El trasplante renal con donante vivo constituye el tratamiento de elección para la insuficiencia renal crónica y la evaluación exhaustiva del donante garantiza el éxito del procedimiento. La diabetes mellitus (DM), la hipertensión arterial y la obesidad representan contraindicaciones clínicas para la donación de vivo, pero no se sabe con claridad cual es el riesgo individual a largo plazo de un potencial donante que presenta alguna de estas alteraciones de origen metabólico agrupadas en el denominado síndrome metabólico (SM), donde la resistencia a la insulina constituye el nexo común etiopatogénico. El SM es muy prevalente en el mundo occidental (20-30%) y se asocia al desarrollo de enfermedad cardiovascular, DM y alteraciones renales. De ahí, la importancia de su detección antes de la donación en aras de evitar tales complicaciones. Independientemente de la aplicación de los criterios clínicos para el diagnóstico del SM, realizar un test de sobrecarga oral de la glucosa, medir los niveles de HbA1c y evaluar la resistencia a la insulina mediante la determinación del HOMA pudieran ayudarnos a desenmascarar este síndrome antes de las donación. En esta revisión profundizaremos en: 1) la prevalencia del SM en la población general; 2) el impacto del SM sobre la DM, la función renal y otras complicaciones cardiovasculares; 3) la evaluación que debe llevarse a cabo para descartar el SM ante la sospecha clínica del mismo; y 4) el tratamiento de los factores de riesgo del SM para disminuir las consecuencias a largo plazo de esta complicación metabólica tras la donación. En cualquier caso, la detección del SM antes de la donación obliga a tomar las medidas profiláctica y terapéuticas oportunas para evitar su progresión. De no conseguirse este objetivo, el SM pudiera ser considerado una contraindicación para la donación de vivo.
Living donor kidney transplantation (KT) is the treatment of choice for end-stage renal disease patients and exhaustive assessment of the potential live kidney donor leads to successful KT in most occasions. Diabetes mellitus (DM), hypertension and obesity make up contraindications to donation because all of them are associated with postsurgical complications and future development of renal failure and cardiovascular (CV) disorders. However, it is unclear how much risk there is for individuals who donate a kidney and then develop some of these complications, which are grouped under the metabolic syndrome (MS.) Indeed, MS is a cluster of CV risk factors such as obesity, dyslipidemia, hypertension where the insulin resistance is the pathogenic mainstay. MS is an entity very prevalent in the western countries (20-30%) and has been associated with the development of CV disorders, DM and renal disease. Thus, it is crucial to detect MS before living donation in order to avoid these complications in the long-term. Regardless of clinical criteria to diagnosis MS, both oral glucose tolerance test and HbA1c levels may be useful clinical tools for unmasking MS before donation. Moreover, determination of insulin resistance by HOMA could help to achieve this objective. This review will outline the next issues: 1) frequency of MS in the general population (potentially, living kidney donor); 2) the impact of MS on DM, renal function and other CV complications; 3) assessment of living donor to unmask MS before donation; and 4) interventions on risk factors for minimizing MS-related threatening complications in the long-term. In any case, if MS is detected prior to donation, prophylactic and therapeutic measurement should be performed to avoid its progression. By contrast, MS could be considered a contraindication to donation.
INTRODUCTION
50 years have gone by since Joseph Murray and his team performed, in Boston, the first kidney transplant (KT) with a living donor through identical twins. Since then, there are several evidences indicating that this therapeutic procedure can be the most suitable substitution treatment for patients with terminal renal failure and with few long-term complications arising from unilateral nephrectomy from the donor.1-3 In fact, international data records and monocentric studies show that the survival rate of grafts from a living donor is far superior to the one from a dead donor (regardless of other developing clinical factors) with a 20 year average life expectancy for the graft.4-6 Without a doubt, modern immunosuppressors and the concept that HLA compatibility is not a requirement for a prolonged survival of KT with a living donor, have had a crucial contribution in the benefits of this therapeutic modality. Obviously, these facts have made KT with a living donor to significantly increase around the world, currently representing among 20-30% of the global activity of KT.
