Introducción: La nefropatía IgM (NIgM) es una glomerulonefritis caracterizada por depósitos mesangiales difusos de inmunoglobulina M (IgM), que suele manifestarse con proteinuria en rango nefrótico y que, según algunos trabajos previos, se presenta más frecuentemente en pacientes que son resistentes o dependientes del tratamiento con esteroides. Objetivo: Realizar una descripción clínica, histológica e inmunopatológica, y evaluar la respuesta al tratamiento esteroideo de los pacientes pediátricos con diagnóstico de síndrome nefrótico y depósitos mesangiales y difusos de IgM. Métodos: Estudio descriptivo, retrospectivo, realizado en dos centros hospitalarios, donde se analizaron los registros clínicos de pacientes pediátricos con diagnóstico de NIgM y se revaluaron los cortes histológicos. Resultados: Trece niños fueron incluidos en el presente estudio. La NIgM correspondió al 5,17 % de todas las biopsias renales pediátricas. La edad de los pacientes estuvo entre 1 y 12 años (mediana: 2 años); el 46,7 % fueron mujeres. El hallazgo morfológico más frecuente fue hipercelularidad mesangial difusa (46,1 %), seguido de glomeruloesclerosis focal y segmentaria (30,8 %) y cambios glomerulares mínimos (23,1 %). Todos los pacientes recibieron esteroides, en 4 de ellos (30,7 %) como el único medicamento inmunosupresor; 3 (23,1 %) recibieron además ciclofosfamida, 5 (38,4 %) micofenolato y 1 (7,7 %) ciclosporina. Siete pacientes (53,8 %) presentaron recaídas frecuentes, 5 (38,5 %) fueron corticorresistentes y 1 (7,7 %) corticodependiente. Dos pacientes (15,38 %) presentaron alteración crónica de la función renal. Conclusión: La presencia de IgM mesangial difusa en pacientes pediátricos con síndrome nefrótico no es un hallazgo muy infrecuente, su presentación clínica se ha asociado con menor respuesta a esteroides. Sin embargo, su pronóstico a largo plazo aún no se conoce.
Introduction: IgM nephropathy (IgMN) is a glomerulonephritis characterised by diffuse mesangial immunoglobulin M (IgM) deposits. It usually presents with nephrotic range proteinuria and, according to some previous work, it occurs most often in patients who are resistant to or dependent on steroid treatment. Objective: To perform a clinical, histological and immunopathological description and assess the response to steroid treatment of paediatric patients diagnosed with nephrotic syndrome and diffuse mesangial IgM deposits. Method: This is a descriptive, retrospective study carried out in two hospitals, where the clinical records of paediatric patients with IgMN were analysed and the histological sections were re-assessed. Results: thirteen children were included in this study. IgMN corresponded to 5.17% of all paediatric renal biopsies. The age of patients ranged from 1 year to 12 years (median: 2 years), 46.7% were women. The most common morphological finding was diffuse mesangial hypercellularity (46.1%), followed by focal segmental glomerulosclerosis (30.8%) and minimal glomerular changes (23.1%). All patients received steroids; in 4 cases (30.7%) as the only immunosuppressant medication, 3 (23.1%) also received cyclophosphamide, 5 (38.4%) mycophenolate, and 1 (7.7%) cyclosporine. Seven patients (53.8%) had frequent relapses, 5 (38.5%) were cortico-resistant and 1 (7.7%) cortico-dependent. Two patients (15.38%) had chronic impairment of renal function. Conclusion: The presence of diffuse mesangial IgM in paediatric patients with nephrotic syndrome is not a very uncommon finding; its clinical presentation has been associated with lower response to steroids. However, the long-term prognosis of these patients is still unknown.
