Chronic kidney disease (CKD) is a real public health problem. It is estimated that by 2040, approximately one in five adults will develop CKD, and that by 2050 it will be the third cause of death in Spain. However, CKD is clearly underdiagnosed, with low screening figures in the population at risk, often delaying its identification until advanced stages of the disease, which worsens prognosis and increases associated costs. In addition, drugs are now available that have been shown to reduce the progression of CKD and the risk of developing vascular complications. In this context, the early detection, diagnosis, and initial management of CKD by health organizations should become a priority, as this would reduce the burden of the disease substantially. Since this pathology is oligo, or paucisymptomatic in the initial stages, a proactive attitude is required from the health professional to detect, diagnose, and treat it early. Several publications have shown that, to date, neither the detection of CKD nor its initial management are optimal. The IntERKit project emerged with the aim of improving the early detection, diagnosis, and initial management of CKD by promoting collaboration with health organizations through tools easily implemented by these organizations. The IntERKit project provides healthcare organizations with a structured framework to optimize early management of CKD efficiently, achieving a real, measurable, sustainable, and scalable impact, which can evolve depending on the results obtained.
La enfermedad renal crónica (ERC) constituye un auténtico problema de salud pública. Se estima que para el año 2040, aproximadamente uno de cada 5 adultos tenga ERC y para 2050 será la tercera causa de muerte en España. Sin embargo, la ERC se encuentra claramente infradiagnosticada, con cifras bajas de cribado en la población de riesgo, retrasando muchas veces su identificación a fases avanzadas de la enfermedad, lo que empeora el pronóstico de los pacientes y los costes asociados. Además, en la actualidad se dispone de fármacos que han demostrado reducir la progresión de la ERC y el riesgo de desarrollar complicaciones vasculares. En este contexto, la detección precoz, diagnóstico y manejo inicial de la ERC por parte de las organizaciones sanitarias debe convertirse en una prioridad, ya que disminuiría la carga de la enfermedad de manera sustancial. Al ser esta patología oligo o paucisintomática en estadios precoces, se exigen del profesional sanitario una actitud proactiva, para detectar, diagnosticar y tratar precozmente está patología. Diversas publicaciones han mostrado que hasta ahora tanto la detección de la ERC como su manejo inicial no es óptimo. El proyecto IntERKit surge con el objetivo de mejorar la detección precoz, diagnóstico y manejo inicial de la ERC promoviendo la colaboración con las organizaciones sanitarias, mediante herramientas fácilmente implementables por dichas organizaciones. El proyecto IntERKit proporciona a las organizaciones sanitarias un marco estructurado para optimizar el abordaje temprano de la ERC de manera eficiente, logrando un impacto real, medible, sostenible y escalable, que pueda evolucionar en función de los resultados obtenidos.
Chronic kidney disease (CKD) is a real public health problem. In fact, it is estimated that worldwide, CKD will go from being the tenth cause of death in 2022 to the fifth in 2025.1 In Spain, the ENRICA study, a population-based epidemiological study, showed a prevalence of CKD in the adult population of 15.1%.2 In the EPIRCE study, the prevalence of stage 3–5 CKD in subjects aged at least 20 years was 6.8%, a figure that rose from 3.3% in individuals aged 40–64 years to 21.4% in those over 64 years.3 In the IBERICAN study, the prevalence of CKD in patients seen in primary care clinics was 14.4% (16.1% in men and 12.9% in women).4 In a population-based study performed in Spain in 2021, the incidence of CKD was 2.1 per 1000 patient-years.5 Furthermore, as a consequence of the aging of the population and a greater prevalence of risk factors for the development of CKD, mainly arterial hypertension and diabetes,6 these figures are expected to increase in Spain, reaching a prevalence of more than 18% in the year 2040.7
The above-mentioned data are very relevant, since CKD not only increases the risk of progression to end-stage CKD and the need for renal replacement therapy, but also markedly increases the risk of presenting vascular complications, accelerated biological aging, and premature death from any cause; in addition, it worsens patient quality of life, increases disability, and leads to loss of employment..8–12 This in turn considerably increases the consumption of resources and the costs associated with CKD.13,14