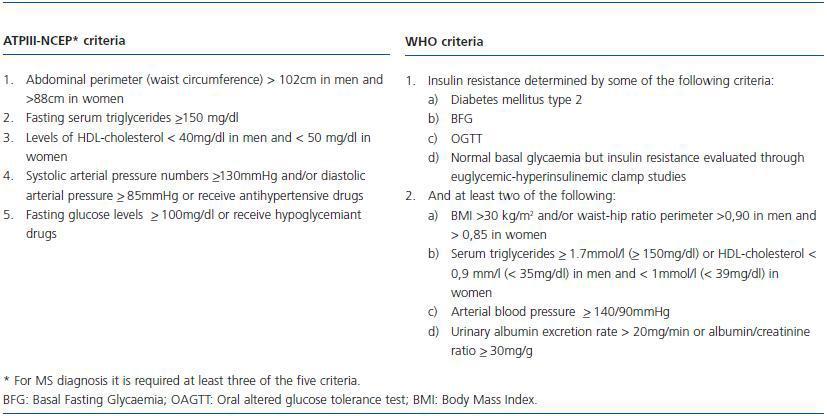
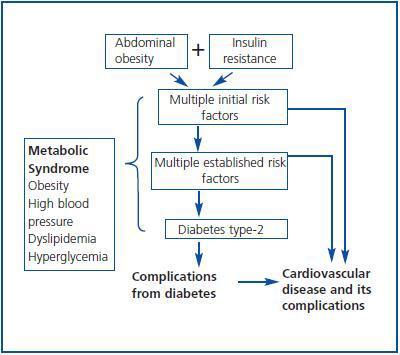
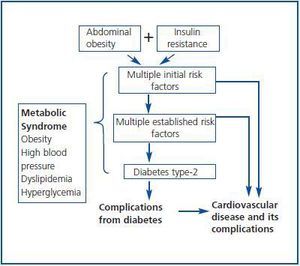
An exhaustive clinical evaluation of the donor reduces the risks of donation and ensures the success of the procedure. In this respect, the Amsterdam Forum establishes that the purpose of the evaluation is to check the health of the donor and rule out all the clinical comorbidities from the donor, including metabolic alterations and heart disorders which may increase post-donation risks.7 Usually, diabetes, high blood pressure and obesity are considered potential contraindications for a living donor, but it is unknown with certainty what is the individual risk for a donor regarding these post-donation changes. In other words, it is unknown if the fact of having only one kidney may accelerate the progression of nephropathy due to these changes in case they are diagnosed after the donation. These clinical entities are classified in the Metabolic Syndrome (MS) according to criteria of ATPIII-NCEP (The Adult Panel of the National Cholesterol Education Program) and the World Health Organization (WHO)8,9 (table 1.) Indeed, the MS is an ensemble of risk factors with a metabolic origin (dyslipemia, high blood pressure, hyperglycaemia, prothrombotic, and inflammatory state) which may lead to the development of DM type-2 and CV disease, where abdominal obesity and resistance to insulin constitute the basic pillars of the pathogenesis (figure 1.) From this point of view, before a living donation, one should question: 1) The MS should be considered a contraindication for living donation given its relation with diabetes, atheromatosis and CV disease? The answer may be positive. During this review these aspects will be fully analysed by approaching the following questions: 1) What is the real prevalence of MS in the general population and, potentially, in the living kidney donor?; 2) What is the impact of MS over DM, renal function and other CV complications?; 3) Who should be evaluated and how should the living donor be evaluated to unmask MS?; and 4) Which modifiable risk factors can intervene to minimize the clinical impact of MS after the donation?
