To determine the incidence of metformin-induced lactic acidosis during the period January 2014 to March 2017 in Aragon Healthcare Area III. To analyze the associated clinical and analytical factors and mortality.
ResultsA total of 31 cases (61.3% males). Incidence: 79.76 cases/100,000 patients-year; mean age 75.39±9.34 years; 23 of them with levels of serum metformin (21.91±15.52mcg/ml); milligrams/day of metformin ingested: 1790.32±499; 96.8% of cases in the context of acute kidney failure; 11 cases with a history of chronic kidney disease (35.5%); 12 required intensive care (38.7%); 13 required purification treatment (41.9%; 3 hemodiafiltration, 10 hemodialysis). There was a significant correlation between daily milligrams of metformin ingested and drug levels; levels of metformin; and peak creatinine, pH and lactate. Mortality was 25.8%. There were only significant differences between the deceased and survivors regarding the duration of stay and final creatinine. Multivariate logistic regression did not detect any study variables associated with mortality.
ConclusionsThe incidence in our healthcare area is higher than in other series, with a 25.8% mortality rate. Virtually all cases were in the context of prerenal acute kidney failure. In 29% of cases, there was an overdose. Patients must be warned about the most common lactic acidosis-inducing situations, especially dehydration, if they continue taking the drug at such times.
Conocer la incidencia de casos de acidosis láctica por metformina durante el periodo enero de 2014 y marzo de 2017 en el Área Sanitaria III de Aragón. Analizar los factores clínicos y analíticos asociados y la mortalidad.
ResultadosTreinta y un casos (61,3% varones). Incidencia: 79,76 casos/100000 pacientes-año; edad media 75,39±9,34 años; 23 de ellos con niveles séricos de metformina (21,91±15,52 mcg/mL); miligramos/día de metformina ingeridos: 1790,32±499; 96,8% de casos en el contexto de fracaso renal agudo; 11 casos con antecedentes de enfermedad renal crónica (35,5%); 12 requirieron UCI (38,7%); 13 requirieron tratamiento depurador (41,9%; 3 hemodiafiltración, 10 hemodiálisis) Existió correlación significativa entre: miligramos diarios ingeridos de metformina y niveles del fármaco; niveles de metformina y: creatinina pico, pH y lactato. La mortalidad fue del 25,8%. Solo hubo diferencias significativas entre los fallecidos y los supervivientes respecto a la duración de la estancia y la creatinina final. La regresión logística multivariante no detectó ninguna variable del estudio asociada con la mortalidad.
ConclusionesLa incidencia en nuestra área sanitaria es más elevada que en otras series, con 25,8% de mortalidad. Prácticamente todos los casos en el contexto de fracaso renal agudo de origen prerrenal. En un 29% de los casos hubo sobredosificación. Es necesario advertir a los pacientes de las situaciones más frecuentes potencialmente inductoras de acidosis láctica, especialmente la deshidratación, si siguen tomando el fármaco durante las mismas.
Metformin is currently one of the most prescribed drugs and everything suggests that in the future it will continue to be so.1 Thus it is particularly important to learn about the adverse effects of this drug. The possibility of inducing lactic acidosis stands out due to its severity, although with metformin this complication occurs to a lesser extent than with other biguanides such as butformin and phenformin, being removed from the market because this problem.2 According to some series, the mortality of this complication is very high (25–50%).2,3
However, the true incidence and practical significance of metformin associated lactic acidosis (MALA) is being debated in the medical literature. The cause–effect relationship between metformin and lactic acidosis has been questioned.4,5
In the present study we intend to analyze what has been the incidence of MALA in recent years in our health area. We have also performed a descriptive analysis of our affected patients and the clinical and biochemical variables associated to an unfavorable outcome.
Material and methodsThe study was preformed in the Health Area III of the Aragón Region which attends a population of 306,200 inhabitants. The reference hospital is Lozano Blesa University Clinical Hospital of Zaragoza (HCULB). Study period: from January 2014 to March 2017.
Source of data: The Toxicology Service of the HCULB, which exclusively perform determinations of metformin levels in our health area, provided us with the list of the 23 patients who during the study period had metformin levels above 5mcg/ml (therapeutic values: 1–2mcg/ml), a limit above which it is considered toxic. In these patients it was also found that they met the analytical criteria of lactic acidosis: pH≤7.35 with lactate level ≥5mmol/l. The Clinical Documentation Service of the HCULB provided us with an additional list of twelve diabetic patients taking metformin with discharge diagnostic codes compatible with lactic acidosis; however in these cases the drug levels had not been determined. After reviewing the medical records of these patients, it was found that five of them did not strictly fullfill the analytical criteria of MALA (pH≤7.35 and lactate level ≥5mmol/l), so only seven out of the twelve patients were included in the study. The HCULB Pharmacy Service provided us with the number of diabetic patients receiving metformin treatment at two time points of the study period, June 2014 (10,636 patients) and January 2017 (12,510 patients). The arithmetic mean of the two values (11,573) was used to perform the calculations of incidence, expressed as cases/100,000 patients-year, with a total sample of 30 cases of MALA.
It should be noted that shortly after completion of the observational period, an additional case was registered in the Nephrology Service, although it did not compute, it was included for the analysis of the clinical and analytical variables because the patient showed the highest levels of the active medication in the whole series (74.04mcg/ml).
The levels of metformin were determined by the liquid chromatography-tandem method (high-performance liquid chromatography coupled with triple-quad tandem mass spectrometry) Agilent Technologies®.
