To evaluate the pressure generated by an adjustable hemostasis clamp on arteriovenous fistulas (AVF) during the hemostasis proccess, and compare it with the direct two-finger pressure applied by the patient. To evaluate the variations of the direct two-finger pressure along the hemostasis process.
MethodsWe analyzed data obtained in 51 hemodialysis procedures from 15 patients. AVF intraaccess pressure was used as indirect indicator of the pressure generated by both methods. It was recorded before venous needle removal (PBasal), at clamp application (P1), after clamp adjustement by a nurse (P2), at the beginning of the direct two-finger pressure by the patient (M0), after 3 min of two-finger pressure (M3) and after 6 min of two-finger pressure (M6).
ResultsIntra-access pressure was lower with the adjusted clamp (P2) than with the direct two-finger pressure by the patient (M0) (variation of −18.57%, 95%CI −14.09 to -4.77 mmHg, P < 0.001). Intraaccess pressure generated by the direct two-finger pressure method showed a decreasing trend along the hemostasis process (M3-M0: −8.82 mmHg, P < 0.001; M6-M0: −12.55 mmHg, P < 0.001).
ConclusionAn adjustable fistula arm clamp generates a lower pressure in AVF than the direct two-finger pressure applied by the patient. The latter showed a decreasing trend along the hemostasis process. These data suggest that some of the recommendations from clinical guidelines could be based on inaccurate premises.
Valorar la presión generada por una pinza ajustable en fístulas arteriovenosas (FAV) durante el proceso de hemostasia, y compararla con la generada por la compresión manual. Evaluar las variaciones de la compresión manual durante el proceso de hemostasia.
MétodosSe analizaron los datos de 51 sesiones de hemodiálisis de 15 pacientes. Se utilizó la presión intraacceso como indicador indirecto de la presión generada por ambos métodos sobre la FAV. La misma se registró antes de retirar la aguja venosa (PBasal), tras retirar la aguja y colocar la pinza (P1), tras ajustar la pinza (P2), al comenzar el paciente a ejercer compresión manual (M0), a los 3 min del inicio de la presión manual (M3), y a los 6 min del inicio de la presión manual (M6).
ResultadosLa presión intraacceso fue menor al aplicar la pinza y ajustarla (P2) que al aplicar presión manual (M0), con una diferencia media de −9,43 mmHg (variación −18,57%, IC95% −14,09 a −4,77 mmHg, p < 0,001). La presión manual mostró una tendencia descendente durante el proceso de hemostasia (M3-M0: −8,82 mmHg, p < 0,001; M6-M0: −12,55 mmHg, p < 0,001).
ConclusiónLa compresión ejercida por una pinza ajustable es inferior o similar a la ejercida de forma manual por el paciente. Esta última muestra una intensidad decreciente durante el proceso de hemostasia. Estos datos sugieren que algunas de las premisas sobre las que se basan algunas de las recomendaciones presentes en las guías clínicas podrían ser imprecisas.
Key concepts
- •
The use of compression devices in the hemostasis process in arteriovenous fistulas is considered negative in current clinical guidelines.
- •
The preferred method of hemostasis is manual compression.
- •
This recommendation is based on expert opinion, but there is no published data to support it.
- •
Our data show that the pressure applied manually by the patient is not constant over time.
- •
Furthermore, it is shown that the use of a compression device that allows to be adjusted by the nurse exerts a pressure similar or lower than that applied manually by the patient.
- •
Our results suggest that some of the premises that support the recommendation of the current clinical guidelines may not be precise.
Removal of the needles after a hemodialysis session in patients with arteriovenous fistulas (AVF) is as important as the cannulation process. An adequate technique protects the wall of fistula from possible injuries and favors adequate hemostasis.1–3
The application of correct pression on the puncture points favors their closure and reduces the risk of complications, such as hematomas, stenosis or thrombosis.1,4–6 These adverse effects makes subsequent punctures difficult, limits the number of options1,4 and, finally, the management of these patients becomes difficult.
For the process of hemostasis, the main clinical guidelines recommend applying compression with the fingertips for at least 10 min after removal of the needles, the pressure has to be applied constantly and maintained until complete hemostasia is achieved.7–9
The use of compression devices (clamps, bands) is also accepted in some of these guidelines,2,7 while others prohibit or discourage their use,1,4,8 arguing that these devices could apply excessive pressure and generate damage of the fistula. This recommendation is based on expert consensus, but to our knowledge, there are no studies that provide data on which method of hemostasis is more appropriate.