PREVALENCE OF THE METABOLIC SYNDROME IN THE GENERAL POPULATION
The prevalence of MS is variable and depends on age, race and Body Mass Index (BMI.) The American population is estimated to have a prevalence of this syndrome oscillating between 20-30% according to the criteria established by ATPIII-NCEP.10 Also, in the European population without DM the prevalence of MS is nearly 15%, according to WHO, as shown in an observational study of cohorts from more than 11.000 individuals, where MS increased 1.4 times the risk of death from any cause as compared with those that did not present this alteration.11
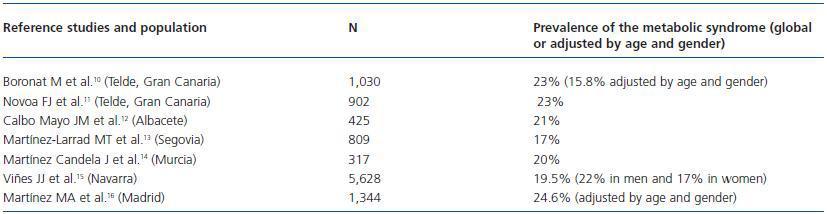
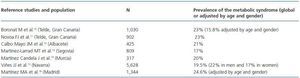
In the Spanish population, the prevalence of MS, according to the ATPIII-NCEP criteria, is around 17-23% with a slight predominance in the general population from the Canary Islands and Madrid over the rest12-18 (table 2.) In any case, the high prevalence of MS in the general population and its important comorbidity justify a deep evaluation of MS before considering a living donation, in order to avoid harmful longterm clinical consequences after the donation.
IMPACT OF THE METABOLIC SYNDROME ON DIABETES MELLITUS, RENAL FUNCTION AND OTHER CV COMPLICATIONS
The MS represents a group of vascular risk factors, which have insulin resistance as a common pathogenic link. Epidemiological studies have demonstrated an association between MS and the development of CV diseases and DM type-2.19 In fact, a recent study of prospective cohorts showed that the global and cardiovascular mortality were considerably higher in patients with MS after an average monitoring period of 9 years. This syndrome provided additional prognostic information; therefore, MS can be considered an independent risk factor for mortality after adjusting other classic cardiovascular risk factors.20 In this line, a metanalysis of 21 studies of prospective cohorts demonstrated that the MS, determined by ATPIII-NCEP and WHO criteria, was an important death and CV disease risk factor. In particular those individuals with MS had a overall increase of 61% of the risk of CV disease.21 This was confirmed in a systematic review with its corresponding meta-analysis of 38 longitudinal studies which included 172.573 individuals, in order for those with MS to have 1.78 more risk of some cardiovascular event and death than those without this alteration.22 So, early detection and treatment of modifiable risk factors for MS should reduce cardiovascular morbidity associated to this syndrome, which can be of considerable importance in potential living kidney donors.
Transversal studies have shown an association between the biological resistance phenomenon to insulin and subclinical atheromatosis, evaluated by performing a carotid intima-media thickness ultrasound test; this was documented in the general population and in renal patients, including those with KT.23 It is very likely that the endothelial dysfunction associated to metabolism alterations of glucose may have a decisive contribution to vascular damage, as observed in patients with DM type-2 after an increase of urinary excretion of 8-iso PGF2a, which is a significant marker of oxidative stress.24
In an observational study carried out in a Portuguese population, the MS was related to structural and functional alterations of the heart. The left ventricular mass and the risk of ventricular disorder increased progressively with the number of MS components (associated to the MS’s severity), regardless the score of the vascular risk of Framingham’s study.25 Also, this syndrome was associated with the development of left ventricular hypertrophy and early changes in the diastolic function in the European population, adjusting the clinical factors that may change the ventricular mass according to age and gender.26 It is possible that the increase of the values of systolic and diastolic arterial pressure may have contributed to these cardiac abnormalities as demonstrated in a recent meta-analysis in healthy individuals that were subjected to an unilateral nephrectomy for a living donation.27 These alterations could justify a larger number of cardiovascular events in individuals with MS. Undoubtedly, this demands a thorough evaluation of the possible existence of MS in potential living renal donors in order to avoid future cardiovascular complications after a donation.