The clinical records of all patients included in the study were reviewed and the following clinical and analytical variables were collected: gender, age, Charlson comorbidity index, metformin daily intake (mg), metformin formulation (alone or in combination), number of active ingredients taken daily, clinical features at admission, admission service, presence of Acute Kidney Failure (AKF), stage of AKF, etiology of AKF, baseline creatinine, baseline glomerular filtration rate (GFR) estimated by the CKD-EPI formula, peak of serum creatinine level during admission, serum creatinine at discharge, days of hospital stay, need for admission to Intensive Care Unit (ICU), need for renal replacement therapy (RRT), type of RRT (hemodialysis [HD]/continuous venovenous hemofiltration), number of sessions of RRT, pH, lactate, bicarbonate, anion gap, pCO2, prothrombin activity, potassium, hemoglobin, plasma levels of metformin, final result of the admission (deceased/not deceased), cause of death, evidence of metformin overdose according to recommendations of the Spanish Agency of Medicines and Health Products6: GFR <30ml/min, metformine is contraindicated; GFR between 30 and 44ml/min, maximum dose of 1000mg/day; GFR between 45 and 59, maximum dose 2000mg/day, GFR between 60 and 89, maximum dose 3000mg/day.
Statistical analysis: Nonparametric Mann Whitney test was used to compare the variables of deceased vs survivors. Categorical variables were analyzed by the Chi square test; if the conditions required for the use of this test were not met, it was replaced by Fisher's exact test. Spearman coefficient was used to analyze correlation between quantitative variables. Finally, a multivariate binary logistic regression analysis was performed to select the variables associated with mortality.