Despite the negative consideration of the use of compression devices in AVF (especially in prosthetics), these are used in clinical practice. There is a large number of this type devices available on the market. Many of them have a regulation system that allows adjusting the compression exerted that could make them suitable for use in AVFs.
It would be useful to have data on the feasibility and safety of the use of this type of device in clinical practice, as well as its comparison with manual compression. The objectives of the present study are: (a) to assess the compression exerted with an adjustable clamp and compare it with that exerted manually, and (b) to assess the intensity and variations of the pressure that is exerted manually on the AVF during the hemostasis process.
MethodsDescriptive cross-sectional study comparing two compression methods in patients undergoing hemodialysis through native or prosthetic AVFs in the Hemodialysis Service of the Segovia Health Care Complex.
Sample, sampling and sample sizeThe patients included in the study are those undergoing hemodialysis through AVF in our hemodialysis Unit. Patients excluded were those with complications in the AVF (subcutaneous hematoma, infection, thrombosis), a situation that implies an increased risk of vascular rupture (according to the practitioner criteria), or those with immature AVF.
The sample unit of the study is the hemodialysis session, in which the intra-access pressure will be determined on several occasions according to the protocol described below. Up to 10 hemodialysis sessions of the same patient are used in the study, which are selected by simple random sampling from all the sessions received by each patient during the duration of the study. Data from sessions in which the presence of fibrin in the system may skew the intra-access pressure reading, as well as those in which a movement or change in position of the patient may alter the readings, will be excluded.
A minimum sample size of 37 hemodialysis sessions has been estimated necessary to evaluate the difference in pressure obtained by doing manual compression and that exerted by a compression device. This was estimated considering a standard deviation of 15 mmHg, a minimum difference to be detected of ±7 mmHg, an alpha error of 0.05, and a statistical power of 20%.
Measurement of intra-access pressureOur Unit has 10 hemodialysis positions, and in the present study we used 4 Gambro monitors: Artis and Artis Physio, and a Premifistola BL170 compression forceps (Bellco, Mirandola, Italy), to which an adjustable flange is attached to allow adjustment of the compression exerted on the AVF. All nurses that participated in the study were experts in AVF cannulation, with more than 3 years of experience in this technique.
The intra-access pressure measured by the monitor in the venous chamber of the system will be used as an indirect indicator of the pressure exerted on the puncture site. It is read 6 times: before removing the venous needle (Basal), after removing the venous needle and placing the clamp (P1), at the time of adjusting the clamp (P2), at the beginning of applying manual compression (M0) at 3 min after starting to exert manual pressure (M3) and at 6 min of manual pressure (M6).
At the end of the hemodialysis session, the blood will be returned from the extracorporeal circuit according to the Unit’s protocol. To obtain the pressure reading in the venous chamber, the venous line will be connected to the arterial needle. To equql the intra-access pressure, the height of the patient’s bed and headboard will be adjusted to match the same level of the venous chamber. In this way, the differences associated with the increase in hydrostatic pressure will be eliminated.10,11
Thereafter, the line that communicates the venous chamber with the dialyzer will be clamped, to prevent pressure changes from dissipating through this line. After 30 s the pressure reading will be recorded, which will be considered the basal pressure (Basal). Then the nurse will remove the venous needle, place the compression device, and adjust it to achieve hemostasis without interrupting flow through the fistula. The presence of the thrill at points immediately before and after will indicate that the flow is sufficient.8 The nurse who places and adjusts the clamp will not have access to the intra-access pressure reading, a second observer will measure the intra-access pressure when placing the clamp (P1) and when adjusting it (P2).
After that, the clamp will be removed and the patient will compress the hole to exert hemostasis. At that time, the pressure reading (M0) will be taken, repeating the reading after 3 min (M3) and at min (M6). Again, the person applying pressure will not have access to the pressure reading provided on the screen, which will be recorded by a second observer. After the 6 min reading (M6) will continue with the normal disconnection process of the patient.
Manual compression will be as sustained and constant as possible, sufficient to interrupt bleeding externally and subcutaneously, but without interrupting the flow through the fistula. The pressure will be exerted for at least 10 min, until complete hemostasis is achieved.7 In our unit, patients are instructed on how to compress after removing the needles. In the event that the patient does not have sufficient cognitive/functional capacity, this pressure is exerted by the nurse.
