Renal tubular calcium reabsorption is one of the principal factors that determine serum calcium concentration and calcium excretion. Calcium excretion is regulated by the distal convoluted tubule and connecting tubule, where the epithelial calcium channel TRPV5 can be found, which limits the rate of transcellular calcium transport. The dynamic presence of the TRPV5 channel on the surface of the tubular cell is mediated by an endosomal recycling process. Different intrarenal factors are involved in calcium channel fixation in the apical membrane, including the anti-ageing hormone klotho and tissue kallikrein (TK). Both proteins are synthesised in the distal tubule and secreted in the tubular fluid. TK stimulates active calcium reabsorption through the bradykinin receptor B2 that compromises TRPV5 activation through the protein kinase C pathway. TK-deficient mice show hypercalciuria of renal origin comparable to that seen in TRPV5 knockout mice. There is a polymorphism with loss of function of the human TK gene R53H (allele H) that causes a marked decrease in enzymatic activity. The presence of the allele H seems to be common at least in the Japanese population (24%). These individuals have a tendency to greater calcium and sodium excretion in urine that is more evident during furosemide infusion. Future studies should analyse if manipulating the renal kallikrein-kinin system can correct idiopathic hypercalciuria with drugs other than thiazide diuretics.
La reabsorción tubular de calcio es uno de los principales factores que determinan la concentración sérica de calcio y su excreción urinaria. El túbulo contorneado distal y conector es donde se produce la regulación fina de la calciuria. A ese nivel se encuentra el canal epitelial de Ca (TRPV5), que es el paso limitante en el transporte transcelular de Ca. La presencia dinámica del canal TRPV5 en la superficie de la célula tubular está mediada por un proceso de reciclado endosómico. Distintos factores intrarrenales intervienen en la fijación del canal de calcio en la membrana aplical, entre ellos la hormona antienvejecimiento klotho y la calicreína tisular (CT). Ambas proteínas son sintetizadas en el túbulo distal y secretadas en el fluido tubular. La calicreína tisular estimula la reabsorción activa de calcio por vía del receptor de bradiquinina tipo 2 que compromete la activación del of TRPV5 por vía de la protein cinasa C. Los ratones deficientes en CT muestran hipercalciuria de origen renal comparable a la pérdida de calcio que se observa en los ratones knockout para el TRPV5. Existe un polimorfismo con pérdida de función del gen de la CT humana denominado R53H (alelo H) que produce una gran disminución de la actividad enzimática. La presencia del alelo H, por lo menos en la población japonesa, parece ser frecuente (24%). Estos individuos tiene una tendencia a excreción más alta de calcio y sodio en orina que se manifiesta más durante la infusión de furosemida. En el futuro habrá que estudiar si la manipulación del sistema calicreína-quinina renal permite corregir la hipercalciuria idiopática con fármacos diferentes a los diuréticos tiazídicos.
Tubular reabsorption of calcium is one of the main factors that determine serum calcium concentration and its urinary excretion. Most of the filtered calcium (60–70%) is reabsorbed in the proximal tubule, primarily by a paracellular mechanism that is not significantly sensitive to calcium regulatory hormones. Another 20–25% of the filtered calcium is reabsorbed in the thick ascending limb of Henle's loop, primarily by paracellular pathway, and involves claudins 16, 19 and 14. The distal convoluted and collective tubule is where fine regulation of hypercalciuria occurs, and where a significant fraction (10–15%) of the filtered calcium load is reabsorbed. In this tubular segment, calcium is reabsorbed by a transcellular mechanism, and enters through calcium channels present in the apical membrane. Under normal conditions, the tubular reabsorption of calcium is tightly regulated. Non-hormonal factors such as ECF volume, acid/base status and plasma concentrations of magnesium and calcium exert an influence on the management of calcium in the renal tubule.1–3 Extrarenal hormonal factors, such as parathyroid hormone and 1,25-dihydroxyvitamin D, also regulate the tubular reabsorption of calcium.4 In contrast, relatively little is known about the possible contribution of intrarenal factors in the regulation of the renal tubular transport of calcium.