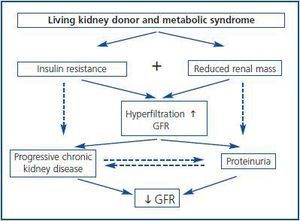
What is the clinical impact of MS over the renal function? Individuals with MS present early hyperfiltration and progressive increase of the Glomerular Filtration Rate (GFR) according to the number of criteria or severity of the MS itself. Possibly this effect is induced through the release of growth factors such as insulin, IGF-1 or the leptin involved in the MS’s pathogenesis, which predispose the emergence of a situation of glomerular hyperfiltration. At the same time, this hyperfiltration was undoubtedly an early marker of the emergence of proteinuria and progressive deterioration of GFR.28 In fact, epidemiological studies demonstrated the narrow relation between MS and expressed renal damage, evidenced as proteinuria and reduction of GFR.29,30 In this sense, it was observed in longitudinal studies that nondiabetic individuals with MS suffer a greater risk of developing chronic renal failure after several years of monitoring, regardless of other risk factors.31
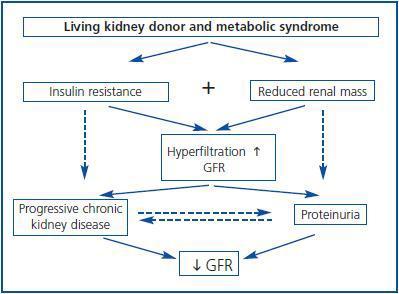
Furthermore, individuals subjected to a unilateral nephrectomy suffer a greater risk of developing proteinuria and reduction of GFR when compared to the controlled ones. This was observed in a systematic review of 48 studies which included 5.048 living kidney donors.32 However, how are these processes connected? In other words, what is the natural history of these changes? Theoretically, in individuals with MS subjected to nephrectomy for living donation, insulin resistance and reduction of renal mass can induce hyperfiltration in the remnant kidney. This is associated to proteinuria and progressive renal disease (figure 2.) Therefore, the combination of these risk factors of renal failure (insulin resistance and reduction of nephron mass) should be avoided in individuals who are candidates to living donation of a kidney in order to minimize the risk of suffering long-term renal complications. In addition, obese patients undergoing a nephrectomy suffer a greater risk of proteinuria and renal disorder than non-obese patients.33 In a similar way, in non-diabetic patients with KT and stable renal function beyond the first year of post-KT, the emergence of MS is more frequently associated with graft loss and mortality than those without this condition. Furthermore, MS was an independent risk factor for loss of renal function during monitoring, which supports the harmful effects that this syndrome causes in renal structure and function.34
Who should be evaluated and how should the living donor be evaluated in order to rule out the presence of metabolic syndrome?
Amsterdam Forum establishes that individuals with a strong family history of DM type-2 or with a definitive diagnosis of it should not be donors due to the potential risk of development and progression of diabetic nephropathy.7 Therefore, it is crucial to evaluate the risk of developing DM type 2 in a potential living donor, especially if there is MS. The American Diabetes Association (ADA) recommends a systematic screening of DM type-2 in the following situations: 1) individuals with family history of DM type-2; 2) obese patients with BMI >30 kg/m2; 3) excessive alcohol consumption; 4) Individuals originating from the black population (African Americans), Native Americans (Hispanic origin) or American Indigenous peoples; 5) and in patients with high blood pressure, dyslipidemia and an age over 40 years old. With these in mind, it is possible to reasonably rule out DM type-2 in an important number of individuals, but on many occasions, the beginning of DM type-2 is uncertain and asymptomatic. Hence, it is crucial to estimate more precisely the probability of developing this disease after the donation.