Although efforts have been made at the institutional level,15 the fact is that CKD is clearly underdiagnosed,16 partly because the patient is asymptomatic until advanced stages of the disease and also because the care process is not optimized and knowledge about the importance and impact of CKD is lacking. In fact, the last National Strategic Plan for the management of CKD in the National Health System dates from 2015 and clearly requires an update.15 The strategy for the approach to chronicity was published in 2021, with mention of the limitations for CKD and its management in the autonomous communities,17 and in 2022 ten scientific societies - the Spanish Society of Nephrology (SEN), Spanish Society of Hypertension- Spanish Leage against Arterial Hypertension (SEH-LELHA), Spanish Society of Internal Medicine (SEMI), Spanish Society of Endocrinology and Nutrition (SEEN), Spanish Society of Cardiology (SEC), Spanish Society of General and Family Physicians (SEMG), Spanish Society of Primary Care Physicians (SEMERGEN), Spanish Society of Laboratory Medicine (SEQC(ML)), Spanish Society of Family and Community Medicine (SEMFyC), and Spanish Society of Diabetes (SED) - signed an important document on the detection and management of CKD,10 which was an update of two previous versions of the document. However, the reality is that its dissemination and implementation in clinical practice in the different Spanish autonomous communities has been limited,18 even after the document was expanded in 2023 with an additional document emphasizing the key role of measuring albuminuria in the early diagnosis of CKD and in the prevention of cardiovascular disease, with the support of 14 scientific societies.19
Consequently, a significant percentage of patients with CKD, especially in its earliest stages, are not receiving adequate treatment to reduce the progression of CKD.5 This is particularly important, since there are currently treatments that in specific clinical trials have been shown to reduce not only the progression toward end-stage CKD, but also the risk of presenting major vascular complications. These treatments comprise mainly renin angiotensin system blockers,20,21 sodium-glucose cotransporter type 2 inhibitors,22,23 some glucagon-like peptide receptor type 124 agonists, and, in patients with CKD associated with diabetes type 2, finerenone, a nonsteroidal mineralocorticoid receptor antagonist.25,26
IntERCede projectThe early detection and management of CKD are fundamental in this pathology, representing a top health priority in Spain. Consequently, specific strategies should be developed to reduce the risk of progression of CKD to more advanced stages, and the development of vascular complications in order to mitigate the burden of the disease and the associated costs. In this context, the IntERCede project arises as a multidisciplinary program that seeks to describe and understand the existing care model in patients with CKD, identifying areas for improvement to optimize the care provided to these patients, focusing on primary and secondary prevention of CKD through the development of specific strategies for prevention, early detection, and delay of disease progression. Specifically, the main objectives include the identification of improvements and optimization of the care process, the identification of gaps in care, the improvement and personalization of services, and the development of a holistic vision of the needs of the patient as a person, with primary care being the fundamental support on which CKD patient care is based, especially in terms of early detection, the approach to progression factors, and the management of the first stages of the disease.27
Within this project, a multidisciplinary consensus was recently published on the challenges and key factors for an optimal model of care in CKD. This work was carried out by a multidisciplinary panel of professionals with experience in the field of CKD, made up of an advisory committee of 15 experts and an additional panel of 44 specialists. In order to be able to optimize the model of care for the CKD patient, the panel identified key factors based on four aspects: 1) development of CKD management models and increased visibility of the disease; 2) prevention, screening, early diagnosis and registration of CKD; 3) comprehensive, multidisciplinary, and coordinated follow-up to achieve a greater optimization of treatment and improved continuity of care; and 4) intensification of training in CKD for both health professionals and patients.28
Among these key factors, and considered a high priority, are the early detection, diagnosis, and initial management of CKD by healthcare organizations. The IntERKit project was born with this objective in mind. This manuscript focuses on presenting the methodology of the IntERKit project, describing the basis and conceptual framework of the project, providing a solid foundation to serve as a first step for its dissemination and development.
IntERKit projectDespite national and international recommendations on the adequate detection of CKD and the ease of performing the necessary diagnostic tests, the reality is that CKD screening is not performed in up to 50% of patients at high risk of developing CKD.7,9,16,29 This has very relevant consequences, since the lack of early detection of CKD delays treatment, worsening the prognosis and increasing health care costs.5,8 In Spain, there are several reasons for this, including care overload, which is particularly important in primary care, a lack of real awareness of the importance of CKD among health professionals, administrative institutions, and the general population, the lack of coordination between different levels of care, the lack of specific symptoms of CKD until the most advanced stages of the disease, the lack of order in the demand (generating order in the existing demand will achieve greater efficiency and effectiveness), and the lack of a proactive work culture.27,28 Consequently, early detection of CKD should be a priority strategy within the national health system, since it offers important advantages at multiple levels (Table 1).7,30–32
Advantages of early detection of chronic kidney disease.