Calcium epithelial channel TRPV5 and its regulation by tubular factorsThe molecular basis of active transcellular transport of calcium in the distal nephron has recently been discovered. This process involves the apical influx of calcium through the calcium epithelial channel (TRPV5), which is the limiting step in the transcellular transport of calcium.5 Consistent with this is the lack of TRPV5 channel resulting in a decrease in the distal tubular reabsorption of calcium and the production of renal hypercalciuria.6 Several calciotropic hormones that are known to alter renal reabsorption of calcium affect the expression of TRPV5; others stimulate TRPV5 channel traffic to the plasma membrane, while a number of ions and associated proteins control the activity of the channel at the plasma membrane level.7 The dynamic presence of the TRPV5 channel on the surface of the tubular cell is mediated by an endosomal recycling process that allows the internalisation of the channel to make it reappear again at the level of the plasma membrane. One of the proteins synthesised by the distal tubule is the klotho anti-ageing hormone. Klotho is a single-pass transmembrane protein, expressed primarily in the kidneys and choroid plexus. Membrane klotho functions as a bound coreceptor of fibroblast growth factor 23 (FGF-23) in the kidney and parathyroid gland. The extracellular domain of klotho is composed of 2 internal repetitions, KL1 and KL2, which can be cleaved and released into the blood and into the tubular lumen and act as hormones. Klotho upregulates TRPV5 both from within and outside the tubular cell. The intracellular action of klotho is likely mediated by an increase in protein trafficking of the channel to the apical membrane, while its extracellular action would be due to inhibition of the endocytosis of the caveolae where the calcium channels are found. Both effects compromise klotho sialidase activity by modifying the glycosylation status of the calcium channel and, therefore, trapping the channel at the cell surface level.8 Consistent with the positive influence of klotho on TRPV5-mediated calcium reabsorption is that the mice without klotho expression have urinary loss of calcium, hyperparathyroidism, and hypervitaminosis D.9
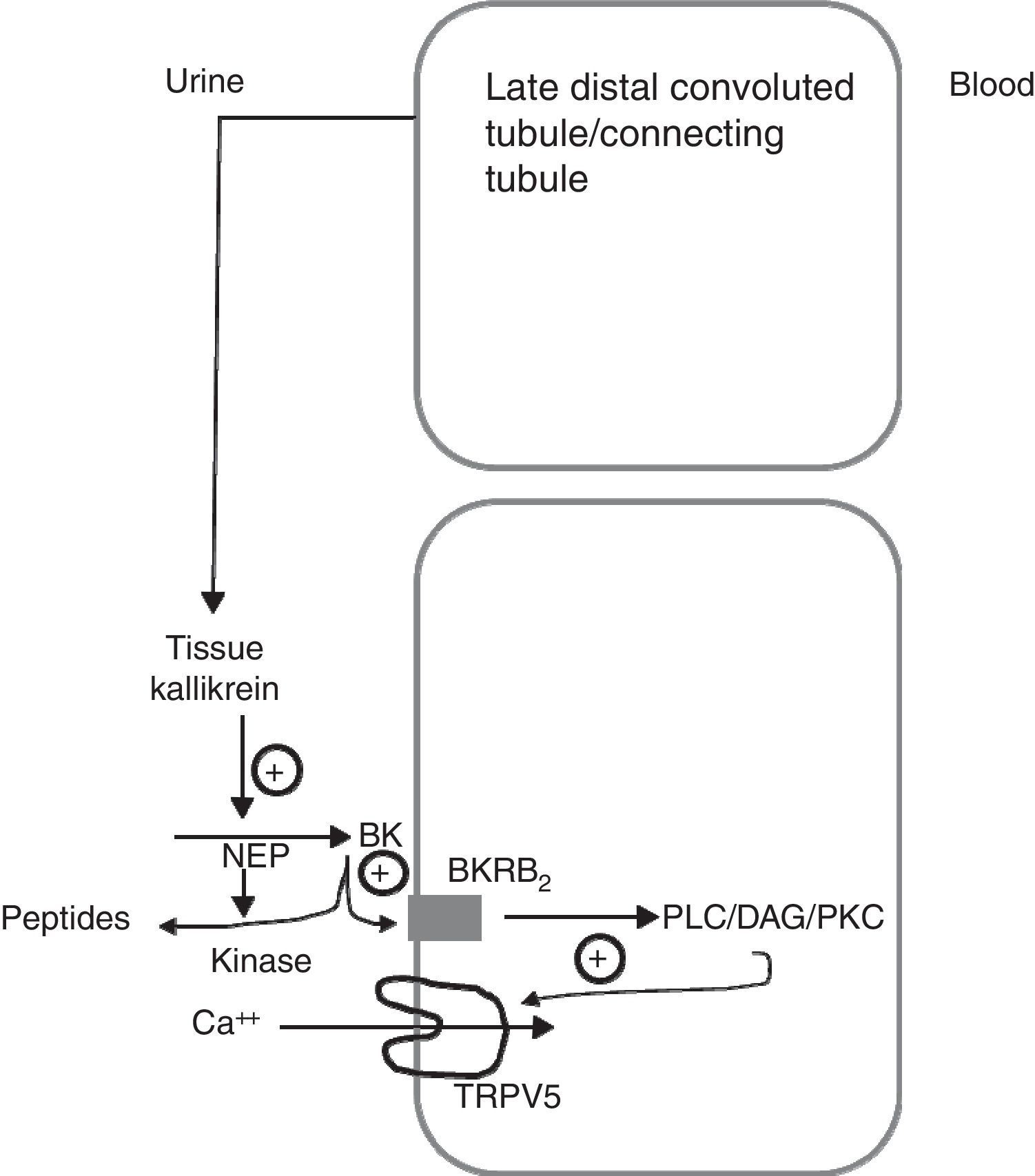
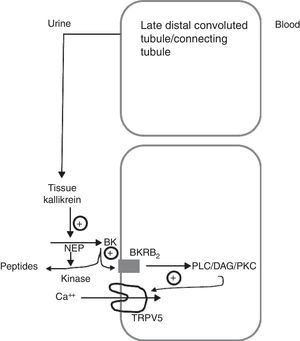