Several risk indices have been developed for estimating the probability of developing DM type-2, but their predictive ability has been highly variable due to the exclusion of important risk factors regarding the development of DM type- 2.35-40 Recently, a European prospective cohorts study with 25,167 individuals determined with precision the risk of developing DM type-2. This risk score was based on the calculation of the statistical weight (β coefficient) of those risk factors that in the multivariate analysis were associated with the emergence of DM type-2 (anthropometric clinical data, diet factors and lifestyle), neatly ranking individuals in different risk levels regarding the development DM type-2 during a five-year period from a certain score.41 For example, for a total score of 300, the probability of developing DM type-2 is 0.3%, while at the other end, with a score of 750, the probability is 23%. Undoubtedly, this risk index is a very useful clinical tool for identifying individuals in risk of DM type-2 or those who have this condition and remain undiagnosed.
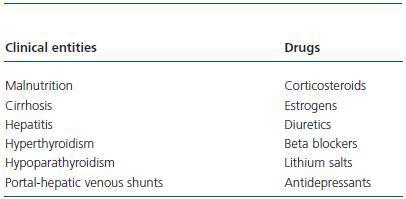
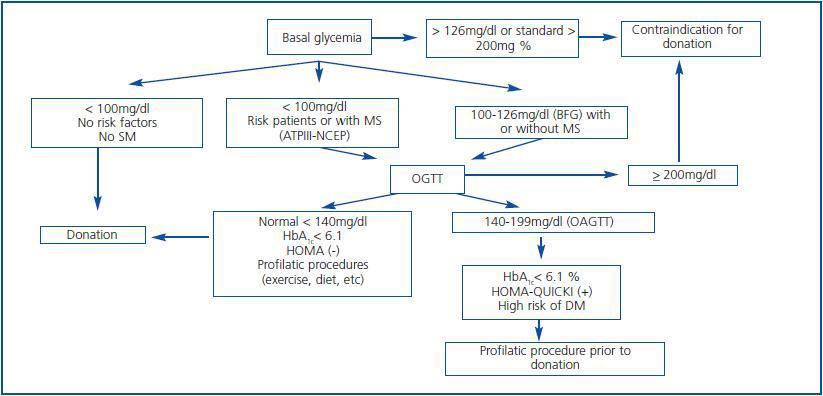
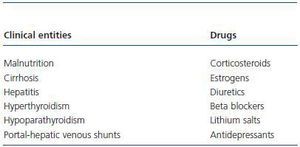
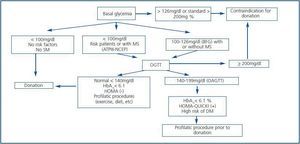
With these premises, the detection of prediabetes is critical to the medical evaluation process of a potential living donor. There are several biochemical and hormonal conditions for the screening of DM type-2, but in clinical practice, basal fasting glucose and Oral Glucose Tolerance Test (OGTT) are standard tests recommended for the diagnosis of DM type-2 including kidney patients.42 For this purpose, these have been adopted by the ADA and WHO. Table 3 shows the criteria for the diagnosis of DM type-2, Basal Fasting Glycaemia (BFG) and Oral Altered Glucose Tolerance Test (OAGTT) from the conditions set by these international organizations.43 A recent report by the UNOS (United Network for Organ Sharing) about living donations showed that most centres in the USA. use OGTT for detecting DM type-2, especially in individuals at risk. This same report also documented that the use of such screening has developed over the years in different centres.44 It is important to consider that certain medical institutions and some drugs may provide a BFG or an OAGTT, which is very important towards the evaluation of the living donor (Table 4.) Figure 3 shows a flow chart for detecting DM type-2 before a living kidney donation. BFG (basal glycaemia > 126mg/dl) or glycaemia > 200 mg/dl at any moment of the day is a contraindication for living donation. If basal glycaemia <100mg/dl and there are no risk factors for developing MS, the donation could be performed with minimum long-term risks to the donor. In the case of a BFG (basal glycaemia 100-126mg/dl), an OGTT should be performed. Furthermore, in individuals with risk of developing MS (obese, hypertensive, etc.) or those with evident MS according to the ATPIII-NCEP or WHO criteria, it should also be performed an OGTT. If this test is normal (glycaemia after two hours of an overload < 140mg/dl) and there are no other additional risk factors (HbA1c < 6,1%, negative HOMA, etc.) a donation could be performed, or if not, carry out a prophylactic measure (weight loss, physical exercise, balanced diet, etc.) to minimize any risk factor in case there are any. However, before performing a OAGTT (glycaemia 140-200 mg/dl after two hours of oral glucose overload) and the possible presence of additional risk factors for the development of MS (HbA1c > 6,1%, positive HOMA, etc.) therapeutic interventions and/or prophylactic measures should be performed to normalize the levels of glycaemia and significantly decrease the risk factors of this syndrome. In any case, if this objective is reached in individuals of risk (MS clinical criteria or OAGTT), it would be advisable to perform an OGTT every year after the donation in order to make an early detection of any glycaemia alteration. However, even if with prophylactic measures it is not possible to normalize the levels of glycaemia, performing a living donation is not advisable given the risk of developing DM type-2 or any of its complications in the future. Finally, if OGTT shows a 200mg/dl glycaemia, then its DM type-2 and therefore the donation is not advisable.