ResultsThe incidence of MALA in our health area during the study period was 79.76 cases per 100,000 patient-year (61.15 cases per 100,000 patients per year if we only consider cases in which metformin serum levels were available).
Our patients were 19 males (61.3%) and 12 females (37.8%). Mean age was 75.39±934 years; males were 75.58±8.7 and females 75.08±10.71 showing no significant difference in age. Mean Charlson index was 6.77±11.71 (males 6.84±11.5, women 6.67±22.1, again without statistical difference). Number of active drugs taken per day 10.19±4.33 (males 9.58±4.1, women 11.17±4.7, no significant difference). Analysis of active ingredients prescribed show that 23 patients were taking an ACEI or ARA II, 22 were on diuretics, 20 on statins, 9 patients took NSAIDs, 5 patients were taking ACEI/ARA II together with diuretic and NSAIDs. A 58% of patients were on metformin alone and the remaining 42% were taking metformin in combination with another oral antidiabetic drug. The most frequent dose of metformin was 850mg every 12h in 10 patients. Six patients also received insulin. The average daily dose of the drug was 1790±499mg. The most frequent clinical condition that led to the consultation was nausea, vomiting or diarrhea in 15 cases, followed by far from chest pain, fever, heart failure or hypoglycemia. These symptoms motivated the consultation in two cases. The rest of the patients consulted for other reasons. Twelve cases were attended in the Nephrology Service, and 11 by the Internal Medicine Service. The rest of patients were admitted in other services: endocrinology, cardiology, psychiatry, general and the gastroenterology surgery received one case. Another patient was treated only in the Emergency Room where he died and finally two patients were admitted directly to the ICU, where they also died. The average duration of hospitalization was 11.23±7.7 days.