INTRODUCTION
Two articles published in 1978 by independent groups,1,2 are considered by many authors to be the first descriptions of a glomerulopathy characterised by diffuse mesangial deposits of immunoglobulin M (IgM) in patients with severe proteinuria. However, four years earlier, a work had been published that described renal biopsies with glomerular deposits predominantly of IgM, but in patients with persistent or recurrent haematuria.3 Although controversial, IgM nephropathy (IgMN) is defined by its immunopathological characteristics: the presence of IgM as the only or dominant immunoglobulin, located in the mesangium and with global and diffuse distribution.1,2,4-18 Its histologic characteristics are highly variable, from a normal glomerular appearance, as in minimal change disease (MCD) to varying degrees of mesangial hypercellularity and focal segmental glomerulosclerosis (FSGS). Most recent publications on IgMN describe its clinical presentation as being characterised by nephrotic syndrome (NS) or severe proteinuria with varying prognosis and response to steroids;1,2,5,14,19-21 few authors describe cases of isolated haematuria.3,13,18
There has been considerable debate regarding the meaning or importance of mesangial IgM deposits. Some authors consider that these deposits are an epiphenomenon of little importance in the aetiology or clinical progression20,22-24 while others view them as nephritogenic and consider IgMN to be a well-defined clinicopathological entity.1,2,4,5,13,14,17,18,21,25-28 Electron microscopy displays mesangial and paramesangial electron dense deposits, which are considered to be a confirmation of diagnosis, in spite of the fact that they are not always present in all cases of IgMN or that in other cases there are few and they are poorly defined.14,15,17
Its incidence varies, in accordance with different series, between 4.8% and 8.6% of all renal biopsies, affecting all ages and without predilection for gender.7,28-30 Some authors report a higher frequency of cortico-resistance or cortico-dependence with relatively few cases of complete remission.9,31,32 However, other authors have not found a long-term prognosis other than MCD.6,19,20,22-24,33-35
Its pathophysiology is unknown, but it has been reported that in some patients there is an increase in serum IgM immune complexes, which could be due to an abnormality in the normal function of T lymphocytes or an abnormality in the clearance of immune complexes by mesangial cells, thereby inducing mesangial activation and hypercellularity measured by IgM deposits which would lead to histological changes similar to those of MCD or FSGS.27
There are currently no precise therapeutic implications for patients with NS and single or predominant diffuse mesangial IgM deposits; however, it is important to determine the clinical and morphological characteristics of this finding to attempt to obtain clarity. Our aim was to conduct a clinicopathological description and assess the response to steroid treatment of paediatric patients diagnosed with NS and with diffuse and global mesangial IgM deposits: IgMN.
MATERIAL AND METHOD
Retrospective descriptive study. From the records of the authors’ institutions, all biopsies of paediatric patients (under 18 years of age) were reviewed between January 2005 and June 2011. We included all cases of patients with NS who were diffuse mesangial IgM positive on immunofluorescence, whether there was single or dominant positivity. The exclusion criteria were simultaneous deposits of various immunoglobins and C1q and C3 fractions of the complement, since this suggested the possibility of lupus erythematosus, the presence of other immunoglobulins or complement fraction with equal (co-dominance) or greater intensity than for IgM on immunofluorescence, and strong and diffuse deposits of IgM in glomerular capillary walls, as this suggests other immune complex mediated diseases, diagnosis of any systemic disease that would compromise the kidneys (secondary glomerulopathy) and biopsies from transplanted kidney. Patients with weak staining for C3 were included. Although the diagnosis of IgMN has historically been defined as a positive immunofluorescence for IgM with the presence of electron dense deposits, other authors have based their diagnosis solely on the presence of positive immunofluorescence for IgM.14, 15, 28.31 The latter was the criterion that we used for the diagnosis of IgMN in the study population.
For the diagnostic study in each case, renal tissue samples were divided and processed for light microscopy and immunofluorescence; of the biopsy tissue selected for conventional light microscopy, we obtained 2 micron thick sections for haematoxylin and eosin stain, Masson’s trichrome stain, periodic acid-Schiff stain and methenamine silver stain. Immunofluorescence (for IgA, IgG, IgM, C3, C1q, κ and λ) was essential for determining the presence of IgM deposits in the mesangium and ruling out other causes of NS.
Fragments selected for immunofluorescence were frozen at -24 ° C in a medium suitable for tissue preservation (OCT Tissue-Tek®, USA) and 3 micron thick sections were immediately made with cryostat for each immunoglobulin, complement fraction and light chain, these sections were fixed in acetone and frozen until the technique was carried out. For the latter, after the microscope slides were defrosted to room temperature, three washes were made with tris-buffered saline (TBS), each slide was incubated with the primary antibodies labelled with fluorescein isothiocyanate (Dako Carpinteria, USA) at a 1:20 dilution for 30 minutes, then three more washes were made with TBS and the coverslip was mounted with aqueous mounting medium: glycerine. Histological sections of all cases were reviewed to determine the percentage of overall glomerulosclerosis, the presence of segmental glomerular sclerosis and the approximate percentages of interstitial fibrosis and tubular atrophy.