The HbA1c levels do not comprise the criteria established for the diagnosis of DM type-2, but previous studies have shown that this determination can be a simple measure and with low economical costs for the screening of this condition. In particular, a HbA1c > 6,1% has a higher sensitivity (61%) than basal glycaemia levels (45%) when using OGTT to identify patients of the risk of developing DM type-2.45
The biochemistry methods, which detect the insulin resistance phenomenon, a MS common pathogenic mechanism, could provide a useful determination to detect prediabetic states before living donation. In this respect, the euglycemichyperinsulinemic clamp is a reference method for quantifying insulin sensitivity in humans, but requires a high time consumption and of resources useful for daily clinical practice. Currently, simpler valid methods used to determine insulin sensitivity are HOMA (Fasting Insulin and Homeostasis Model Assessment) and QUICKI (Quantitative Insulin-Check Sensitivity Index), which evaluate insulin sensitivity from mathematical transformations of fasting glycaemia and insulin levels. These determinations have proved to be good predictors of insulin sensitivity in individuals with and without obesity, as well as in patients with DM type-2 and high blood pressure, using as gold standard the glucose euglycemic-hyperinsulinemic clamp.46,47 There is a narrow relation between obesity and inflammation, in such a way that some inflammatory markers such as TNFalpha, IL-6 and PCR are associated with insulin resistance and involved in the pathogenesis of DM type-2, mainly in the obese population. Furthermore, low levels of adiponectin are closely related with the development of DM type-2.48 In this sense, several reviews have shown that genetic polymorphisms of TNF-alpha (G-308A), IL-6 (174 G/C) and adiponectin (11391 G/A and 276 G/T) are strictly associated to the development of DM type-2,49-51 which can be a very useful clinical tool for detecting individuals at risk of this disease.
Lastly, given the close relation between MS and cardiovascular disease, it should be carried out a thorough evaluation of potential injuries in target organs from potential living donors with this syndrome. This would include an echocardiography, evaluation of the renal function and levels of microalbuminuria, a carotid echography to determine the carotid artery intima-media thickness and assess the presence of peripheral vascular disease.