Regarding the baseline renal function, the majority, 20 cases (64.5%), had no history of chronic kidney disease (CKD), with abaseline GFR >60ml/min. Three patients presented stage 3a (GFR between 60 and 45ml/min), 6 patients had stage 3b (GFR between 44 and 30ml/min) and finally 2 patients had stage 4 (FG <30). Metformin was overdosed in 9 patients (29% of the patients); two in stage 4 in which metformin was contraindicated, 6 cases in stage 3b and one case in stage 3a.
There was only one case of voluntary overdose and it was the one without deterioration of renal function. All other patients presented an episode of AKF, in 3 cases of parenchymal origin and in 27 it was prerenal. In 25 cases (83.3%) the AKF reached stage III according to the KDIGO classification, in three cases it reached stage II and in the two remained in stages I.
A total of twelve patients (38.7%) required admission to the ICU, and 19 cases (61.3%) did not require it. In 13 cases (41.9%) it was necessary to use RRT and 18 cases (58.1%) did not need it. Regarding the type of RRT, HD was used in 10 cases (76.9%), the number of sessions was 2.3±1.1 (range 1–5). Continuous venovenous hemofiltration was used in 3 cases (23.1%), and the number of sessions were 2.33±1.53 (range 1–4). The duration of the HD sessions performed by the Nephrology Department was 4h. The duration of continuous venovenous hemofiltration sessions in the ICU was around 6h.
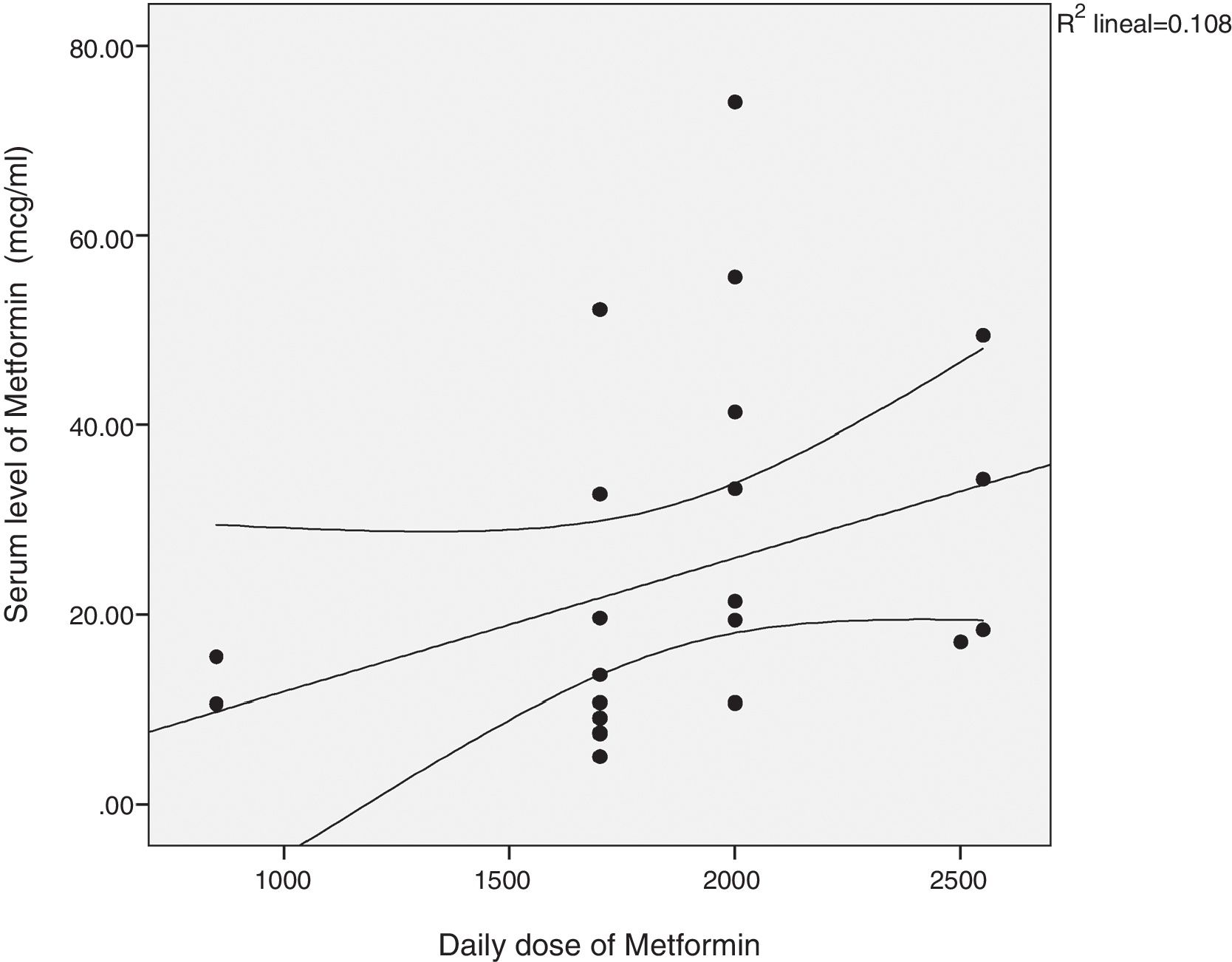
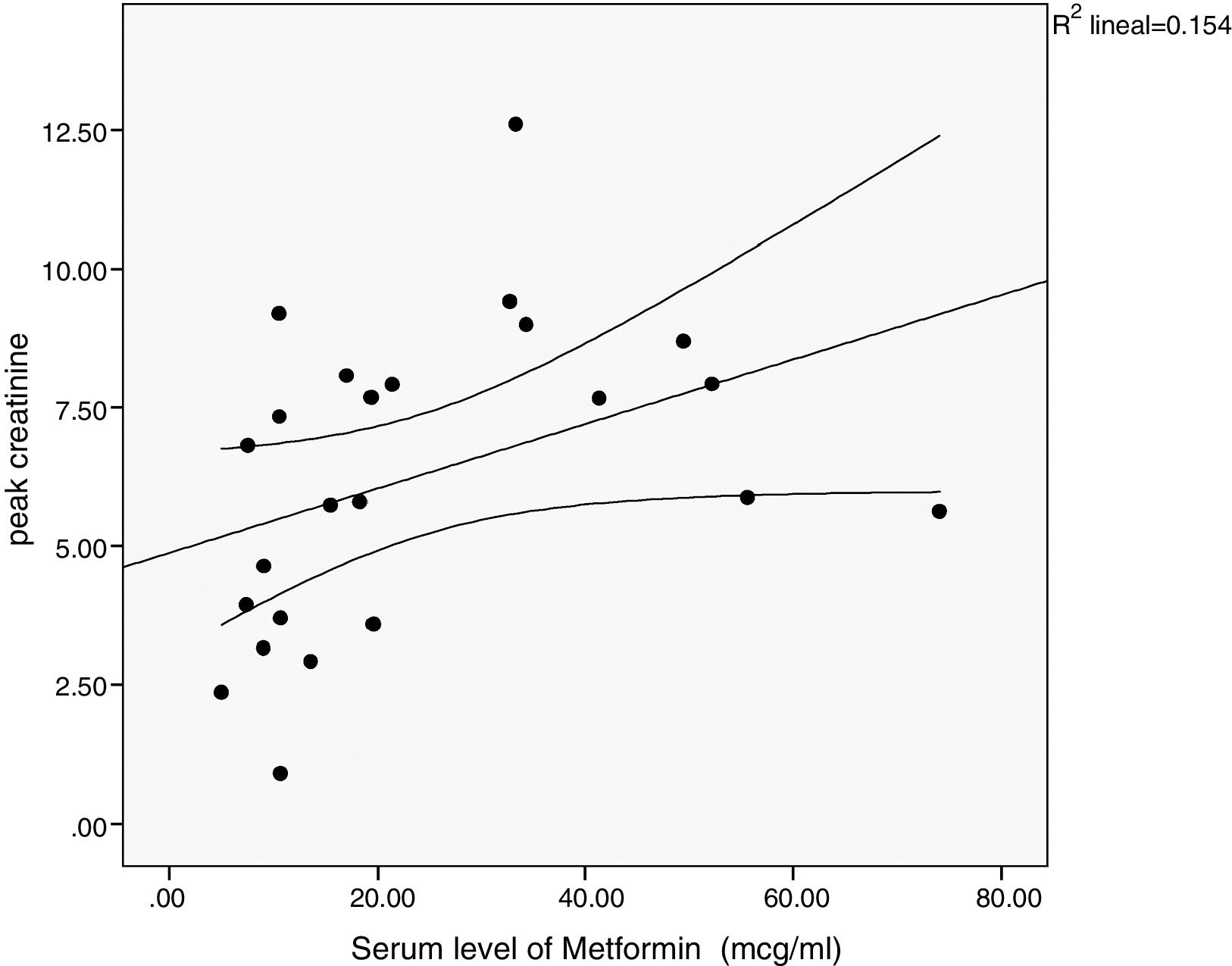
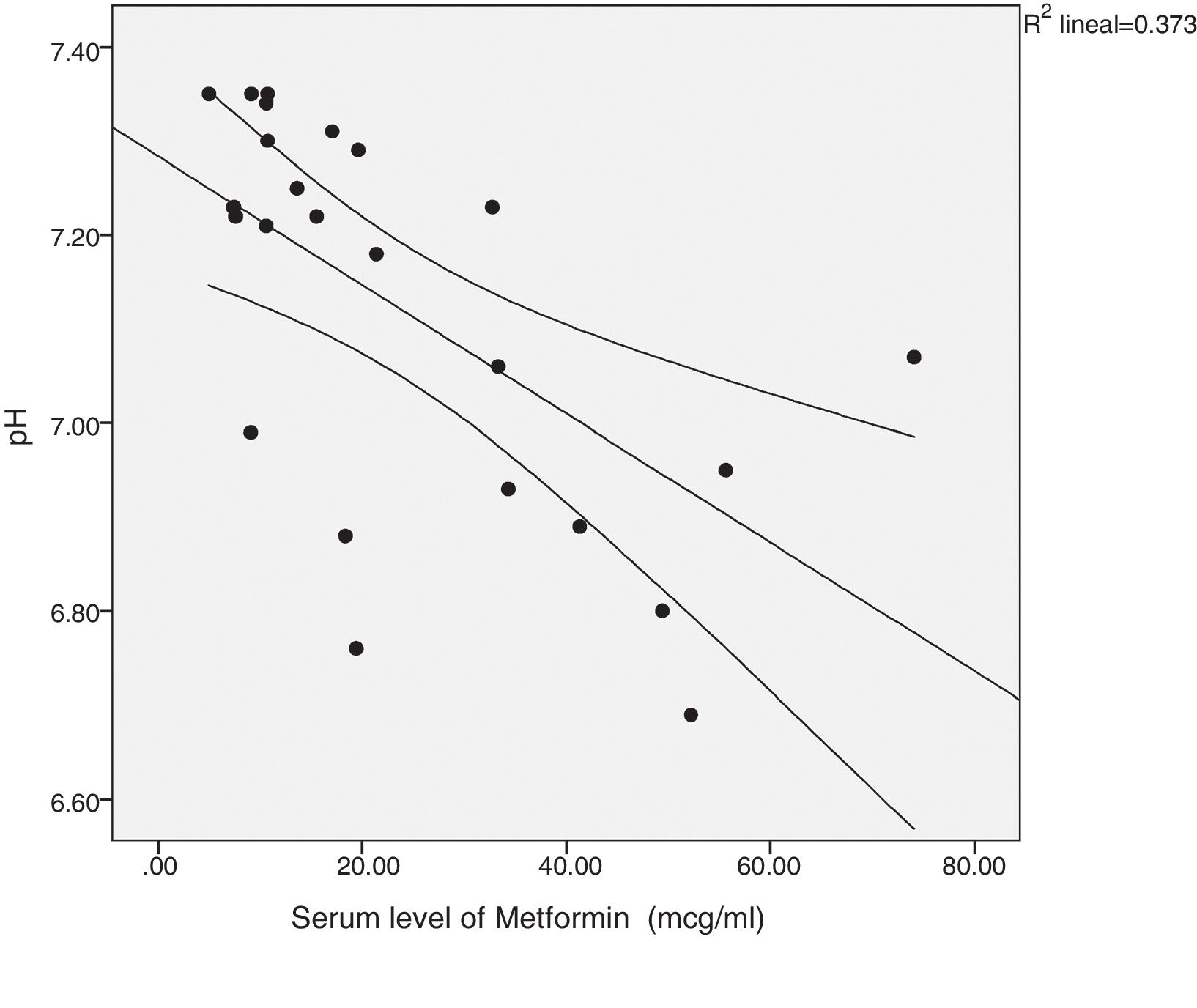
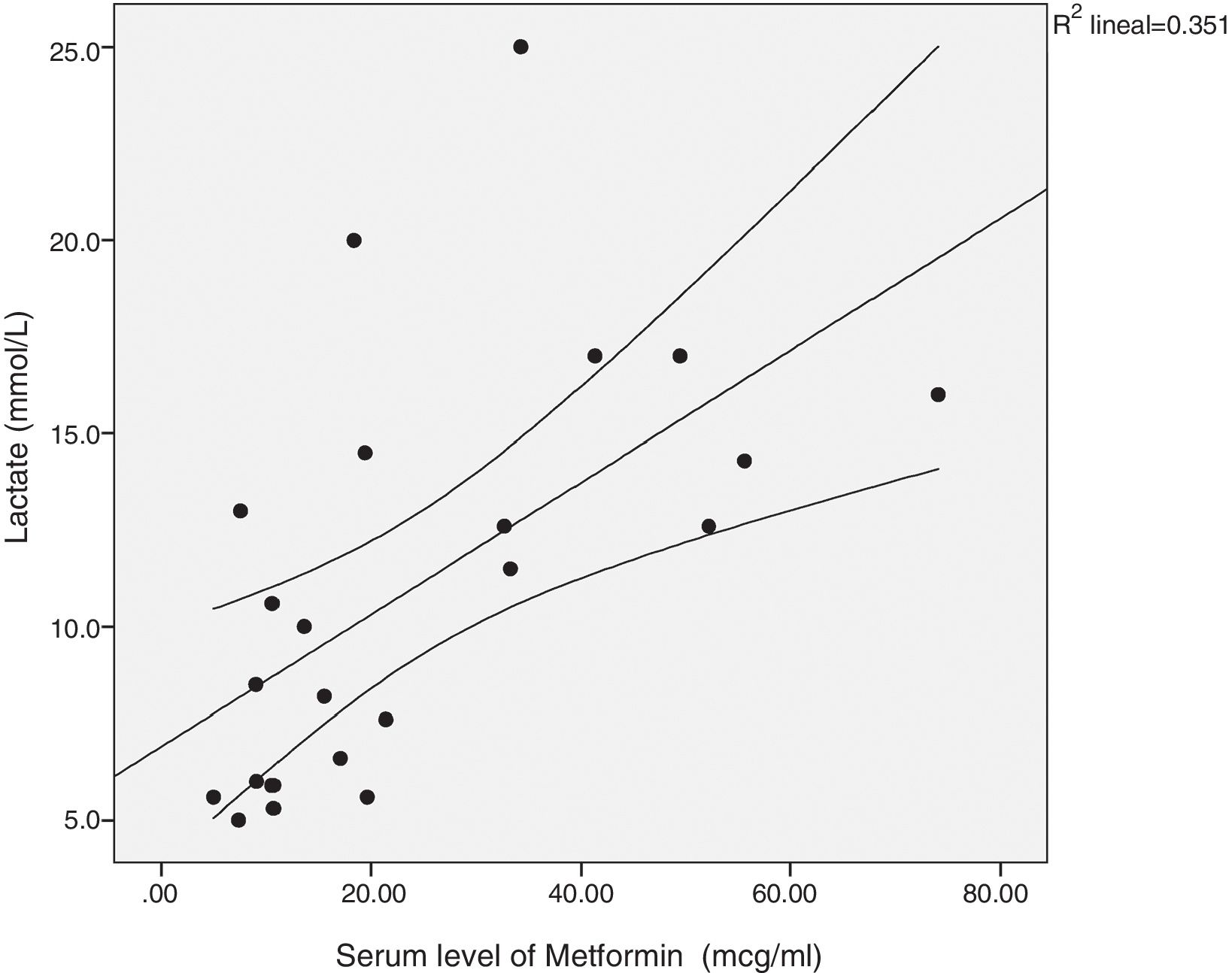
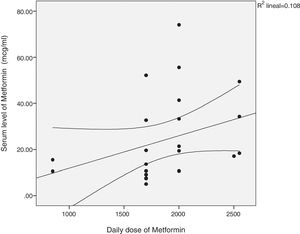