VariablesThe differences in the values of pressures recorded (Basal, P1, P2, M0, M3 and M6) will be calculated. In addition, sociodemographic and clinical variables will be recorded, such as the sex and age of the patient, the type of AVF (native or prosthetic), location and age of the fistula, treatment with antiplatelet aggregation, anticoagulant or erythropoiesis stimulator treatment, and parameters such as urea, hemoglobin and the value of Kt.
Complications arising in the area of the fistula during the 48 h after the hemodialysis sessions attributable to the process of hemostasis will be also recorded, such as external bleeding, subcutaneous hematoma, marked decrease in the flow of access, infection, stenosis or new onset pseudoaneurysm, thrombosis, vascular rupture, etc.
Statistical analysisSPSS software v.20.0.0 (IBM Corporation, ©2011, USA) will be used for data analysis. In the descriptive analysis of the results, means and standard deviations will be used for the quantitative variables and percentages for the qualitative ones. All significance tests will be bilateral and a p value <0.05 is considered as statistically significant. For estimates a confidence interval of 95% (CI be provided 95%). The Saphiro–Wilk test will be used to determine if the distribution of the data corresponding to each variable conforms to the normal distribution.
Comparison of intra-access pressure differences (P2-M0, P2-Basal, M0-Basal, etc.), are analyzed by using Student’s t test for paired samples there is a normal distribution. Otherwise, will use the Wilcoxon test for comparisons of related samples.
ResultsData was collected from a total of 51 hemodialysis sessions in15 patients (mean age, 71.33 ± 12.66 years, 9 females). The mean value of urea prior to the hemodialysis session was 113.41 mg/dl (range 88−180 mg/dl). The average Kt was 48.42 ± 5.70 l (range 36−60 l). A value of Kt below the optimum was observed in 2 (3.9%) sessions. The mean value of hemoglobin before hemodialysis was 10.81 ± 1.21 g/dl (range 9.60–13.20 g/dl).
There were 4 patients that did not complete the study: one was discontinued for presenting critical stenosis in the venous anastomosis and 3 patients died (one sudden death and 2 due to advanced neoplasia). The reasons for withdrawal of these 4 patients were not associated with the hemostasis process in the AVF.
Out of the 15 patients included, 2 (13.3%) had a Gore-Tex prosthetic fistula implanted, both of humerus-axillary location, and results were obtained from 9 (17.6%) of the 51 sessions evaluated. The remaining 13 (86.6%) patients had a native fistula, in which data from 42 (82.3%) sessions were collected. Of these 13 fistulas, 8 were humerus-cephalic, 3 radio-cephalic, and 2 humerus-basilic. The median age (range) of the fistulas was 592 (3,589) days.
Regarding the usual daily treatment, 6 patients were on of acetylsalicylic acid 100 mg, 2 on 100 mg of acetylsalicylic acid and 75 mg of clopidogrel, and 2 were anticoagulated with 20 mg of enoxaparin. All patients were anticoagulated with sodium enoxaparin at the beginning of the hemodialysis session, with doses between 20 and 80 mg. All patients received erythropoietin as a treatment for anemia. During the development of the study, none required transfusion of packed red blood cells. In all the sessions analyzed, an absorbable gelatin dressing (Surgispon®) was used as coadjuvant hemostatic agent to apply pressure to the puncture site, both with the forceps and with the patient’s fingers.
Of the 79 hemodialysis sessions initially included in the study, 2 (2.5%) were excluded due to the fibrin deposition in the system that prevented the correct reading of intra-access pressure and 26 (32.9%) due to movements or changes in the position of the patient that affected the reliability of the readings. Therefore, in the final analysis, the data from 51 sessions were considered. In none of them there were complications that could be associated with the hemostasia process.
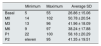
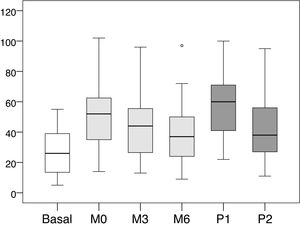
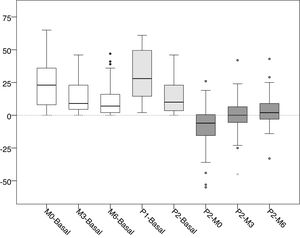
The mean values and standard deviations of the intra-access pressure in each of the readings are shown in Table 1, and the mean values of the differences between each of the readings are presented in Table 2, The distribution of these values are shown in Figs. 1 and 2.