APPLICATION OF RISK FACTORS OF THE METABOLIC SYNDROME TO REDUCE ITS CLINICAL IMPACT
Presumably, the therapeutic intervention of MS risk factors may modulate the clinical impact of this syndrome in a long term. However, what modifiable factors may be involved to achieve this objective? At present, it is unknown if whether reducing insulin resistance per se can prevent the emergence of diabetes, cardiovascular complications and renal disease. However, it is feasible to believe that improving insulin sensitivity through exercise, weight loss, stop smoking and a balanced diet are appropriate strategies to minimize the negative consequences of this clinical syndrome. Furthermore, the administration of drugs that reduce insulin resistance or appetite and the blockage of the Reninangiotensin system (RAS) can contribute to this goal. Figure 4 shows some therapeutic measures that can improve insulin sensitivity in individuals with MS. Regarding this issue, a controlled European study that included randomized individuals with OAGTT, showed a reduced risk of DM of nearly 58% with non-pharmacological measures such as changes in daily life, diet and physical exercise after a threeyear monitoring.52
Drugs that increase insulin sensitivity may reduce considerably the clinical risks inherent to MS. In a randomized study with 3,234 individuals with OAGTT or BFG (basal glycaemia 100-126mg/dl), the administration of metformin significantly decreased the incidence of DM after four years of monitoring compared to the placebo. However, changes in daily life also mimic the beneficial effects of metformin.53 Also, thiazolidinedione (such as rosiglitazone) also represent new drugs known to increase insulin sensitivity. In this respect, a controlled study showed that the administration of rosiglitazone (8mg/day) decreased the incidence of DM or death (60%) after three years of monitoring in individuals with BFG or OAGTT versus the placebo.54
Rimonobant is a drug that blocks cannabinoid receptors B1, considerably reducing the appetite, which translates into a reduction in body weight. In a randomized clinical trial performed with obese individuals (BMI > 27kg/m2), this drug was able to significantly reduce body weight and the abdominal perimeter, as well as improve the metabolic and lipid profile when compared to the placebo.55
Other drugs such with sibutramine (anorectic), acarbose (inhibitor of intestinal absorption of carbohydrates) or orlistat (inhibitor of fat absorption), amongst others, can prevent or delay the development of DM in individuals with MS. In this respect, a meta-analysis that included 17 randomized clinical trials and 8,084 individuals with OAGTT showed that the majority of the drugs used for MS, as well as changes in daily life, could achieve these objectives in a similar way (risk reduction of 56% and 49%, respectively).56
Recently, it has been observed that co-administration of metformin and dipeptidyl peptidase-4 inhibitors (sitagliptin) prevents enzymatic degradation and lethargy of incretins (GLP-1 and insulinotropic peptides involved in glucose homeostasis) and can improve the levels of glycaemia and HbA1c in individuals with carbohydrate alterations. This behaviour could also be an alternative therapy to temporarily improve insulin resistance in individuals with MS before the donation.57
Furthermore, other measures such as controlling blood pressure, administration of statins, avoid the abuse of alcohol and smoking, as well as blocking SRA, can help reduce the risk of progression of MS. Indeed, drugs that block SRA (ACE inhibitors and ARBs) increased hepatic glucose production and improved insulin sensitivity, reducing the risk of DM type-2 in individuals with MS. A meta-analysis of 11 clinical trials involving 66,608 patients showed that the use of ACE inhibitors / ARBs prevented the emergence of DM, regardless of the indication for high blood pressure, ischemic heart disease or ventricular disorder.58 Therefore, these drugs may be indicated for living donors at the risk of developing DM after donation. If to this is added the selective modulation activity (partial agonist) of PPAR-g (peroxisomes proliferator-activated receptor gamma), improving insulin sensitivity of some ARBs such as telmisartan, could provide an additional clinical benefit for preventing DM.59
To summarise, MS is very common in the general population and, presumably, in living kidney donors (evidence level B). This syndrome prompts the development of DM type-2, cardiovascular disease and renal disorders (evidence level A.) These facts, along with a small renal mass after donation, could accelerate the development of proteinuria and longterm progressive renal failure (evidence level B.) Therefore, a thorough evaluation of the donor for an early detection of this syndrome, the treatment of this alteration with proper medication and changes in daily life will make these individuals viable for living donation (evidence level B.)
Table 1. Clinical criteria for diagnosis of metabolic syndrome according to ATPIII-NCEP criteria (The Adult Panel of the National Cholesterol Education Program) and the World Health Organization (WHO)
Figure 1.
Table 2. Prevalence of the metabolic syndrome in Spain¿s general population
Figure 2.
Table 3. Criteria from the American Diabetes Association and World Health Organization for the diagnosis of alterations in carbohydrates
Table 4. Clinical entities and drugs that can provide a basal fasting glycaemia or a positive oral glucose tolerance test
Figure 3.