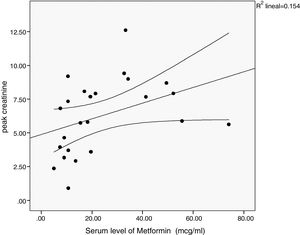
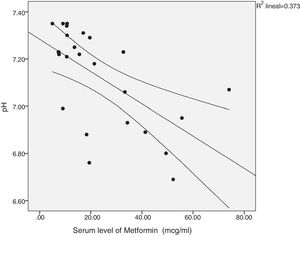
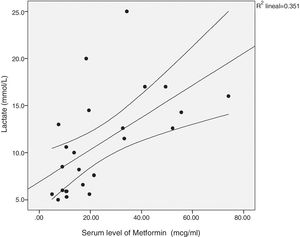
There was a significant correlation between the daily dose of metformin and the serum levels of the drug (Fig. 1). Correlation was also found between the levels of metformin and three other variables: peak creatinine, pH and serum lactate concentration (Figs. 2–4). Likewise, a correlation was detected between the Charlson comorbidity index and the number of active drugs taken, as well as between the pH and the serum potassium level.
A total of 8 patients (25.8%) died during admission, compared to 23 (74.19%) who survived. Three patients died in the ICU, one of them due to asistolia refractory to resuscitation maneuvers, the second as a consequence of refractory shock and multi-organ dysfunction and the third due to sepsis. Another patient died in the emergency department due to cardiorespiratory arrest without response to resuscitation maneuvers, an underlying mesenteric ischemia was suspected. The rest of deaths occurred in the hospital ward. One of them due to palliative sedation in the context of disseminated neoplasia, another case due to acute pulmonary edema with respiratory failure, the third presented heart and respiratory failure with poor outcome and the last patient presented a bilateral pneumonia with unfavorable evolution leading to sepsis and death.
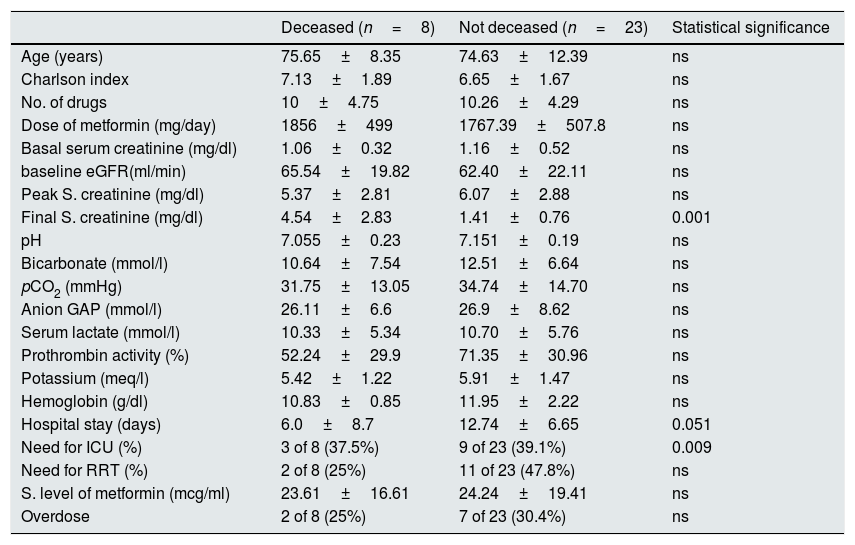
Table 1 shows the comparison between the clinical and analytical variables in patients deceased versus the survivors. The only significant differences were the final serum creatinine level and the length of hospital stay. None of the variables was independently associated with mortality in the multivariate logistic regression analysis.
