Introducción: Los inhibidores de mTOR (del inglés mammalian target of rapamycin), sirolimus y everolimus, utilizados como tratamiento inmunosupresor en el trasplante de órganos sólidos, pueden producir efectos adversos graves, como la neumonitis intersticial. Incidencia y presentación clínica: La incidencia de neumonitis intersticial se ha estimado entre el 4 % y el 11 %, aunque podría ser mayor. La mayoría de los casos publicados se ha producido en pacientes trasplantados renales en tratamiento con sirolimus. La presentación clínica es heterogénea, lo que dificulta el diagnóstico. Se acostumbra a observar alteraciones en la tomografía axial computarizada torácica, como opacidades en vidrio deslustrado. La fisiopatología es poco conocida. Sin embargo, se ha observado una mayor incidencia en pacientes con función renal alterada y en pacientes que habían recibido inhibidores de calcineurina previamente. La relación entre aparición de neumonitis y concentraciones plasmáticas de inhibidores de mTOR no está bien definida. Tratamiento: La suspensión del fármaco y la administración de dosis altas de corticoides parecen ser efectivos. Otras alternativas terapéuticas, aunque más discutidas, son la reducción de la dosis del inhibidor de mTOR y el cambio de sirolimus a everolimus. Conclusión: Se debe sospechar de neumonitis iatrogénica en pacientes trasplantados en tratamiento con inhibidores de mTOR y con síntomas respiratorios. Faltan datos concluyentes en cuanto a estrategias de tratamiento. Parece que everolimus podría ser mejor tolerado que sirolimus.
Introduction: mTOR (mammalian target of rapamycin) inhibitors sirolimus and everolimus, used as immunosuppressants in solid organ transplantation, may cause severe adverse effects, such as interstitial pneumonitis. Incidence and clinical presentation: The estimated incidence of interstitial pneumonitis is 4-11% although it may be higher. Most reported cases have occurred in renal transplant recipients treated with sirolimus. Clinical presentation is heterogeneous, which makes diagnosis difficult. Abnormalities, such as ground glass opacities, are often found in computerised axial tomography scans of the chest. Physiopathology is not well-known. However, patients with abnormal renal function and those with previous calcineurin inhibitor treatment display a higher incidence. The relationship between pneumonitis and mTOR inhibitor plasma concentrations is not well defined. Treatment: Drug discontinuation and administration of high doses of corticosteroids seems to be an effective treatment. mTOR inhibitor dose reduction and replacing sirolimus with everolimus are other alternatives, but they are still under discussion. Conclusion: Iatrogenic pneumonitis must be suspected when a transplant recipient being treated with mTOR inhibitors presents respiratory symptoms. There is lack of conclusive data on treatment strategies. It appears that everolimus may be tolerated better than sirolimus.
INTRODUCTION
mTOR (mammalian target of rapamycin) inhibitors, sirolimus (rapamycin) and everolimus, act by inhibiting T and B lymphocyte proliferation. They were proposed as a safer alternative to immunosuppressant treatment of solid organ transplantations, compared with calcineurin inhibitors already widely used, such as tacrolimus and cyclosporine. However, in clinical practice, it has been demonstrated that mTOR inhibitors are not exempt from toxicity.1 At the time of their introduction to the market, the absence of nephrotoxicity was highlighted as its main advantage over calcineurin inhibitors but subsequent studies on patients with a kidney transplant called into question this assertion.2,3
The pharmacokinetics of sirolimus and everolimus are very similar in terms of metabolism and excretion. Both are metabolised by CYP3A4 to inactive metabolites, which are fundamentally eliminated through faeces, with excretion through the kidney being minimal. The pharmacokinetic parameters, which are altered in the event of liver failure, are not affected by renal failure. Both drugs are also substrates of P-glycoprotein, which is involved in the clearance processes. The elimination half-life is about 30 hours for everolimus and around double for sirolimus. These drugs are extensively distributed around the body, as their high volume of distribution suggests.4,5 A new mTOR inhibitor, temsirolimus, has been recently introduced to the market, which is indicated in the treatment of advanced renal cell carcinoma.