Mean, minimum and maximum values of the intra-access pressure readings (mmHg).
| Minimum | Maximum | Average SD | |
|---|---|---|---|
| Basal | 5 | 55 | 26.86 ± 15.06 |
| M0 | 14 | 102 | 50.78 ± 20.54 |
| M3 | 13 | 96 | 41.96 ± 18.09 |
| M6 | 9 | 97 | 38.24 ± 17.88 |
| P1 | 22 | 100 | 58.18 ± 20.29 |
| P2 | eleven | 95 | 41.35 ± 19.51 |
Basal: without exerting compression on the arteriovenous fistula; M0: at the start of manual compression; M3: minute 3 of manual compression; M6: minute 6 of manual compression; Q1: when placing the clamp, without being adjustited; P2: after adjusting the compression using the adjustable system; SD: standard deviation.
Differences in the values of pressure (mmHg).
| Value | Average ± SD | Change % | 95% CI | p |
|---|---|---|---|---|
| M0-Basal | 23.92 ± 17.30 | +89.05 | 19.06 to 28.79 | <0.001 |
| M3-M0 | −8.82 ± 10.44 | −17.36 | −11.76 to −5.89 | <0.001 |
| M6-M0 | −12.55 ± 12.91 | −24.71 | −16.18 to −8.92 | <0.001 |
| P1-Basal | 31.31 ± 18.33 | +116.56 | 26.16 to 36.47 | <0.001 |
| P2-Basal | 14.49 ± 13.67 | +53.94 | 10.64 to 18.33 | <0.001 |
| P2-P1 | −16.82 ± 14.55 | −28.91 | −20.91 to −12.73 | <0.001 |
| P1-M0 | 7.39 ± 12.88 | +14.55 | 3.77 to 11.01 | <0.001 |
| P2-M0 | −9.43 ± 16.57 | −18.57 | −14.09 to −4.77 | <0.001 |
| P2-M3 | −0.61 ± 14.25 | −1.45 | −4.62 to 3.40 | 0.901 |
| P2-M6 | 3.12 ± 12.32 | +8.15 | −0.35 to 6.58 | 0.102 |
Basal: without exerting compression on the arteriovenous fistula; M0: at the start of manual compression; M3: minute 3 of manual compression; M6: minute 6 of manual compression; Q1: when placing the clamp, without adjustement; P2: after adjusting the compression using the adjustable system; SD: standard deviation; Change %: percentage of variation; 95% CI: 95% confidence interval for the difference; p: statistical significance for the difference, according to the Wilcoxon test for related samples.
Distribution of values intra-access pressure (mmHg).
Basal: without exerting compression on the arteriovenous fistula; M0: at the start of manual compression; M3: minute 3 of manual compression; M6: minute 6 of manual compression; Q1: when placing the clamp, without adjusting it; P2: after adjusting the compression using the adjustable system.
Distribution of the values of differences of the intra-access pressure (mmHg).
Basal: without exerting compression on the arteriovenous fistula; M0: at the start of manual compression; M3: minute 3 of manual compression; M6: minute 6 of manual compression; Q1: when placing the clamp, without being adjusted; P2: after adjusting the compression using the adjustable system.
The differences between basal and manual pressure are shown in white. Medium gray shows the differences between basal pressure and adjustable clamp. Differences between adjustable clamp and manual pressure are shown in dark gray.
When placing the clamp to perform hemostasis, and before being adjusted by the nurse (P1), the increase in pressure was +31.31 mmHg (+116.56 %, p < 0.001) with respect to the basal pressure. Subsequently, after being adjusted by the nurse (P2), the pressure decreased −16.82 mmHg (−28.91% compared to P1, p < 0.001). At that time (P2) represented an increase of +14.49 mmHg (+53.94%, p < 0.001) with respect to the basal pressure.
The increase in pressure when puncture point was first pressed manually (M0) was +23.92 mmHg (p < 0.001), representing an increase of +89.05% as compared to baseline intra-access pressure. From that moment, the pressure exerted showed a downward trend, with an average decrease of −8.82 mmHg (−17.36%, p < 0.001) at 3 min (M3) and −12, 55 mmHg (−24.71%, p < 0.001) at 6 min (M6).
After the clamp was adjusted by the nurse the pressure (P2) was lower than the values registered with the patient's manual pressure at minute zero (M0); the mean difference was -9.43 mmHg (−18.57%, p < 0.001). However, it was observed that these values tended to be equal with M3 (−0.61 mmHg, −1.45%, p = 0.901) and M6 (+3,12 mmHg, +8.1%, p = 0.102), with a very similar distribution of values.