Comparison of various variables in the group of deceased patients and survivors.
| Deceased (n=8) | Not deceased (n=23) | Statistical significance | |
|---|---|---|---|
| Age (years) | 75.65±8.35 | 74.63±12.39 | ns |
| Charlson index | 7.13±1.89 | 6.65±1.67 | ns |
| No. of drugs | 10±4.75 | 10.26±4.29 | ns |
| Dose of metformin (mg/day) | 1856±499 | 1767.39±507.8 | ns |
| Basal serum creatinine (mg/dl) | 1.06±0.32 | 1.16±0.52 | ns |
| baseline eGFR(ml/min) | 65.54±19.82 | 62.40±22.11 | ns |
| Peak S. creatinine (mg/dl) | 5.37±2.81 | 6.07±2.88 | ns |
| Final S. creatinine (mg/dl) | 4.54±2.83 | 1.41±0.76 | 0.001 |
| pH | 7.055±0.23 | 7.151±0.19 | ns |
| Bicarbonate (mmol/l) | 10.64±7.54 | 12.51±6.64 | ns |
| pCO2 (mmHg) | 31.75±13.05 | 34.74±14.70 | ns |
| Anion GAP (mmol/l) | 26.11±6.6 | 26.9±8.62 | ns |
| Serum lactate (mmol/l) | 10.33±5.34 | 10.70±5.76 | ns |
| Prothrombin activity (%) | 52.24±29.9 | 71.35±30.96 | ns |
| Potassium (meq/l) | 5.42±1.22 | 5.91±1.47 | ns |
| Hemoglobin (g/dl) | 10.83±0.85 | 11.95±2.22 | ns |
| Hospital stay (days) | 6.0±8.7 | 12.74±6.65 | 0.051 |
| Need for ICU (%) | 3 of 8 (37.5%) | 9 of 23 (39.1%) | 0.009 |
| Need for RRT (%) | 2 of 8 (25%) | 11 of 23 (47.8%) | ns |
| S. level of metformin (mcg/ml) | 23.61±16.61 | 24.24±19.41 | ns |
| Overdose | 2 of 8 (25%) | 7 of 23 (30.4%) | ns |
ns: not significant difference; TRS: renal replacement therapy; ICU: Intensive Care Unit; eGFR: estimated glomerular filtration rate.
Since its description in 1957 by Jean Stern, metformin has become one of the most prescribed drugs in the world.2 Currently, it is estimated that 150 million patients are being treated with metformin worldwide for control of diabetes.1 The study United Kingdom Prospective Diabetes Study showed that in type 2 diabetic patients the use of this drug was associated with a decrease in mortality of all causes and of cardiovascular origin as compared to those treated with sulfonylureas or insulin.7 In addition to its hypoglycemic effect, there are many other potential positive effects that have been described in recent years: cáncer,8 polycystic ovary,9 among others. It is also noteworthy the potential benefit against aging and neurodegenerative diseases even in non-diabetics, a fact that is currently being evaluated by a clinical trial.10 Therefore it would not be surprising that in the near future the use of this drug will increase even more. In this context it is important to remember that metformin can also produce side effects, among which traditionally the possible induction of lactic acidosis has been highlighted, which in some cases can be lethal. However, there is a debate in the literature about the true incidence and importance of this problem. In a 2010 a Cochrane review concluded that there is no clear evidence that metformin is associated with an increased risk of lactic acidosis compared to other hypoglycemic treatments.4
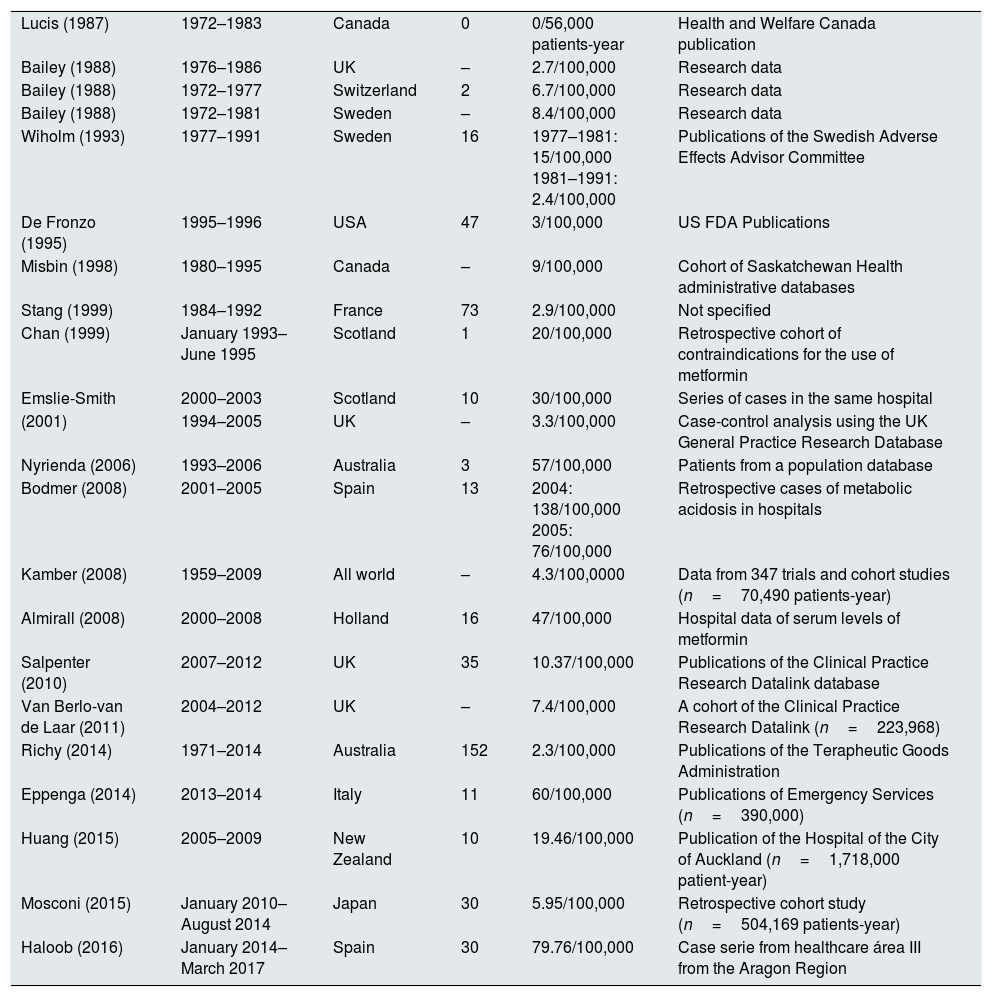
By contrast, in our study, the incidence of lactic acidosis is certainly high, well above what has been reported in other previous studies, as shown by the data presented in Table 2,4,11–29 adapted and expanded from Lalau,5 so that in our opinion we are facing a real an important clinical problem that should not be minimized. We did not obtain an average estimate of the patients treated with metformin during the entire study period because just collecting the data from the two specific dates of interval used was already extremely laborious. Therefore, calculations were made using the arithmetic mean of the two values available. With this strategy of data collection for estimation of the incidence, if there was any bias, it would have resulted in underestimation, because there could have been some case not detected in the search of the Clinical Documentation Service due to a fault in coding. However, all cases in which metformin levels were determined are adequately collected and without possibility of error since there is only one toxicology laboratory that centralizes these requests for quantification of drug levels. Therefore, we believe that the values obtained truly reflect the incidence of these events in the health area of our hospital during the study period.