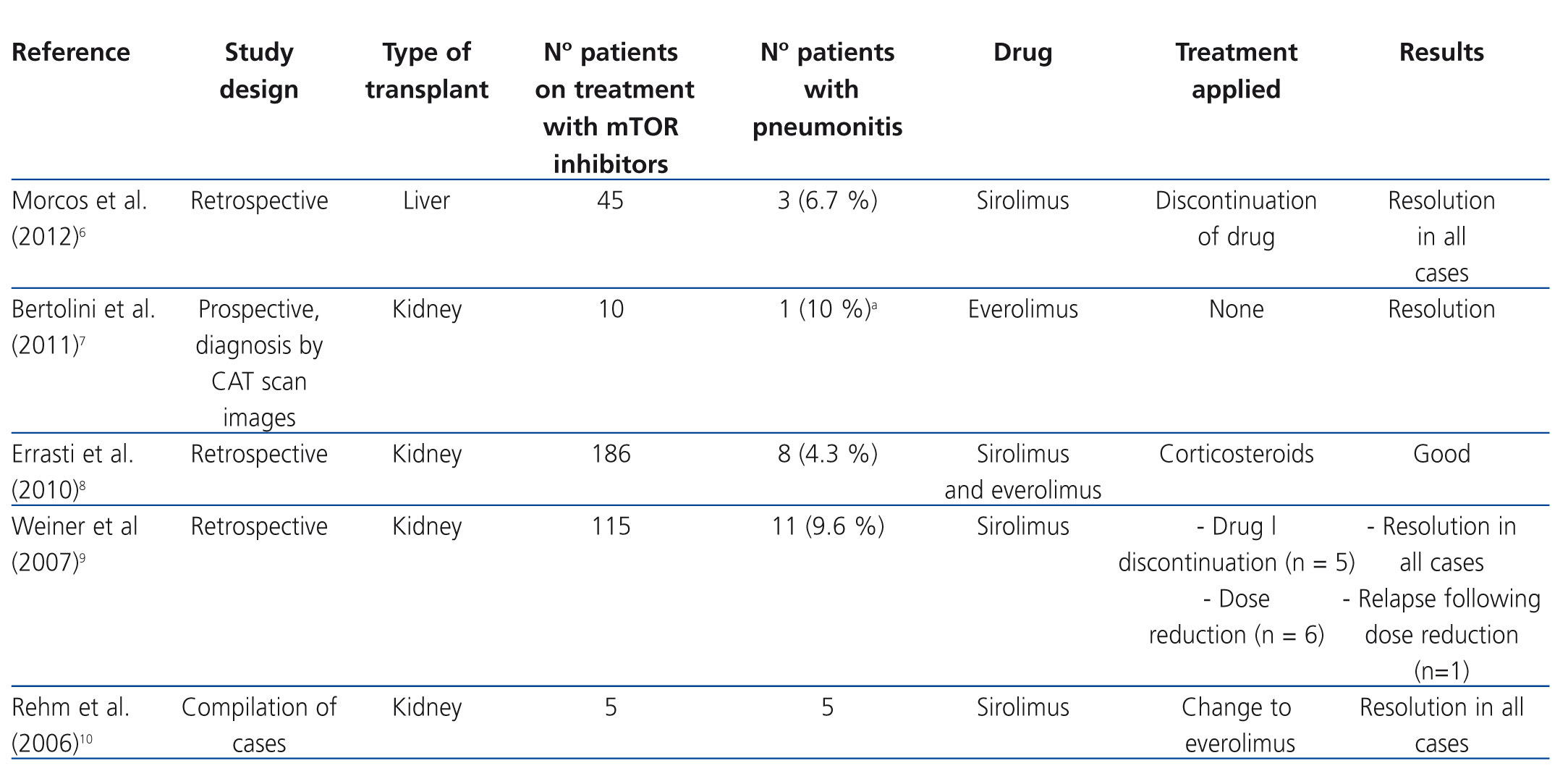
It has been shown that mTOR inhibitors stimulate the production of some proinflammatory cytokines in monocytes and macrophages and promote the proliferation of memory T cells. This indicates that they possess certain proinflammatory effects that may be the cause of some of its adverse effects, such as pneumonitis, which is the inflammation of lung tissue.2 Table 1 shows the main studies on pneumonitis in patients treated with mTOR inhibitors.6-10
INCIDENCE
A lot of cases of pneumonitis due to mTOR inhibitors have been reported since they were first marketed, most associated with sirolimus treatment, although cases have also been described that involve everolimus and temsirolimus.3,11 The incidence of this adverse effect was estimated at 4-11%.6,8,9 However, the recent introduction of this group of drugs, specifically everolimus and temsirolimus as antineoplastic treatment, provides more case studies and suggests that the incidence is probably higher. Given that cancer patients periodically undergo computed axial tomography (CAT), we have been able to observe pulmonary abnormalities suggestive of pneumonitis in asymptomatic patients. The overall incidence of pneumonitis for mTOR inhibitors in this type of patient, symptomatic or not, seems to be over 20%.11,12 Mortality associated with pneumonitis due to sirolimus and everolimus is estimated at around 5%.2
The overwhelming majority of cases described occurred in kidney transplant patients, since it is in this type of transplantation that mTOR inhibitors were first administered. Nevertheless, cases of pneumonitis have also been reported in liver and heart transplant patients who presented similar characteristics.6,13,14
CLINICAL PRESENTATION
Pneumonitis caused by mTOR inhibitors has heterogeneous clinical manifestations and may begin with fever, fatigue, coughing and dyspnoea, nonspecific signs and symptoms, which does not facilitate diagnosis. It may appear at the beginning or after several years of treatment. In many cases, the chest X-ray did not show abnormalities, which were only observed in CAT scans. The most common findings in CAT scans were ground glass opacities, although additional peripheral infiltrates and patterns of bronchiolitis obliterans with organising pneumonia have also been observed. In some cases, signs of pulmonary fibrosis were also observed. Diagnosis is difficult and tends to be made by excluding other causes, such as infections, autoimmune diseases or toxicity due to other products.8,9,15,16 Clinical improvement upon discontinuation of the drug can confirm the diagnosis.8
The pathophysiology of pneumonitis associated with mTOR inhibitors is not very well understood. Direct toxicity by drugs, immunological toxicity or a combination of both mechanisms has been proposed. In lung biopsies of affected patients, lymphocytic alveolitis has been observed, which favours the immune mediated toxicity hypothesis. However, the rapid response to drug discontinuation favours the direct toxicity hypothesis.2,15-17
Interestingly, it has been observed that patients who began post-transplant immunosuppression directly with mTOR inhibitors have a lower incidence of pneumonitis than those who started treatment with calcineurin inhibitors and subsequently changed to sirolimus or everolimus. The reason for this lower incidence is not known, but some authors suggest that it may be due to differences in renal function, since an increased incidence of pneumonitis in patients with abnormal renal function has been observed.9,18 Sometimes the reason for switching from calcineurin inhibitors to mTOR inhibitors is nephrotoxicity due to the former. Therefore, when switching to treatment with mTOR inhibitors, patients typically present worse renal function. This hypothesis is not very plausible since sirolimus and everolimus are metabolised by CYP3A4 and their renal excretion is negligible.4,5 However, other mechanisms by which renal function modifies tolerance to mTOR inhibitors cannot be discarded and it has been questioned whether a metabolite of renal elimination may be the cause of symptoms.