All the cases included were classified as primary glomerular disease, since one of the exclusion criteria was another disease that could be the cause of glomerulopathy.
The clinical information and follow-up were recorded for analysis. All the information was obtained from the records of the Pathology Department or the Paediatric Nephrology Section of the authors’ institutions. The following information was recorded: age, sex, clinical presentation, renal function at diagnosis (by serum creatinine or creatinine clearance), disease progression time, systemic high blood pressure and subsequent progression on the basis of renal function (by serum creatinine or creatinine clearance) and 24-hour urine proteinuria.
The beginning of the disease or presentation was considered as the moment in which proteinuria was detected for the first time. Nephrotic range proteinuria was considered as values above 40mg/m2/hour; complete remission was defined as proteinuria under 4mg/m2/hour; partial remission was defined as proteinuria between 4 and 39mg/m2/hour. Haematuria was defined as the presence of >3 red blood cells per high power field on urinalysis or by the presence of red cell casts in the urine sediment. Relapse was defined as the presence of proteinuria greater than 40mg/m2/hour or three crosses in a urinary smear for three consecutive days after being in remission; cortico-resistance was defined as the presence of nephrotic proteinuria after eight weeks of treatment with steroids; cortico-dependent was when the patient had two consecutive relapses during the decrease of steroid treatment or relapse within 14 days of its discontinuation. The pharmacological treatment received was also recorded. The minimum follow-up period was six months.
The frequency data are presented as percentages; the values in which we determined dispersion measurements are presented as medians and minimum and maximum values, owing to the non-normal distribution of data.
RESULTS
Demographic and clinical data
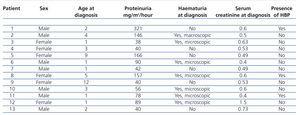
Out of a total of 251 paediatric renal biopsies during the study period, 13 biopsies (5.17%) were found with NS diagnosis and diffuse IgMN mesangial deposits. Six of the cases correspond to females (46.15%); the median age was 2 years (range: 1 to 12). All patients were diagnosed with NS, of which 7 (53.8%) were categorised as frequent relapse patients, 5 (38.4%) cortico-resistant and one (7.7%) cortico-dependent. The median level of proteinuria at diagnosis was 78mg/m2/hour (range: 38-321) (Table 1); all patients presented nephrotic range proteinuria at some time in the progression of their disease. There was haematuria in 4 of the 13 patients (30.7%), which was episodic microscopic in one of them and persistent microscopic in the three remaining patients. Two patients (15.3%) presented high levels of blood pressure at the time of diagnosis (blood pressure levels above the 95th percentile for weight and height). The level of serum creatinine at the time of diagnosis was normal in 92.3% of patients (12/13). During the follow-up, 2 patients (15.3%) presented persistently high creatinine levels in the first year following diagnosis (non-end-stage chronic renal failure).
Histological and immunopathological findings
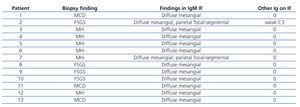
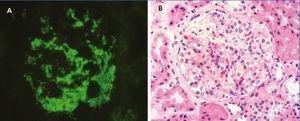
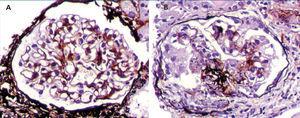
The median total number of glomeruli per biopsy was 21 (range 7-34), the most frequent glomerular morphological finding was diffuse mesangial hypercellularity: in 46.1% of patients. In 30.8%, FSGS was found and in 23.1% there were no glomerular abnormalities (minimal glomerular changes). All biopsies had diffuse mesangial positivity for IgM and in two cases there were also focal and segmental deposits of the same immunoglobulin in some capillary walls. In one case, there was weak mesangial positivity for C3 (Table 2). Tubular atrophy was found in 3 patients (23.1%), which was mild (<25%) in all of them; in the 10 remaining cases, there were no tubulointerstitial or chronic vascular abnormalities (Figure 1 and Figure 2).
Clinical progression
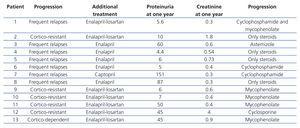
During the follow-up, at six months we found that 7 patients (53.8%) continued with nephrotic range proteinuria, 2 maintained non-nephrotic range proteinuria and in 4 patients, proteinuria had disappeared. However, at the end of the follow-up, 7 patients were classified as having NS with frequent relapses (53.8%), five were cortico-resistant (38.5%) and one was cortico-dependent (7.7%).