Incidence of MALA published in various works.
| Lucis (1987) | 1972–1983 | Canada | 0 | 0/56,000 patients-year | Health and Welfare Canada publication |
| Bailey (1988) | 1976–1986 | UK | – | 2.7/100,000 | Research data |
| Bailey (1988) | 1972–1977 | Switzerland | 2 | 6.7/100,000 | Research data |
| Bailey (1988) | 1972–1981 | Sweden | – | 8.4/100,000 | Research data |
| Wiholm (1993) | 1977–1991 | Sweden | 16 | 1977–1981: 15/100,000 1981–1991: 2.4/100,000 | Publications of the Swedish Adverse Effects Advisor Committee |
| De Fronzo (1995) | 1995–1996 | USA | 47 | 3/100,000 | US FDA Publications |
| Misbin (1998) | 1980–1995 | Canada | – | 9/100,000 | Cohort of Saskatchewan Health administrative databases |
| Stang (1999) | 1984–1992 | France | 73 | 2.9/100,000 | Not specified |
| Chan (1999) | January 1993–June 1995 | Scotland | 1 | 20/100,000 | Retrospective cohort of contraindications for the use of metformin |
| Emslie-Smith | 2000–2003 | Scotland | 10 | 30/100,000 | Series of cases in the same hospital |
| (2001) | 1994–2005 | UK | – | 3.3/100,000 | Case-control analysis using the UK General Practice Research Database |
| Nyrienda (2006) | 1993–2006 | Australia | 3 | 57/100,000 | Patients from a population database |
| Bodmer (2008) | 2001–2005 | Spain | 13 | 2004: 138/100,000 2005: 76/100,000 | Retrospective cases of metabolic acidosis in hospitals |
| Kamber (2008) | 1959–2009 | All world | – | 4.3/100,0000 | Data from 347 trials and cohort studies (n=70,490 patients-year) |
| Almirall (2008) | 2000–2008 | Holland | 16 | 47/100,000 | Hospital data of serum levels of metformin |
| Salpenter (2010) | 2007–2012 | UK | 35 | 10.37/100,000 | Publications of the Clinical Practice Research Datalink database |
| Van Berlo-van de Laar (2011) | 2004–2012 | UK | – | 7.4/100,000 | A cohort of the Clinical Practice Research Datalink (n=223,968) |
| Richy (2014) | 1971–2014 | Australia | 152 | 2.3/100,000 | Publications of the Terapheutic Goods Administration |
| Eppenga (2014) | 2013–2014 | Italy | 11 | 60/100,000 | Publications of Emergency Services (n=390,000) |
| Huang (2015) | 2005–2009 | New Zealand | 10 | 19.46/100,000 | Publication of the Hospital of the City of Auckland (n=1,718,000 patient-year) |
| Mosconi (2015) | January 2010–August 2014 | Japan | 30 | 5.95/100,000 | Retrospective cohort study (n=504,169 patients-year) |
| Haloob (2016) | January 2014–March 2017 | Spain | 30 | 79.76/100,000 | Case serie from healthcare área III from the Aragon Region |
Metformin acts by inhibiting hepatic neoglucogenesis as a consequence of a relative energy deficit caused by the decrease in adenosine triphosphate produced in the mitocondria.30 Other important actions of the drug are mediated by the activation of the enzyme AMPK, responsible for the pleiotropic effects of the drug.1,31 A beneficial effect on the intestinal microbiota has also been described.32 In the mitochondria, the drug inhibits complex I of the respiratory chain by an unknown mechanism.30 It is better described the action on the inhibition of the mitochondrial glycerol-3-phosphate dehydrogenase enzyme that also causes a uncoupling of the mechanism of cell respiration.33 In the presence of tissue hypoxia, pyruvic acid produced as a final product of cytoplasmic glycolysis is metabolized by the enzyme lactic acid dehydrogenase to lactic acid (type A lactic acidosis). In the absence of hypoxia pyruvic acid is incorporated into the mitochondria and is used as a substrate of the Krebs cycle that is coupled to the respiratory chain to produce adenosine triphosphate by oxidative phosphorylation. The final effect of biguanides is similar to that produced by hypoxia (type B lactic acidosis).34 In the case of metformin when used at therapeutic doses this effect is negligible, but when taken in excess, intentionally, or at normal doses in a certain clinical context (heart failure, hypoxia situations, liver failure, dehydration, CKD) and if there is associated with certain factors (radiological contrasts, drugs such as NSAIDs, ACE inhibitors or ARA II) there is an increased risk of overdosage and induction of lactic acidosis,35 especially if the clinical context is that of an AKF, since the drug is eliminated mainly by the kidney as unaltered molecule.36
The debate also extends to the definition of such a clinical situation. Some authors such as Lalau distinguish in clinical practice up to three different situations5: (1) the so-called Metformin-unrelated hyperlactatemia – MULA, in the absence of metformin accumulation. It is especially true if blood levels of metformin are normal, below normal or even undetectable. Lalau maintains that this group is the most numerous and the one that carries worse prognosis. (2) Metformin-induced hyperlactatemia – MILA. It refers to hyperlactacidemia caused only by metformin. A typical case is the voluntary intoxication by metformin in the absence of concomitant drugs. A similar case is the acute accumulation of metformin due only to acute renal failure. (3) Metformin-associated hyperlactatemia – MALA – is the most complex case. The word “associated” means that the induction of hyperlactataemia is a consequence (although to a different degree, perhaps) of the accumulation of metformin and one or more associated concomitant diseases. According to Lalau, this group would be less frequent than suspected and its prognosis would be better than that of MULA, granting metformin a “protective” factor. The difficulty of applying these definitions, somewhat cumbersome in itself, also lies in having available the determination of metformin levels in real time, a fact that usually does not occur in routine practice. In any case and for the sake of simplicity, patients could be divided into two groups, those in which metformin is not related to the induction of lactic acidosis (the so-called MULA) and those in which the drug is the main responsible (MILA) or share that responsibility with another series of concomitant pathologies (MALA).
