemodialysis573–574. However, the albumin levels are affected by the characteristics of these patients, with multiple disease conditions and hospital admissions that are often associated to infectious / inflammatory processes577,578 which have a profound and sustained impact, reducing albumin synthesis and increasing its catabolism. Furthermore, the albumin levels exhibit variability and are known to increase with patient age579. These factors cannot be modified by the dialysis techniques571,580; consequently, although the albumin levels should be measured periodically as mentioned in Chapter 7 of the Guides corresponding to the year 2006, we consider that the quantitative determination of albumin should not be used for the assessment and follow-up of the quality of care in patients on hemodialysis.
Mean weekly erythropoietin (EPO) dose
Rationale:
The mean EPO dose indirectly measures the efficiency of management of anemia and adherence to the guides and recommendations of the scientific societies. It is considered to be an alert indicator in the general management of anemia. Changes in this indicator can alert us to problems of resistance, quality of the water, poor management of iron therapy, etc.
On an orientative basis, the EPO maintenance dose in Spanish studies ranges from 110-125 U/kg/week, depending on the administration route. The European DOPPS trial established a dose of 109 U/Kg/week (with predominance of the subcutaneous route).
Formula:
Numerator: mean weekly dose of rh-EPO (U/kg/week) administered in the study period to prevalent patients on hemodialysis in the period.
Denominator: patients / month at risk (patients treated with rh-EPO).
Units: U/kg/week.
Periodicity: monthly.
Standard: to de defined, distinguishing between subcutaneous (s.c.) and intravenous (i.v.) route.
Mean weekly darbepoetin dose (U/kg/week)
Rationale:
The same as above. On an orientative basis, the weekly darbepoetin dose in patients on hemodialysis is between 30- 45 ug/week.
Formula:
Numerator: mean weekly darbepoetin dose (ug/kg/week) administered to prevalent patients on hemodialysis in the period.
Denominator: patients / month at risk (patients treated with darbepoetin).
Units: ug/kg/week.
Comment
The response to the administration of EPO in patients on hemodialysis depends on a range of factors that are not related to the quality of treatment, and which are therefore difficult to modify by the healthcare team. Inflammatory factors (C-reactive protein, IL-6, tumor necrosis factor alpha), nutritional parameters such as low albumin levels, the number of hospital admissions, and the characteristics of the patients, such as age or the female gender, are involved213,577,578.
Financial supportThe drafting of this document has been entirely supported by the Spanish Society of Nephrology (Sociedad Española de Nefrología [S.E.N.]).
The coordinators, authors and editors of this Hemodialysis Centers Guide wish to thank all the people who have made this publication possible and who have participated in the planning, development, drafting and review of its contents.
Special mention must be made of all the authors and reviewers, as well as of the Spanish Society of Nephrology (S.E.N.) and its members, for the time and effort they have all dedicated to discussion of the more controversial aspects and to the contribution of topics of great value. We believe that the multidisciplinary perspective of all the professionals involved has made it possible to address different subjects from different points of view395,491,575,576,583,584,585.
Additional material to this article is available in electronic form at this link.
Rafael Pérez García (1)
Francisco Maduell (2)
(1) Department of Nephrology. Hospital Universitario Infanta Leonor. Madrid. (2) Department of Nephrology. Hospital Clinic. Barcelona.
María Dolores del Pino Pino. Chairperson of the Spanish Society of Nephrology (Sociedad Española de Nefrología).
Carlos Quereda Rodríguez-Navarro. Coordinator of clinical practice guides and consensus documents.
(1) Alcalde Bezhold, Guillermo; (2) Alcázar Arroyo, Roberto; (3) Angoso de Guzmán, Manuel; (4) Del Pino y Pino, M. Dolores; (5) Hernández Marrero, Domingo; (6) Maduell Canals, Francisco; (7) Otero González, Alfonso; (8) Pérez García, Rafael.
(1) Hospital Universitario Araba, Department of Nephrology, Vitoria. (2) Hospital Universitario Infanta Leonor, Department of Nephrology, Madrid. (3) Hospital Vithas Consuelo, Department of Nephrology, Valencia. (4) Complejo Hospitalario Torrecárdenas, Department of Nephrology, Almería. (5) Hospital Regional Universitario de Málaga, Department of Nephrology, Málaga. (6) Hospital Clinic, Department of Nephrology, Barcelona. (7) Complejo Hospitalario Universitario de Ourense, Department of Nephrology, Ourense. (8) Hospital Universitario Infanta Leonor, Department of Nephrology, Madrid.
(1) Arenas Jiménez, Dolores; (2) Arias Guillén, Marta; (3) Martín de Francisco Hernández, Ángel; (4) Martín Malo, Alejandro; (5) Muñoz González, Rosa Inés; (6) Díaz-Tejeiro Izquierdo, Rafael; (7) Fernández Lucas, Milagros; (8) Gutiérrez Martínez, Eduardo; (9) Ojeda López, Raquel; (10) Prieto Velasco, Mario; (11) Rodríguez Benítez, Patrocinio; (12) Sáenz Santolaya, Alfredo José; (13) Slon Roblero, María Fernanda; (14)Teruel Briones, José Luis; (15) Tornero Molina, Fernando.
(1) Hospital del Mar, Department of Nephrology, Barcelona. (2) Hospital Clinic, Department of Nephrology, Barcelona. (3) Hospital Universitario Marqués de Valdecilla, Department of Nephrology, Santander. (4) Hospital Universitario Reina Sofía, Department of Nephrology, Córdoba. (5) Hospital de Galdakao, Department of Nephrology, Galdakao (Vizcaya). (6) Complejo Hospitalario Universitario de Toledo, Department of Nephrology, Toledo. (7) Hospital Universitario Ramón y Cajal, Department of Nephrology, Madrid. (8) Hospital Universitario Doce de Octubre, Department of Nephrology, Madrid. (9) Hospital Universitario Reina Sofía, Department of Nephrology, Córdoba. (10) Complejo Asistencial de León, Department of Nephrology, León. (11) Hospital General Universitario Gregorio Marañón, Department of Nephrology, Madrid. (12) Hospital Universitario Infanta Leonor, Department of Nephrology, Madrid. (13) Complejo Hospitalario de Navarra, Department of Nephrology, Pamplona. (14) Hospital Universitario Ramón Cajal, Department of Nephrology, Madrid. (15) Hospital Universitario del Sureste, Department of Nephrology, Arganda del Rey.
- Home
- All contents
- Publish your article
- About the journal
- Metrics
- Open access
- Léalo en español
- Download PDF
- Bibliography
- Additional material