The potential relationship between the occurrence of pneumonitis and plasma drug concentrations has also been researched. In the sirolimus efficacy studies published to date, different target treatment concentrations have been used. While some authors have used predose concentrations of 5-10ng/ml, others opt for 10-15ng/ml or greater, depending on whether or not the treatments were combined with calcineurin inhibitors, as well as the post-transplant period.19 In the study by Weiner et al., although in most cases of pneumonitis, sirolimus concentrations were higher than both treatment margins (mean 16.7ng/ml), cases were also observed with concentrations below 10ng/ml. As such, plasma concentrations within therapeutic margins do not allow pneumonitis caused by mTOR inhibitors to be ruled out.9-15
TREATMENT
The first measure to be taken in a potential case of pneumonitis due to mTOR inhibitors is the suspension of the drug involved in the process. This eliminates symptoms completely in many cases.8,9,20,21 The administration of high doses of corticosteroids is also advised, although no recommended dose has been established. It is necessary to bear in mind that most of these patients are already treated with low levels of corticosteroids to avoid graft rejection. However, in some case series, patients have evolved favourably without having received corticosteroids.9 Therefore, some authors suggest administering corticosteroids only in the most serious cases.8
Likewise, cases have been described in which the reduction of the mTOR inhibitor dose has been sufficient to eliminate pneumonitis.3 As an alternative to sirolimus discontinuation, switching to everolimus has also been proposed and has yielded good results in several cases. Everolimus is a more hydrophilic drug than sirolimus, making it easier to eliminate from the body and less likely to cause hypersensitive reactions.10,22 It seems that it presents a lower incidence of pneumonitis with respect to sirolimus3, although some studies have not found any differences.1
CONCLUSION
In solid organ transplant patients who are treated with mTOR inhibitors and have respiratory symptoms, after ruling out infection and other causes, we should suspect iatrogenic pneumonitis, in order that the appropriate treatment may begin as soon as possible: discontinuation or a change of immunosuppressive treatment and addition of corticosteroid treatment if necessary.
Given that pneumonitis due to mTOR inhibitors is more common in patients with renal failure and that cases have been described in which dose reduction eliminates clinical manifestations, we may be led to believe that this toxicity is dependent on concentrations of the drug in the body. However, patients with plasma concentrations within the treatment margin have presented this complication. Details of the pathophysiology of this disease are still unknown, but for drugs with a high volume of distribution, one possibility would be specific accumulation of the mTOR inhibitor or its metabolites in the lung parenchyma. This could explain the presence of serum concentrations within the therapeutic interval in patients with pneumonitis.
It seems that the mTOR inhibitors, although they have the same mechanism of action, seem to differ in terms of their adverse effects, with there being greater tolerance to everolimus. However, there is still a lack of data to confirm this.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Main studies on pneumonitis in patients treated with mTOR inhibitors