There is also disparity between the studies on the relationship between metformin levels and the degree of lactic acidosis, as the results between different studies are discordant.37–41 However, the findings of the recent study by Boucaud-Maitre et al. of large sample of patients showed that the correlation between both parameters is clear,42 as we have also reported in our study. The controversy also extends to recommendations for the dosage of the drug in patients affected by renal failure since they differ according to scientific societies and the opinion of some experts.43 In this line, it is pertinent to recall the results of a Taiwanese study that compared the mortality of a large cohort of CKD stage 5 patients treated with metformin vs untreated.44 The treated group showed significantly higher mortality that paradoxically was not related to a higher incidence of lactic acidosis in this group. The authors argue that the alteration of the mitochondrial respiratory chain could be more harmful in patients with advanced chronic kidney disease, even without causing lactic acidosis.
In our study, we detected 29% of cases of overdosage according to GFR. This is a feature that must be emphasized, because it would be easily avoidable with an adequate prescription, for which the clinician who prescribes the drug should previously request a blood biochemistry to determine the baseline renal function of the patient and make appropriate adjustments in the dosage. In subsequent follow-up, renal function should be monitored periodically as recommended by the guidelines. We want to highlight that probably a high number of MALA episodes detected in our study has been due to inadequate monitoring of dose prescribed relative to the renal function (in all cases except one that was at the patient's will).
Nevertheless, in defense of prescribing doctors, it can be argued that recently there has been disagreement between the latest European guidelines, which are more flexible for treatment with metformin in CKD45 and American guidelines, which still contraindicate metformone administration if GFR is below 30ml/min.46 In fact, until recently the data sheet pointed out that metformin should not be used in patients with GFR <60ml/min, but it was often prescribed below the GFR limits. Recently the metformin data sheet has been modified by the European Medicines Agency.47 In the recent Spanish medical literature there are also guides and recent reviews on this subject.48,49
From our data, a typical case presentation could be an elderly patient with important and polymedicated comorbidity, in many cases using drugs that can produce AKF, that undergoes dehydration due to gastrointestinal disorder but the patient continues to take metformin. The consequence is an episode of AKF stage III with metformin accumulation of elevation of the serum levels of the drug and an increase in lactate levels with reduction of the pH. Approximately 40% of the patients will require admission to the ICU and a similar percentage need RRT illustrating the clinical severity of the situation, which in our patients caused a 25% of mortality. The majority of patients were treated in the nephrology and internal medicine services. Between the deceased and the survivors the only significant differences were the final serum creatinine level, higher in deceased patients, and the duration of the hospital stay that was lower in the deceased ones. No variable maintained its significance in the multivariate analysis, perhaps reflecting the limited sample size. In the aforementioned study by Boucaud-Maitre, the lactate level was independently associated with mortality.42
Metformin is a small molecule (129Da) with a low protein binding, so it diffuses freely through dialyzers and hemofilters. The limiting factor for its extracorporeal clearance is its relatively large volume of distribution (1–5l/kg) although this may be reduced in the context of AKF. The justification of the RRT goes beyond the elimination of the drug from the organism since it also includes: (1) fast, predictable and effective correction of acidosis that is achieved with administration of bicarbonate, (2) improvement of hyperlactataemia, (3) correction of the electrolyte abnormalities, and (4) renal replacement therapy.36 Regarding the modality of the RRT to be used, a recent systematic review of the working group of the extracorporeal treatment of intoxications highlights the superior efficacy of hemodialysis as compared to continuous techniques, so if the patient's hemodynamic stability allows, it is preferable to perform hemodialysis with bicarbonate in the dialysis fluid with exhaustive monitoring during the sesión.36 An elegant work recently published has shown that the duration of the dialysis sessions should be prolonged to 5 or 6h, and thereafter the levels of metformin and lactate should be monitored due to the possibility of rebound at the end of the dialysis session. There are no specific recommendations with respect to conductivity or other technical specifications.50
This study presents a series of limitations. First, it is retrospective, which has led to biases in the selection of patients and errors in the collection and interpretation of data from clinical records, despite the attention paid when performing this task. Secondly, there is no control group, but it can argued that it would have been difficult to gather 31 cases from our hospital affected by lactic acidosis not attributable to MALA during the study period to be used as controls. Despite this, the sample size of our work is considerable and provides clinically relevant information.
In conclusion, in this study we have detected a high incidence of MALA in our health area, although if we apply the definitions of Lalau, a good part of our cases could be included within the group called MILA. Our incidence is much higher than traditionally published, so we believe that we should be alert to detect and treat this clinical situation that may have serious consequences. We have had a 25% of deaths in our patients during admission. Base on the data extracted from our case series, and the substantial change issued by the European Medicines Agency in 2016,47 it is important to warn patients at the time of prescription of the harmful effects that can be produced if the treatment is continued if there is a risk of extracellular volume depletion with AKF with accumulation of the drug. Therefore it is important to consult with the doctor in situations of gastrointestinal disorders with vomiting and diarrhea. Likewise, it is also necessary to perform an adequate initial dosage, according to the patient baseline renal function.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Peña Porta JM, Villafuerte Ledesma HM, Vicente de Vera Floristán C, Ferrer Dufol A, Salvador Gómez T, Álvarez Lipe R. Incidencia, factores relacionados con la presentación, evolución y mortalidad de la acidosis láctica asociada a metformina en el área sanitaria de un hospital de tercer nivel. Nefrologia. 2019;39:35–43.