| • Prevention/delay of kidney disease progression, delaying or reducing the need for kidney replacement therapy |
| • Reduction in associated vascular and non-vascular complications |
| • Improvement in patient quality of life |
| • Patient education and empowerment |
| • Reduction in associated costs (direct and indirect) |
| • Optimization of the use of healthcare resources |
Source: table prepared with data from references.7,30–32
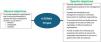
IntERKit is an initiative that forms part of the IntERCede project, which aims to improve early detection, diagnosis, and initial management of CKD by promoting collaboration with healthcare organizations through tools that can be easily implemented by these organizations. In other words, IntERKit aims to provide healthcare organizations with a structured framework to optimize the early approach to CKD in an efficient manner, achieving a real, measurable, and sustainable impact, which can evolve depending on the changes and improvements made in accordance with specific indicators and which are scalable, facilitating the replication and extension of the model throughout the Spanish healthcare system (Fig. 1).
To achieve these objectives, various tools are applied to improve diagnosis based on the action taken by primary care and hospitals, since areas for improvement are being detected, specific tools are then provided that can be implemented, together with a monitoring system and standardized clinical practices for the management of CKD. However, given that IntERKit addresses the early management of CKD, it is expected that primary care will be the central focus of the interventions to be carried out, with health centers constituting the specific framework for implementation. In CKD, the role of primary care is fundamental in terms of health promotion, risk detection and, of course, screening tests for early diagnosis. Additionally, IntERKit is expected to have a direct impact on the entire healthcare process, since the project has been developed so that its implementation is led by the directors of healthcare organizations and involves not only professionals from primary care but also from hospitals.
In order to obtain the tools resulting from the IntERKit project, a multidisciplinary scientific committee was formed, including national experts in CKD care and executive managers, both in primary care and hospital care, as well as representatives of other key agents who are also involved, directly or indirectly, in the optimal development of the process of detection, diagnosis, and early treatment of CKD, such as laboratory and information systems professionals. This scientific committee comprises specialists in family and community medicine, nephrology, cardiology, endocrinology, clinical analysis, family and community specialist nursing and primary care pharmacy, health managers, experts in digital health and representatives of patient associations. Based on different meetings of the scientific committee and personal interviews, a consensus was reached on the critical points and areas for improvement in the development of the project, as well as the generation of the different tools (toolkit) for the effective implementation of IntERKit.
In addition, the IntERKit project has the scientific endorsement of the main scientific and management societies, namely, SEN, SEMERGEN, SEMG, SEMFYC, SEC, SEEN, SEMI, SEQC-ML, the Spanish Society of Primary Care Managers (SEDAP), the Network of Diabetes Study Groups in Primary Health Care (redGDPS), Spanish Society of Health Managers (SEDISA), and the Spanish Society of Health Informatics (SEIS), as well as patient associations (ALCER Federation).
In short, the IntERKit project has resulted in the design of a set of tools that make it easier for healthcare organizations to address the areas identified for improvement. These areas mainly include:
- 1
Increasing awareness of the impact of CKD among healthcare professionals.
- 2
Improving the knowledge of healthcare professionals about CKD, including the criteria for diagnosis, classification, and treatment.
- 3
Inclusion of renal function tests in routine clinical analyses, to facilitate early detection of the disease.
- 4
Automatic generation of alert systems that notify health professionals early and effectively about the presence of CKD.
- 5
Translation of abnormal laboratory results into the diagnostic registry and specific actions in the electronic medical record.
- 6
Development of the role of nursing in early detection, initial management, and patient education about CKD.
The tools designed to act on the areas of improvement indicated above can be classified into three groups: implementation tools, evaluation tools, and support tools for the development of action plans (Table 2).
- -
Implementation tools. Their purpose is to itemize the necessary requirements, the organization, and the actions to be developed for adequate deployment of the toolkit in healthcare organizations. These tools are:
- o
The user guide, which constitutes the fundamental implementation tool of IntERKit and contains the presentation of the toolkit. The user guide describes the tools that are included, explains the benefits of its implementation and the requirements that healthcare organizations must meet for the utilisation of the toolkit, and defines its implementation model.
- o
The IntERKit implementation model, which is based on the development of two groups made up of professionals from the healthcare organization: the Management Committee, responsible for leadership and global approach, and the Implementation Team.
- o
- -
Evaluation tools. These are essential to define both the baseline situation and assess the impact and effectiveness of the interventions. Two evaluation tools have been defined:
- o
Scorecard: includes a set of 9 easily used indicators from primary care information systems based on available and accessible data, as well as the formulas for their measurement and the expected temporal trend.