100% of patients received steroids and antiproteinuric medication; in 4 of them (30.7%) this was the only medication they received; 3 out of 13 (23.1%) received cyclophosphamide, 5 (38.4 %) mycophenolate, 1 astemizole (7.7%) and 1 patient (7.7%) received cyclosporine.
On comparing the findings of the renal biopsy with the progression of the patients, we found that all patients with FSGS were cortico-resistant; however, in patients with diffuse mesangial hypercellularity, 83.3% (5/6) were classified as frequent relapse patients, and 16.6%, as cortico-resistant (Table 3). With regard to the treatment used on cortico-resistant patients, 1 received cyclosporine, 3 mycophenolate and 1 only steroids and enalapril; the latter patient presented a rapid loss of renal function, which may explain the non-use of a second immunosuppressant (Table 3).
Two patients (14.3%) presented deterioration in renal function (not terminal), one had a histological finding of FSGS and another was diagnosed with diffuse mesangial hypercellularity. The three hypertensive patients continued to have high blood pressure in spite of treatment.
DISCUSSION
According to the review of databases, this is the first study carried out in our country on clinical, histological and immunopathological characteristics of children with IgMN. Out of the total renal biopsies during the study period, IgMN corresponded to 5.17%, which is similar to that which had been reported in previous studies, where prevalence varies between 4.8-7.8%.7,28-30
The characteristics of our patients suggest that IgMN usually presents with cortico-resistance or cortico-dependence. However, there is a bias inherent in the indications for carrying out a renal biopsy in paediatric patients with NS: only those who have atypical clinical features, cortico-dependence, frequent relapses or cortico-resistance are biopsied. Therefore, it is not possible to know with certainty how many patients with NS and response to treatment have diffuse mesangial IgM deposits (IgMN). However, clinical follow-ups of many years, comparing patients with a biopsy that shows MCD or FSGS without IgM deposits and patients with IgMN would allow the importance of mesangial deposits of this immunoglobulin to be determined in response to steroids and the long-term prognosis.
The contradictory results in the literature with regard to the clinical importance of IgM deposits need to be analysed with caution due to the relatively short follow-up period in many studies; most patients with MCD or FSGS who develop chronic kidney disease do so after many years. The persistent increase in serum creatinine levels in two of our cases suggests that IgM could be important in the progression of the disease. However, one of these patients presented histological disorders of FSGS, a lesion that has demonstrated that it has an adverse prognosis for renal function. Thus far, the rigorous review of literature has not allowed us to draw objective conclusions on the long term prognosis of IgM.
Zeis et al.28 compared patients with presence or not of IgM in the renal biopsy and found a greater progression to FSGS on carrying out a second renal biopsy in patients with mesangial IgM, which suggests the possibility of histological transition from MCD or mesangial hypercellularity to FSGS. The authors consider the possibility of IgM being a pathogenic factor involved in a higher degree of glomerular damage and suggest that it may be a severity marker with a higher probability of requiring a second line of immunosuppressant treatment. Other authors did not find in their work that IgMN would involve a worse and statistically significant prognosis.6,19,20,22-24,33-35
The presence of haematuria, which is an uncommon finding in patients with NS due to MCD, was common in our patients (50%), which is similar to that found in some previous studies;7,9,10,25 nevertheless, we cannot determine if mesangial IgM has a direct relationship with or is the cause of this haematuria; in fact, in the other 50% of patients with IgMN, haematuria was not found. In most series, patients present complete NS or variable proteinuria; only some authors include cases with isolated haematuria.3,13,18 In our centre, we did not find cases of patients with isolated haematuria (without proteinuria) and IgMN immunopathological characteristics.
In conclusion, this study demonstrates that the presence of diffuse mesangial IgM in patients with nephrotic syndrome is not a very uncommon finding; its clinical presentation has been associated with a lower response to steroids and more aggressive behaviour. Nevertheless, its long-term prognosis is still unknown and comparative studies with a greater follow-up period are required for clear conclusions to be obtained.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Demographic and clinical data
Table 2. Histological and immunopathological findings
Table 3. Histological findings and treatment received
Figure 1. Renal biopsy findings
Figure 2. Renal biopsy findings