Another of the proteins that are synthesised in the distal tubule and secreted in the tubular fluid is tissue kallikrein (TK).10–12 TK is a serine protease involved in the generation of kinins in many organs, including the kidney.13 The kinins generated in the collecting tubule through TK inhibit the reabsorption of sodium chloride, through the activation of the bradykinin B2 receptors located along the epithelial cells of the collecting tubule. These kinins are immediately inactivated by 2 kidney-specific enzymes (kininases), the carboxypeptidase Y-like (CPY) exopeptidase, and the neutral endopeptidase (NEP)14 (Fig. 1). In addition, TK, through its catalytic activity, acts directly on the epithelial sodium channel (ENac) to modulate its activity, without being critical for the renal regulation of sodium homeostasis. TK-deficient mice exhibit a net transepithelial absorption of K+ in the cortical collecting tubule due to an abnormal activation of colonic-type H+, K+-ATPase in intercalated cells and reduced secretion of K+ by the principal cells secondary to a decrease in ENac activity. Therefore, TK is a kaliuretic factor that, independent from aldosterone, provides rapid protection against hyperkalemia after dietary K load.15 Partial deficiency of TK in humans precludes proper adaptation to a load of potassium.16 TK-deficient mice have recently been shown to exhibit hypercalciuria of renal origin17 comparable to the leak of calcium observed in knockout mice for TRPV5. TK distribution mainly overlaps that of the TRPV5 channel in the distal nephron, and the expression of the kallikrein gene is increased in low calcium diets. Recently, TK has been shown to stimulate active reabsorption of calcium via the type 2 bradykinin receptor that compromises the activation of TRPV5 via protein kinase C.18 This indicates that kallikrein could be a physiological modulator of the tubular reabsorption of calcium.
Schematic model showing how tissue kallikrein participates in the regulation of the TRPV5 calcium epithelial channel in the late distal convoluted tubule. The tissue kallikrein produced by the connecting tubule is released into the urinary fluid. It is there that it acts on the filtered or locally secreted kininogen (KN) and finally produces bradykinin (BK). BK acts on its B2 receptor (BKRB2), activating the phospholipase C/diacylglycerol/protein kinase C (PLC/DAG/PKC) pathway by inducing the TRPV5 calcium channel localisation at the apical membrane level and favours the reabsorption of tubular calcium. BK is degraded by neutral endopeptidase (NEP) and renal CYP kinase.