- o
Self-diagnosis questionnaire on the initial approach to the detection, diagnosis, and early treatment of CKD, enabling the identification of areas for improvement, as well as the automatic generation of recommendations related to the areas identified as having the greatest potential for development (Fig. 2).
- o
- -
Support tools for the development of action plans. They are designed to organize and guide the implementation of specific strategies aimed at improving the detection, diagnosis, and early treatment of CKD. These tools include:
- o
Clinical follow-up model, which provides a detailed sequence of actions that healthcare professionals should carry out to optimize both the early detection of CKD and the management of the disease in its early stages.
- o
Tools for intervening in specific areas of improvement, which include a variety of resources designed to improve early detection, diagnosis, and treatment of CKD.
- o
Action plan template, which is a tool designed to facilitate planning, follow-up, and implementation in the different areas of improvement identified (Fig. 3).
- o
The IntERKit implementation model comprises five development phases (Fig. 4):
- 1
Project launch.
- 2
Diagnosis of the initial situation, using the assessment tools, scorecard, and self-diagnosis questionnaire, which enable each organization to identify the starting point, both quantitatively and qualitatively, and areas for improvement.
- 3
Definition and implementation of the action plan, with concrete solutions to improve the early diagnosis of CKD. For this purpose, an action plan template is used. This is a tool designed to facilitate the planning, monitoring, and implementation of action plans to address identified areas for improvement, including actions to be developed in specific areas, necessary resources, responsibilities, deadlines, and monitoring indicators.
- 4
Follow-up and monitoring in the development of the action plan, through periodic meetings of the implementation team, who evaluate how the action plan is being developed. With this information and the indicators, the Steering Committee updates the action plan on an ongoing basis.
- 5
Evaluation of the implementation and scalability through critical indicators. These will be analyzed to determine who successful implementation has been, making it possible to identify new areas for improvement and enable action adjustments and modifications.
After an initial implementation period of 14 months, the process is restarted from phase 3 in such a way as to promote the continuous improvement of care for people with CKD through the development of successive action plans, fostering the long-term sustainability of the results achieved.
From a practical point of view, steps are being taken to contact different health organizations at the national level to explain in detail the IntERKit project and enable its implementation. Subsequently, an analysis will be carried out to evaluate the effects of the implementation, and the results will be published.
ConclusionsIntERKit provides a simple, understandable, and accessible implementation model that facilitates early, sustainable, and scalable diagnosis of CKD in Spain, through the implementation of specific plans to improve diagnosis in healthcare organizations, with a special focus on primary care.
CRediT authorship contribution statementThe authors meet the criteria for authorship recommended by the International Committee of Medical Journal Editors (ICMJE). All authors have contributed significantly to the work presented in this article, contributing in the conception, design or acquisition of information, or in the analysis and interpretation of data. All authors have participated in the drafting and/or revision of the manuscript and agree to its publication.
FundingThe intERKit project was funded by Boehringer Ingelheim.
The intERKit project was developed with the consulting firm CROWE, funded by Boehringer Ingelheim.
Content Ed Net provided medical writing assistance and editorial assistance in the preparation of this manuscript, working with the authors and funded by Boehringer Ingelheim. Boehringer Ingelheim had the opportunity to review the manuscript for medical and scientific accuracy, as it relates to Boehringer Ingelheim drugs and involves considerations of intellectual property.
AO's research activity on the topic is funded by Comunidad de Madrid en BiomedicinaP2022/BMD-7223, CIFRA_COR-CM. Instituto de Salud Carlos III (ISCIII) RICORS program to RICORS2040 (RD21/0005/0001) co-funded by European Union and SPACKDcPMP21/00109, FEDER funds, COST Action PERMEDIKCA21165, supported by COST (European Cooperation in Science and Technology); PREVENTCKD Consortium Project ID: 101101220 Programme: EU4H DG/Agency: HADEA. KitNewCare, Project ID: 101137054, Call: HORIZON-HLTH-2023-CARE-04, Programme: HORIZON. DG/Agency: HADEA.
The authors have not received honoraria related to the present manuscript.
- José Luis Górriz declares funding in relation to clinical trials from AstraZeneca, Bayer, Boehringer Ingelheim, and Novo Nordisk (all to the INCLIVA Research Institute); consulting fees from AstraZeneca, Bayer, Boehringer-Ingelheim, and Novo Nordisk; payment of conference fees from AstraZeneca, Novo Nordisk, Bayer, Menarini, Boehringer-Ingelheim, and Eli Lilly.
- Fran Adán Gil declares no conflicts of interest.