None of the patients included in the study showed complications associated with the process of hemostasis within 48 h after the puncture of the fistula.
DiscussionThe results of this study show that the pressure exerted on the AVF by an adjustable compression system is equal to or less than that exerted manually by the patient. In addition, it has been shown that the manual pressure applied by the patient is not uniform over time and shows a descending pattern as the hemostasis process progresses.
Although these results may seem somewhat predictable, they needed to be evaluated since there is not objective data in the literature and, there is not information about which method of hemostasis would be more convenient. In fact, due to this lack of data, the recommendations by clinical guidelines are currently based only on the consensus of experts.
The recommendations assume that manual compression is the most appropriate and safe, since it is moderate and constant over time.2,12 However, our data reveal that manual pressure is not constant but decreases over time, possibly due to a reduction of tone or to circumstances that decrease the concentration of the patient. One of the advantages of compression devices would be that they would not be affected by these factors.
The use of compression devices in AVF has a negative consideration in the guidelines, so it is generally discouraged, or allowed with restrictions in some situations.1,2,8,13 This is based on the premise that they may exert excessive pressure on the fistula, favor the development of complications and shorten its survival. In any case, as our data suggests, this appears not to be the case with all devices. The risk of a device applying inadequate compression to achieve hemostasis (by excess or by defect) is only expected in those devices that do not allow pressure adjustment, or in adjustable devices that are not adequately supervised.
As our results show, the pressure exerted by a device that allows adjustment is similar to or less than that exerted manually by the patient. This reveals several issues: (1) that the premise of excessive pressure, assumed in clinical guidelines, would not be true in all devices, only in those that do not have a system that allows adjustment; (2) the importance of being used by trained personnel, capable of determining the appropriate compression in each patient and in each session, and (3) the need of a system that allows adjustment compression in all devices marketed for this purpose.
However, despite the usefulness of the data provided in this study, they do not allow us to know the effect of the regular use of these devices in aspects as relevant as the occurrence of complications and survival of the fistula. These are issues that must be addressed in future clinical trials, the results of which would provide the first evidence on which to base the recommendations of the clinical guidelines.
Although our results do not allow us to provide data regarding the safety of the continued use of these devices, we did observe an absence of complications in the hemostasis process. This has been the case even in patients with a more unfavorable uremic situation that has been associated with a greater risk of bleeding.14
Finally, it should be mentioned that, although the compression method is an important factor in determining the success of the hemostasis process, it also depends on a correct cannulation technique which includes, the use of the most appropriate type of needle for each patient (length, gauge, fenestration),15 the use of the most appropriate technique16 or the performance of an ultrasound-guided puncture when necessary.17 This can reduce the risk of a troublesome hemostasis process and the appearance of complications.
Our study has some limitations. Although we have used a sample size sufficient to determine the existence of pressure differences between the two methods, this study has not evaluated the safety of the regular use of adjustable devices and its effects on the lifespan of the fistulas, an issue that has been commented already. Furthermore, the small number of prosthetic fistulas included in the study did not allow us to analyze whether the results obtained would be applicable to this type of fistula. In addition, we have evaluated only one of the many compression devices on the market. Although we think that our conclusions could be extended to any adjustable device, there could be differences depending on the type of regulation system used (continuous band,18 discontinuous band,19 bandage20 or clamp21 ). We have evaluated a clamp system, that could have disadvantages as compared to other systems, such as the possibility of displacement during the hemostasis process. In this regard, it would be interesting to be able to analyze the use of compression band systems, which could be more effective in avoiding this problem.
To conclude, the results of this study have shown that the intensity of manual compression on the AVF decreases throughout the hemostasis process, and that the compression exerted by a forceps after being adjusted by the nurse is lower or similar to that applied manually. These data suggest that the premises on which some of the recommendations in the clinical guidelines are based could be imprecise, such as that the compression exerted manually is constant over time, or that all the devices exert excessive compression on the fistula. However, the role of routine use of adjustable compression devices in patient safety and fistula survival are issues that still need to be evaluated in future clinical trials.
Conflict of interestsNone.
Please cite this article as: Álvaro Cristóbal A, París Boal C, Blanco Velasco N, Matesanz Sanchidrián S, Mayoral-Peñas A, Arévalo Manso JJ, et al. Hemostasia en fístulas arteriovenosas: comparación de la presión manual con la ejercida por un dispositivo regulable. Nefrologia. 2021;41:566–572.