Secretion of renal kallikreins by collecting tubule cells may be increased by ATP-dependent potassium (KATP) channel blockers. Thus, oral administration of glibenclamide (a KATP channel blocker) or U18177, a kidney-selective KATP channel blocker, induces natriuresis and an increase in secretion of kallikreins that suppress the elevation of systolic blood pressure in Sprague Dawley rats who received 8% sodium chloride in their diet. These effects are counteracted by coadministration of a bradykinin B2 receptor antagonist (B2RA), FR17365. This indicates that KATP channel blocking induces diuresis and natriuresis through the release of renal kallikreins.14
Ebelactone B and poststatin are inhibitors of CPY renal kininase. Administration of these drugs to a DOCA-salt-induced hypertension rat model prevents the development of salt-dependent hypertension.14 A neutral endopeptidase enzyme inhibitor, BP102, also suppresses systolic blood pressure elevation, but its effect is weaker than ebelactone B. Interestingly, young, genetically hypertensive SHR rats exhibiting hypercalciuria have an attenuated ability to secrete renal kallikreins as compared to normotensive WKY rats.19 To date, there are no studies in which the renal kallikrein-kinin system is manipulated with the above drugs to see if hypercalciuria is modified in these animals.
Tissue kallikrein deficiency in humans and its effect on hypercalciuriaThere is a polymorphism with loss of function of the human TK gene called R53H (H allele) that produces a significant decrease in enzymatic activity. In a crossover study in young white males, 30 of the subjects were homozygous for R53H and 10 were heterozygous for the same gene. They were randomised to 7-day periods with a low calcium diet (10mmol/day) associated with a low Na/high K diet or a high Na/low K diet to modulate the synthesis of TK.20 On the seventh day of each diet, participants were studied before and during a 2-hour infusion of furosemide, which functionally excludes the thick ascending limb of Henle's loop and increases the supply of calcium to the distal tubular segments. Urinary kallikrein activity was 50–60% lower in participants with R53H than in those without R53R. The adaptation of urinary calcium excretion to the contrasting Na/K diets was not affected in the participants who were carriers of R53H. In contrast, after the infusion of furosemide, R53H participants had significantly lower serum ionised calcium concentrations than R53R participants (p<0.0001) and a non-significant tendency to greater calcium urinary excretion than R53R participants. These effects were more marked with low-Na/high-K diets.
Another recent study analysed TK polymorphisms in Japanese volunteers and examined the association between the H allele in the promoter region, which is shown to produce a decrease in promoter activity, and urine kallikrein activity.21 Ninety and 73 volunteers were analysed for the promoter and coding regions of the tissue kallikrein gene, respectively. The presence of the H allele in the Japanese population was common, a 24%. One synonymous and three non-synonymous polymorphisms were found in the coding regions. They studied the physiological parameters in subjects treated with an ad libitum diet. Urinary kallikrein activity was not significantly decreased in subjects with the H allele as compared with individuals without the H allele, although it was low in 2 homozygotes for the H allele. However, urinary excretion of calcium and sodium were higher in subjects with the H allele than in those without that allele.
From these studies, it follows that the polymorphism with loss of function of the human TK gene (H allele) is relatively frequent. These individuals have a tendency to a high urinary excretion of calcium and sodium and this defect is more evident during the infusion of furosemide, exaggerating the defects of reabsorption in the distal tubule.
End-noteIn the future, it will be necessary to study whether the renal kallikrein-kinin system is altered in animals with hypercalciuria and in humans with idiopathic hypercalciuria. This system could be modified by kidney-selective KATP channel blockers that increase the secretion of renal tissue kallikrein or through the increase of urinary kinin by renal CPY kininase inhibitors, such as ebelactone B and poststatin. Thus, it is conceivable that recurrent renal lithiasis caused by idiopathic hypercalciuria could be treated with drugs other than thiazide diuretics.
Conflicts of interestThe author has no conflicts of interest to declare.
Please cite this article as: Luis A. ¿Es el sistema calicreína/quinina renal un factor modulador de la calciuria? Nefrologia. 2017;37:5–8.