- Manuel A. Botana López has received consulting fees from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Esteve, Roche, Sanofi; fees for presentations, papers, or reports from AstraZeneca, Almirall, Boehringer Ingelheim, Daiichi-Sankyo, Novo Nordisk, Novartis, Lilly, Sanofi, Servier; invitations to congresses, courses, or educational events organized by the pharmaceutical industry, with assistance from AstraZeneca, Boehringer Ingelheim, Lilly, Novo Nordisk, Novartis, Menarini and Sanofi.
- Antonio Buño Soto declares no conflict of interest.
- Francisco José Campos Cabrera has participated in consulting work for Boehringer-Ingelheim.
- Ángeles Cisneros declares no conflicts of interest.
- Silvia Cobo Guerrero has received fees for conferences from AstraZeneca, GlaxoSmithKline, and Chiesi; for attending symposia, conferences, and training activities for Boehringer-Ingelheim, AstraZeneca, GlaxoSmithKline, Lundbeck, and Chiesi. The author has participated in consensus documents with the support of Boehringer-Ingelheim and AstraZeneca without receiving fees.
- María Dolores Conejos declares funded attendance at congresses, conferences, and training activities from Boehringer-Ingelheim and Novo Nordisk; fees for participation in congress round tables from Boehringer-Ingelheim.
- Isabel Egocheaga Cabello declares having received consulting fees from Bayer, Boehringer-Ingelheim, and Viatris; fees for training activities from AstraZeneca, Rovi, Boehringer-Ingelheim, and Eli Lilly.
- M. Concepción Fernández Planelles declares no conflicts of interest.
- Lisardo García-Matarín has received honoraria for conferences from AstraZeneca, Boehringer-Ingelheim, Lilly, Chiesi, GlaxoSmithKline, Bial, Menarini, Teva, Novartis, Pfizer, Sanofi Aventis, FAES, Novo Nordisk, and Vifor Pharma. He has served on advisory boards for Boehringer-Ingelheim, AstraZeneca, Daiichi Sankyo, and GlaxoSmithKline.
- Natalia Jiménez is an employee of Boehringer Ingelheim Spain.
- Juan Carlos Julián Mauro states that his organization (ALCER) receives grants that are not related to this project for its participation in advisory boards or for social and health education projects from the following companies: Astellas, AstraZeneca, Baxter, BMS, Boehringer-Ingelheim, CSL Vifor, Diaverum, Fresenius, GSK, Hansa Biopharma, Ipsen, MSD, Novartis, Novonordisk, and Pfizer.
- David León Jiménez has received speaker fees from AstraZeneca Pharmaceuticals LP, Boehringer Ingelheim Pharmaceuticals Inc., Eli Lilly and Company, Janssen Pharmaceuticals Inc., Mundipharma, Sanofi, Esteve, Novo Nordisk, Mylan (Viatris), Bayer, Novartis, Rovi and Amgen. He has also participated on advisory boards for Sanofi, Boehringer Ingelheim Pharmaceuticals Inc., and Eli Lilly and Company.
- Daniel Martínez Gamote has received consulting fees from Boehringer-Ingelheim.
- Pilar Mazón Ramos reports participation in conferences/consultancy for AZ, Esteve, Boehringer-Ingelheim, Bayer, BMS-Pfizer, Daichii-Sankyo, Servier, Ferrer, and Novo-Nordisk.
- Alberto Ortiz has received grants from Sanofi. He has received consulting fees, speaking fees, and travel support from Adviccene, Alexion, Astellas, AstraZeneca, Amicus, Amgen, Bioporto, Boehringer Ingelheim, Esteve, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Sobi, Menarini, Mundipharma, Kyowa Kirin, Lilly, Freeline, Idorsia, Chiesi, Otsuka, Novo-Nordisk, Sysmex, and Vifor Fresenius Medical Care, Renal Pharma, and Spafarma, and is Director of the UAM-AstraZeneca Chair in Chronic Kidney Disease and Electrolytes. He owns shares in Telara Farma.
- Gemma Palau is an employee of Boehringer Ingelheim Spain.
- Julia Quevedo Rivera participated in the consensus document on behalf of Boehringer-Ingelheim.
- Emilio Sánchez-Álvarez declares no conflicts of interest.
- Rita Tristancho Ajamilha participated in the consensus document on behalf of Boehringer-Ingelheim.
- Cristina Varga declares no conflicts of interest.
- Roser Vallés Fernández reports funding to attend conferences, workshops, and training activities from Pfizer, Daiichi-Sankyo, and GSK; speaker fees from GSK; and participation in the consensus document on behalf of Boehringer-Ingelheim.